Abstract
Purpose
To prospectively compare survival between human immunodeficiency virus (HIV)-infected versus HIV-uninfected cervical cancer patients who initiated curative chemoradiation therapy (CRT) in a limited-resource setting.
Methods and Materials
Women with locally advanced cervical cancer with or without HIV infection initiating radical CRT in Botswana were enrolled in a prospective, observational, cohort study from July 2013 through January 2015.
Results
Of 182 women treated for cervical cancer during the study period, 143 women initiating curative CRT were included in the study. Eighty-five percent of the participants (122 of 143) had stage II/III cervical cancer, and 67% (96 of 143) were HIV-infected. All HIV-infected patients were receiving antiretroviral therapy (ART) at the time of curative cervical cancer treatment initiation. We found no difference in toxicities between HIV-infected and HIV-uninfected women. The 2-year overall survival (OS) rates were 65% for HIV-infected women (95% confidence interval [CI] 54%-74%) and 66% for HIV-uninfected women (95% CI 49%-79%) (P = .70). Factors associated with better 2-year OS on multivariate analyses included baseline hemoglobin >10 g/dL (hazard ratio [HR] 0.37, 95% CI 0.19-0.72, P = .003), total radiation dose ≥75 Gy (HR 0.52, 95% CI 0.27-0.97, P = .04), and age <40 years versus 40-59 years (HR 2.17, 95% CI 1.05-4.47, P = .03).
Conclusions
Human immunodeficiency virus status had no effect on 2-year OS or on acute toxicities in women with well-managed HIV infection who initiated curative CRT in Botswana. In our cohort, we found that baseline hemoglobin levels, total radiation dose, and age were associated with survival, regardless of HIV status.
Introduction
Cervical cancer is one of the most common cancers in women globally, with sub-Saharan Africa (SSA) bearing the highest burden (1). The most important risk factor for cervical cancer development is chronic persistent human papillomavirus (HPV) infection (2). Women with human immunodeficiency virus (HIV) infection are more likely to have persistent HPV infection causing cervical abnormalities and cancer (3). This is particularly important in SSA, where HIV infection is hyperendemic (4) and access to cervical cancer screening and HPV vaccination is limited (5).
Botswana, a country in SSA (Fig. 1), has had one of the most severe HIV epidemics globally, with more than 50% of women aged 25 to 45 years living with HIV and consequently at risk for developing cervical cancer (4, 7, 8). In response, Botswana developed the first public HIV treatment program in Africa, with coverage now exceeding that of even the best-performing high-income countries (9). However, despite expanding access to antiretroviral therapy (ART), cervical cancer incidence has not declined (10, 11). As a result, public health interventions, such as a national cervical cancer screening program and an HPV vaccination program, are being implemented in Botswana to reduce the burden of locally advanced cervical cancer in the future (11, 12). However, it will take several years to observe the benefits of such interventions.
Fig. 1.
Map of Botswana in Sub-Saharan Africa (6), high-lighting Botswana and Gaborone, the capital city, where the single radiation therapy department in Botswana is based.
High rates of HIVand cervical cancer in SSA underscore the urgent need to identify the optimal therapy for HIV-infected women with cervical cancer, particularly as life expectancy for HIV-infected women is increasing.
Treatment of locally advanced cervical cancer with radiation therapy given concurrently with cisplatin-based chemotherapy has become the global standard (13–15). However, the outcomes of curative chemoradiation therapy (CRT) among HIV-infected patients are not well described. Studies from the United States (16) and from Botswana (17) indicate that survival may be poorer for HIV-infected compared with HIV-uninfected women with cervical cancer; however, those studies had limited treatment data and included women unable to receive CRT.
We sought to prospectively compare survival between HIV-infected versus HIV-uninfected cervical cancer patients who initiated curative CRT in a limited-resource setting.
Methods and Materials
This prospective, observational, cohort study included women with cervical cancer who presented for CRT between July 2013 and January 2015 at the only radiation oncology facility in Botswana, located in the capital city of Gaborone (Fig. 1). Initial patient data, including demographics, distance from the treatment facility, marital status, cancer screening history, initial presenting symptoms, history of HIV, delays in presentation (defined as the duration of symptoms before treatment and the duration between the first physician visit and presentation for treatment), time to treatment initiation (defined as the time from biopsy to treatment initiation), and treatment prescribed, were collected at the initial consultation through patient interviews and review of medical charts. Chemotherapy cycles received were recorded during weekly treatment visits. At the end of treatment the total radiation dose received to point A was calculated using the radiobiological equivalent dose (EQD2) formula (18). Tumor response was recorded from patient medical records at the end of treatment.
All the data were collected electronically using predesigned data forms. Research software available locally in Botswana was used to develop the study forms and save the data.
This study was reviewed and approved by the institutional review board at the University of Pennsylvania and by the Ministry of Health in Botswana.
Main outcome
The primary endpoint of this study was overall survival (OS), defined as the time from the scheduled end of CRT until death or until last contact with the patient. If a patient or next of kin could not be reached by telephone, medical records were searched to determine vital status or the date of the patient’s last visit to a health care facility, at which point the patient was censored if vital status was not available. At the time of analysis, less than 10% of all patients (or patients’ next of kin) were not reachable by phone, and their vital status was obtained through the electronic medical records.
Cervical cancer treatment
Botswana’s government provides free publically delivered healthcare for all of its citizens. However, radiation therapy is not currently available in the public sector. Therefore, patients are referred to a private hospital for CRT, which is fully funded by the government (6, 11).
Cervical cancer for each patient was staged clinically according to the International Federation of Gynaecology and Obstetrics staging criteria (19). Basic laboratory studies (ie, complete blood count, renal function test), chest x-ray, and abdominal ultrasound were done before treatment. Patients with hemoglobin levels <10 g/dL received blood transfusions when blood was available (limitations in the blood bank infrastructure translate into frequent national shortages of blood). Women with good performance status (ie, Karnofsky performance score of ≥70 [20], baseline hemoglobin level >8 g/dL, receiving ART if HIV-infected, without renal dysfunction, or with distant metastatic disease) were offered radical CRT, which consisted of 45 to 50 Gy whole-pelvis radiation, weekly concurrent cisplatin treatment (35-40 mg/m2) for 5 cycles, and high-dose-rate (HDR) brachytherapy (7 Gy × 3 fractions or 6 Gy × 4 fractions) using either a tandem and ring applicator or a tandem and ovoid applicator. Computed tomography–based treatment simulation with a 16-slice computed tomography scanner was routinely performed for external beam treatment planning and for the first brachytherapy fraction. Patients were treated on a linear accelerator with either 4 fields (anterior/posterior and right/left lateral) or 2 fields (anterior/posterior). Organs at risk were contoured, and dose was prescribed to point A. Subsequent brachytherapy fractions used the plan from the initial fraction and were delivered by an HDR 192Ir afterloader (6, 21).
Antiretroviral treatment
The Botswana National ART program provides ART free of charge to all citizens (4, 7, 8). During the study period, patients with CD4 cell counts of ≥350 cells/μL, with cervical cancer, or with other World Health Organization HIV stage 3 or 4 conditions (22) were eligible for ART. Standard first-line ART included a combination of tenofovir, emtricitabine, and efavirenz (5). All study participants with a negative or unknown HIV status were tested for HIV before the initiation of cancer treatment. Antiretroviral therapy was started for all HIV-infected patients who had not yet received it before cervical cancer diagnosis.
Toxicity
During cancer treatment the patients were evaluated weekly to determine hematologic toxicity, renal function, and nonhematologic toxicity according to Common Terminology Criteria for Adverse Events version 4.0 grading (23). Hematologic function was evaluated weekly from complete blood counts, hemoglobin levels (g/dL), white blood cell counts (×109/L), and absolute neutrophil counts (×109/L). Renal function was measured as serum creatinine (μmol/L). Patients were evaluated weekly for the following: performance status using Karnofsky performance score, fatigue, weight loss, gastrointestinal toxicity (eg, nausea, vomiting, diarrhea), urinary toxicity (eg, frequency, urgency, incontinence), and radiation dermatitis.
Follow-up
Follow-up care after cancer treatment is not routinely done in Botswana owing to limited personnel in oncology and challenges (distance and cost) for patients to travel to the oncology clinic. For this study, follow-up after treatment was done by telephone at 6 weeks after treatment and then every 3 months thereafter; questions were asked to assess toxicity or cancer recurrence. Patients with any symptoms consistent with a recurrence (eg, new pain or bleeding) were asked to return to the clinic.
Statistical analysis
Patient demographics, clinical and treatment characteristics, and type and severity of acute toxicity were compared between HIV-infected and HIV-uninfected patients using χ2 tests. Student’s t tests or nonparametric tests were used, as appropriate. Overall survival was estimated using the Kaplan-Meier method (24). Cox regression models were used to identify factors associated with OS. Univariate analyses were done after purposeful selection, and factors with P values of ≤ .10 or with clinical significance were included in a multivariate analysis. The final model included 143 patients and 46 events. No collinearity was noted between any of the factors in the final model. All tests were 2-tailed, and P values of < .05 in multivariate analysis were considered statistically significant. All statistical analyses were carried out using commercially available software (STATA version 13; StataCorp, College Station, TX).
Results
Of the 182 patients with cervical cancer expected to receive CRT during the study period, 39 were excluded (30 for not receiving any chemotherapy, 6 for treatment with a palliative radiation dose, and 3 who did not return for treatment), resulting in 143 patients (79%) included in this analysis. The median follow-up time for all patients was 630 days (interquartile range [IQR], 349-842 days), including 633 days (IQR, 348-819 days) for HIV-infected patients and 627 days (IQR, 396-885 days) for HIV-uninfected women. Forty-six of the women (32%) died during the follow-up period.
Patient, disease, and treatment characteristics are shown in Tables 1 and 2.
Table 1.
Patient demographic characteristics by HIV status
| Characteristic | HIV-infected (n = 96 [67%]) | HIV-uninfected (n = 47 [33%]) | P |
|---|---|---|---|
| Age (y) | .004 | ||
| 24-39 | 36 (37.5) | 9 (19.2) | |
| 40-59 | 53 (55.2) | 26 (55.3) | |
| >60 | 7 (7.3) | 12 (25.5) | |
| Marital status | .001 | ||
| Single | 65 (68.4) | 16 (34.0) | |
| Married/partnered | 19 (20.0) | 19 (40.4) | |
| Divorced/widowed | 11 (11.6) | 12 (25.5) | |
| Previously screened for cervical cancer | 69 (71.9) | 21 (44.7) | .002 |
| Age at first sexual activity (y) | 18 (16-20) | 18 (16.5-20) | .72 |
| Duration of symptoms before diagnosis (mo) | 5 (3-12) | 5 (2-12) | .15 |
| Time to treatment presentation* (mo) | 3 (2-6) | 4 (3-8) | .21 |
| Time to treatment initiation† (d) | 116.5 (77-153) | 114 (66.5-152) | .67 |
| Distance from treatment facility (km) | 310 (94.6-310) | 94.6 (59.2-310) | .03 |
Abbreviation: HIV = human immunodeficiency virus.
Values are presented as number (percentage) or median (interquartile range).
Interval between first physician visit and presentation for radiation therapy.
Time from biopsy to treatment initiation.
Table 2.
Clinical and treatment characteristics of patients by HIV status
| Characteristic | HIV-infected (n = 96 [67%]) | HIV-uninfected (n = 47 [33%]) | P |
|---|---|---|---|
| Cervical cancer histology | .29 | ||
| Adenocarcinoma | 7 (7.3) | 6 (12.8) | |
| Squamous cell carcinoma | 89 (92.7) | 41 (87.2) | |
| Disease stage | .04 | ||
| I (IA, IB) | 14 (14.9) | 3 (6.4) | |
| II (IIA, IIB) | 57 (60.6) | 24 (51.1) | |
| III (IIIA, IIIB) | 23 (24.5) | 18 (38.3) | |
| IV | 0 (0) | 2 (4.3) | |
| Symptoms at presentation | |||
| Vaginal bleeding | 66 (68.8) | 28 (59.6) | .28 |
| Post coital bleeding | 35 (36.5) | 12 (25.5) | .19 |
| Vaginal discharge | 69 (71.9) | 35 (74.5) | .74 |
| Pelvic/back pain | 70 (72.9) | 28 (59.6) | .11 |
| Bowel/bladder | 5 (5.2) | 2 (4.3) | .80 |
| Edema | 3 (3.1) | 7 (14.9) | .01 |
| Baseline laboratory values | |||
| Creatinine (μmol/L) | 50 (41-57) | 54 (47-62) | .02 |
| Hemoglobin (g/dL) | 10.6 (9.2-12.1) | 11.3 (10.5-13) | .04 |
| ANC (×109/L) | 3.55 (2.50-6.67) | 4.63 (3.01-6.36) | .35 |
| WBC count (×109/L) | 5.77 (4.29-8.85) | 6.83 (4.75-8.91) | .49 |
| Baseline performance status (KPS) | .18 | ||
| ≥90 | 66 (68.8) | 27 (57.4) | |
| <90 | 30 (31.2) | 20 (42.6) | |
| HIV characteristics | |||
| CD4 (cells/μL) | 481 (351-579) | - | |
| CD4 category | |||
| ≥500 | 40 (47.1) | ||
| ≥350-<500 | 24 (28.2) | ||
| <350 | 21 (24.7) | ||
| On ART | 92 (95.8) | - | |
| Months on ART | 84 (24-120) | ||
| Treatment characteristics | |||
| No. of chemo cycles received | 4 (3-5) | 4 (2-4) | .45 |
| No. of chemotherapy cycles completed | .40 | ||
| 1 | 10 (10.4) | 4 (8.5) | |
| 2 | 8 (8.3) | 9 (19.2) | |
| 3 | 22 (22.9) | 9 (19.2) | |
| 4 | 29 (30.2) | 15 (31.9) | |
| 5 | 27 (28.1) | 10 (21.3) | |
| ≥4 | 56 (58.3) | 25 (53.2) | .56 |
| Received EBRT dose ≥45 Gy | 95 (99.0) | 45 (95.7) | .21 |
| Received brachytherapy dose ≥20 Gy | 73 (76.0) | 32 (68.1) | .31 |
| EQD2 (Gy) | |||
| Median (IQR) | 79.8 (74-79.8) | 79.8 (68.8-79.8) | .90 |
| Mean (standard deviation) | 75.6 (8) | 74.7 (10) | |
| Treatment duration (d) | 45 (41-52) | 48 (42-54) | .34 |
| Tumor response | .39 | ||
| Complete | 44 (45.8) | 20 (42.6) | |
| Partial | 12 (12.5) | 3 (6.4) | |
| Not available | 40 (41.7) | 23 (51.1) |
Abbreviations: ANC = absolute neutrophil count; ART = antiretroviral therapy; EBRT = external beam radiation therapy; EQD2 = radiobiological equivalent dose; KPS = Karnofsky performance score; WBC = white blood cells. Other abbreviation as in Table 1.
Values are presented as number (percentage) or median (interquartile range), unless otherwise noted.
Two-thirds (67% [96 of 143]) of the patients were HIV-infected. Relative to HIV-uninfected patients, HIV-infected patients were younger, were more likely to be single, were more likely to have been screened for cervical cancer at least once, and were more likely to live a greater distance from the treatment facility. Patients with HIVinfection presented with earlier-stage disease than HIV-uninfected patients (P = .04).
In terms of other patient characteristics, baseline hemoglobin levels were higher in the HIV-uninfected group (median 11.3 g/dL; IQR, 9.2-12.1 g/dL) than in the HIV-infected group (median 10.6 g/dL; IQR, 10.5-13 g/dL) (P = .04), and baseline serum creatinine levels were also higher in the HIV-uninfected group (median 54 μmol/L; IQR, 47-62 μmol/L) than in the HIV-infected group (median 50 μmol/L; IQR, 41-57 μmol/L) (P = .02).
Among the HIV-infected patients, the median CD4 cell count at the time of cervical cancer diagnosis was 481 cells/μL (IQR, 351-579 cells/μL). At the time of presentation for CRT, 96% (92 of 96) of the HIV-infected patients had already been taking ART for a median of 84 months (IQR, 24-120 months). The 4 remaining HIV-infected patients began ART before they initiated cervical cancer treatment.
No difference was found between HIV-infected and HIV-uninfected groups in treatment duration or cancer treatment received (Table 2).
Toxicities
Hematologic values and nonhematologic toxicity during and after treatment for the HIV-infected and HIV-uninfected groups are shown in Table 3. No differences were found in nadir hemoglobin, absolute neutrophil count, or white blood cell count during or after treatment according to HIV infection status; serum creatinine levels were higher for the HIV-uninfected group after treatment (P = .008), but all values remained within normal limits. Overall, radiation dermatitis and gastrointestinal symptoms were the most common nonhematologic forms of toxicity, with 55% of both groups having grade ≥2 radiation dermatitis and more than half of both groups having grade ≥2 gastrointestinal symptoms.
Table 3.
Toxicity and performance status score by HIV status
| Variable | HIV-infected | HIV-uninfected | P |
|---|---|---|---|
| Hematologic values during treatment | |||
| Nadir hemoglobin level (g/dL), mean (SD) | 10.4 (1.79) | 10.4 (1.70) | 1.0 |
| Nadir ANC (×109/L) | 1.9 (1.58-2.85) | 2.16 (1.73-3.48) | .15 |
| Nadir WBC (×109/L) | 2.95 (2.39-4.03) | 3.12 (2.4-4.5) | .28 |
| Hematologic values at end of treatment | |||
| Hemoglobin (g/dL) | 10.7 (1.42) | 11 (1.67) | .23 |
| Renal function during treatment | |||
| Creatinine (μmol/L) | 52 (47-62) | 56 (47-64) | .40 |
| Renal function at the end of treatment | |||
| Creatinine (μmol/L) | 48 (41-54) | 53 (46-66) | .008 |
| Grade ≥2 nonhematologic toxicity during treatment | |||
| Fatigue | 12 (12.6) | 6 (12.8) | .98 |
| Weight loss | 6 (6.3) | 7 (14.9) | .10 |
| Urinary | 6 (6.3) | 3 (6.4) | .99 |
| Dermatitis | 52 (55.3) | 27 (57.4) | .81 |
| Gastrointestinal | 52 (54.7) | 26 (55.3) | .95 |
| Minimum performance status (KPS) | .16 | ||
| ≥90 | 40 (42.1) | 14 (29.8) | |
| <90 | 55 (57.9) | 33 (70.2) |
Survival
With a median follow-up interval of 630 days, the 2-year OS rate for all patients was 65.5% (95% confidence interval [CI] 56%-73%) overall, 65% (95% CI 54%-74%) for HIV-infected patients, and 66% (95% CI 49%-79%) for HIV-uninfected patients (P = .70) (Fig. 2). On univariate Cox regression analysis, a baseline hemoglobin level of >10 g/dL, receipt of a radiation dose ≥75 Gy, and receipt of ≥4 cycles of chemotherapy (compared with 1 cycle) were associated with a better 2-year OS (Table 4). Human immunodeficiency virus status, however, had no effect on 2-year OS. In a multivariate analysis, a baseline hemoglobin level >10 g/dL (hazard ratio [HR] 0.37, 95% CI 0.19-0.72) and receipt of a total radiation dose ≥75 Gy (HR 0.52, 95% CI 0.28-0.99) were associated with better 2-year OS, whereas older age (40-59 years vs <40 years) (HR 2.17, 95% CI 1.05-4.47) was associated with worse 2-year OS. Human immunodeficiency virus status was not associated with 2-year OS in this analysis (Table 4).
Fig. 2.
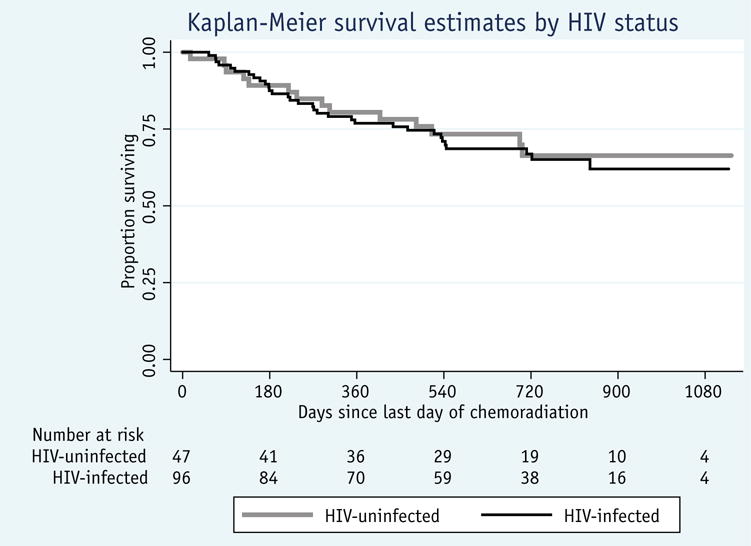
Overall survival by human immunodeficiency virus (HIV) status for cervical cancer patients treated with curative-intent chemoradiation.
Table 4.
Univariate and multivariate Cox regression analysis of predictors of death
| Bivariate
|
Multivariate
|
|||
|---|---|---|---|---|
| Variable | Hazard ratio | 95% CI | Hazard ratio | 95% CI |
| HIV status | ||||
| Uninfected | Reference | |||
| Infected | 1.12 | 0.60-2.12 | 1.12 | 0.57-2.20 |
| Disease stage | ||||
| I (IA, IB) | Reference | |||
| II (IIA, IIB) | 2.21 | 0.67-7.32 | 1.65 | 0.47-5.79 |
| III (IIIA, IIIB)/IV | 2.87 | 0.84-9.81 | 2.32 | 0.65-7.54 |
| Age (y) | ||||
| <40 | Reference | |||
| 40-59 | 1.48 | 0.74-2.96 | 2.17* | 1.05-4.47* |
| >60 | 1.22 | 0.45-3.29 | 2.46 | 0.80-7.54 |
| Baseline hemoglobin level (g/dL) | ||||
| ≥10 | Reference | |||
| >10* | 0.39* | 0.22-0.70* | 0.37* | 0.19-0.72* |
| No. of chemotherapy cycles | ||||
| 1 | 2.51* | 1.12-5.61* | 2.25 | 0.99-5.13 |
| 2 | 0.86 | 0.30-2.49 | 0.67 | 0.22-2.06 |
| 3 | 1.08 | 0.52-2.27 | 1.10 | 0.52-2.35 |
| ≥4* | Reference | |||
| Final EQD2 dose (Gy) | ||||
| <75 | Reference | |||
| ≥75* | 0.56* | 0.31-1.01* | 0.52* | 0.27-0.97* |
Abbreviation: CI = confidence interval. Other abbreviation as in Table 2.
Statistically significant.
Discussion
In this large, prospective, observational cohort study of women with locally advanced cervical cancer beginning curative CRT in Botswana, we did not identify differences in 2-year OS rates between women who were HIV-infected and taking ART and women who were HIV-uninfected. Furthermore, we did not identify any significant differences in hematologic or nonhematologic toxicity according to HIV status. A baseline hemoglobin level >10 g/dL, receipt of a total radiation dose ≥75 Gy, and age <40 years were all associated with improved survival, independent of HIV status.
Our results are consistent with both prospective and retrospective studies of other malignancies in the United States, such as anal cancer and lung cancer, which have demonstrated similar stage-specific survival in HIV-infected versus HIV-uninfected patients starting curative therapy in the modern ART era (25–27). However, our findings are discordant with results from a previous study in Botswana by Dryden-Peterson et al (17) (2016), from an analysis of administrative datasets of women in the United States with cervical cancer by Coghill et al (16) (2015), and from a retrospective study in Brazil by Ferreira et al (28) (2017). Those studies estimated a 1.7- to 2.0-fold increased risk of death associated with HIV infection. There are, however, several key differences between our study and those prior studies that may explain our discordant findings (16, 17, 28). In contrast to our study in which only women being treated with curative CRT were included, the 3 previous studies included women being treated with both palliative and curative intent. Many patients in the 3 previous studies were not receiving ART, and the patients in the 3 previous studies had lower CD4 cell counts overall. For example, in Ferreira et al (28) (2017), the median CD4 count was 263 cell/μL (95% CI 137-368 cell/μL), and only 63% of the HIV-infected patients were known to be taking ART. In our study, all of the HIV-infected patients were receiving ART for a median of 84 months when the cancer treatment was initiated, and the median CD4 count was 481 cells/μL. An initial exploratory analyses of all 182 enrolled patients (including those who subsequently did not receive CRT) showed reduced survival among HIV-infected women, a finding similar to those reported in the studies mentioned above (Fig. E1 and Table E1; available online at www.redjournal.org). However, when the analysis was restricted to those women who initiated curative CRT, HIV status was not found to be a predictor of OS. Furthermore, both Dryden-Peterson et al (17) (2016) and Ferreira et al (28) (2017) were also limited by their inability to adjust for hemoglobin levels, an important clinical predictor, as well as by missing details about chemotherapy and radiation treatment history. Our study demonstrates that patients with well-controlled HIV infection who are receiving ART and initiate curative CRT have outcomes similar to those of HIV-uninfected individuals and also highlights the critical importance of prospectively measuring and accounting for all clinical-and treatment-related confounders known to affect treatment outcomes and OS. However, HIV infection may be associated with an inability to initiate curative treatment owing to immune dysfunction, with poor performance status from inadequately managed HIV, or with physician bias against administering chemotherapy to HIV-infected women (29–32).
We also found that for women receiving ART with well-controlled HIV infection, as demonstrated by high median CD4 counts and high rates of ART coverage, the toxicity associated with curative CRT is equivalent to that in HIV-uninfected women. Another recent study from the SSA region noted a similar difference in treatment-related toxicity for HIV-infected and HIV-uninfected women who initiated curative CRT (33). However, those findings stand in contrast to previous studies from SSA, showing that HIV-infected women had worse rates of treatment completion (external beam radiation, brachytherapy, and chemo-therapy), treatment response, and treatment tolerance compared with HIV-uninfected patients (30, 31). It is important to note that in those studies, most patients were started on ART when treatment for cervical cancer was initiated, which may also account for worse treatment outcomes and poor tolerance of treatment due to immune reconstitution before beginning cancer treatment (34–37).
Baseline hemoglobin levels were associated with 2-year OS. This finding is consistent with observations in several previous studies of patients with locally advanced cervical cancer (38, 39). Hemoglobin levels are thought to influence outcomes in cervical cancer through factors including a hypoxic tumor micro-environment leading to radiation resistance or worsened biology, larger tumors, and more-aggressive phenotypes (40–42).
Notably, there was no difference in treatment received between HIV-infected and HIV-uninfected patients in our cohort. Essentially, every patient who initiated curative CRT completed it in <56 days, and more than 70% of all patients were able to complete brachytherapy. These rates are higher than those reported in the United States (43) and are also higher than those reported in other studies of HIV-infected women (44). In the present analysis, the total EQD2 radiation dose was associated with 2-year OS. For locally advanced cervical cancer, the recommended total EQD2 dose to the tumor volume is >85 Gy, and a dose–tumor response relationship has been repeatedly demonstrated (45). However, such doses are difficult to achieve with HDR brachytherapy for locally advanced tumors while still respecting normal tissue limits without magnetic resonance imaging–guided brachytherapy, which is not readily accessible in most limited-resource settings where the incidence of cervical cancer is highest (46).
In our cohort, HIV-infected women presented with earlier-stage disease than HIV-uninfected women: 25% of the HIV-infected women versus 43% of the HIV-uninfected women presented with stage III-IV disease. It was also noted in our data that more HIV-infected women than HIV-uninfected women had been screened for cervical cancer at least once in their lifetime. This imbalance may reflect the increased health access for cervical cancer screening available to women who are HIV-infected in Botswana (47, 48).
A key strength of this study is that it is one of the largest cohort studies of women with HIV infection who are well managed on ART and receiving curative CRT for cervical cancer, with complete treatment data available, systematic review of toxicity during treatment, and robust follow-up data. Although other studies have examined the outcomes of HIV-infected versus HIV-uninfected women with cervical cancer receiving radiation therapy, to our knowledge this is the first study to report that HIV status does not affect 2-year OS in women with well-managed HIV infection and receiving ART being treated with curative CRT for cervical cancer, and that standard of care CRT was well tolerated in HIV-infected patients with cervical cancer.
Our findings are, however, subject to some limitations. Survival was better than anticipated; consequently, the power to detect clinically important effects of HIV on survival was limited. Although the results do not suggest impaired outcomes in HIV-infected women receiving CRT, it is possible that our study was underpowered to identify more modest effect sizes. A relative lack of power to identify factors associated with survival in this cohort was further evidenced by the trends found between the association of cancer stage (P = .092) and the number of chemotherapy cycles (P = .053) with OS. An additional limitation is that median follow-up for our study was 630 days. Our conclusions are limited to this time period, and we have limited power to comment on longer-term differences in survival (beyond 2 years) in the 2 groups. The study design was also limited in its ability to determine whether HIV infection was associated with the initiation of curative CRT among patients who were intended to receive it, a factor that will be investigated in future studies. All of our HIV-infected patients were receiving ART before starting CRT, with a median CD4 count of 481 cells/μL. A lack of effect of HIV infection over clinical outcomes could be due to high rates of ART coverage and well-preserved immune system function in our cohort. However, our cohort does not allow for testing this specific hypothesis, given the lack of a control group (HIV-infected cervical cancer patients not receiving ART). In Botswana, as in other limited-resource settings, radiographic imaging is not widely available. As a result, up to 60% of the women in our study may have had extrapelvic metastases, which could influence the tolerability of treatment as well as survival (49). Furthermore, follow-up was largely limited to telephone calls, and as a result, data on tumor recurrence are limited. Nonetheless, the findings of this study are significant and shed light on the lack of influence of HIV status on the 2-year OS of patients receiving curative CRT for cervical cancer.
In summary, no differences were detected in 2-year OS rates between HIV-infected women receiving ART with cervical cancer and HIV-uninfected women with cervical cancer who were able to initiate curative CRT in Botswana. Hemoglobin levels at baseline, total radiation dose, and age were associated with survival in these patients. No differences in acute toxicity were noted in the HIV-infected versus HIV-uninfected women. The results of this study indicate that HIV-infected women receiving ART with well-managed HIV and newly diagnosed cervical cancer should receive standard-of-care radical CRT, just like HIV-uninfected women, for the best possible outcomes.
Supplementary Material
Summary.
Human immunodeficiency virus (HIV) status did not impact 1-year overall survival of cervical cancer patients who initiated curative chemoradiation in Botswana. Women with well-managed HIV infection and taking antiretroviral therapy, who were able to initiate curative chemoradiation therapy (CRT), tolerated CRT similarly to HIV-uninfected patients.
Acknowledgments
The authors thank Rosemarie Mick, Ponatshego A. Gaolebale, Babe Eunice Gaolebale, Thabo Moloi, Stephen M. Hahn, Anthony T. Russell, Patricia Eifel, Erle Robertson, Kathleen Schmeler, Robert Gross, Hannah M. Simonds, E. Paul Wileyto, Keba Ngoni, Gita Suneja, Mosepele Mosepele, Lame Bakwenabatsile, Kesego Phologo, and Tapologo Leselwa for their contributions.
This study was funded by the Center for AIDS Research (5-P30-AI-045008-17), a Conquer Cancer Foundation Young Investigator Award, and the Sub-Saharan African Collaborative HIV and Cancer Consortia-U54 (1 U54 CA190158-01).
Footnotes
Conflict of interest: none.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.World Health Organization. Cervical Cancer, Mortality and Prevalence Worldwide in 2012. Geneva: World Health Organization GLOBOCAN; 2012. [Google Scholar]
- 2.Bosch FX, Manos MM, Munoz N, et al. Prevalence of human papillomavirus in cervical cancer: A worldwide perspective. International Biological Study on Cervical Cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 3.Singh DK, Anastos K, Hoover DR, et al. Human papillomavirus infection and cervical cytology in HIV-infected and HIV-uninfected Rwandan women. J Infect Dis. 2009;199:1851–1861. doi: 10.1086/599123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Botswana Ministry of Health. Botswana HIV/AIDS Impact Survey III Results. Available at: http://www.gov.bw/Global/NACA_Ministry/wana/BAIS_III_StatsPress.pdf. Accessed August 15, 2016.
- 5.Denny L, de Sanjose S, Mutebi M, et al. Interventions to close the divide for women with breast and cervical cancer between low-income and middle-income countries and high-income countries. Lancet. 2017;389:861–870. doi: 10.1016/S0140-6736(16)31795-0. [DOI] [PubMed] [Google Scholar]
- 6.Efstathiou JA, Bvochora-Nsingo M, Gierga DP, et al. Addressing the growing cancer burden in the wake of the AIDS epidemic in Botswana: The BOTSOGO collaborative partnership. Int J Radiat Oncol Biol Phys. 2014;89:468–475. doi: 10.1016/j.ijrobp.2014.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Statistics Botswana. Botswana AIDS Impact Survey IV (BAIS IV), 2013 Summary Results. Available at: http://www.cso.gov.bw/images/aids_summary.pdf. Accessed August 15, 2016.
- 8.UNAIDS. HIV and AIDS Estimates, Botswana. Available at: http://www.unaids.org/en/regionscountries/countries/botswana. Accessed August 15, 2016.
- 9.Gaolathe T, Wirth KE, Holme MP, et al. Botswana’s progress toward achieving the 2020 UNAIDS 90-90-90 antiretroviral therapy and virological suppression goals: A population-based survey. Lancet HIV. 2016;3:e221–e230. doi: 10.1016/S2352-3018(16)00037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al. Cancer incidence following expansion of HIV treatment in botswana. PLoS One. 2015;10:e0135602. doi: 10.1371/journal.pone.0135602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grover S, Raesima M, Bvochora-Nsingo M, Chiyapo SP, Balang D, Tapela N, et al. Cervical Cancer in Botswana: Current State and Future Steps for Screening and Treatment Programs. Front Oncol. 2015;5:239. doi: 10.3389/fonc.2015.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raesima MM, Forhan SE, Voetsch AC, et al. Human papilloma-virus vaccination coverage among school girls in a demonstration project–Botswana. MMWR Morb Mortal Wkly Rep. 2013;64:1147–1149. doi: 10.15585/mmwr.mm6440a5. 2015. [DOI] [PubMed] [Google Scholar]
- 13.Chuang LT, Temin S, Camacho R, et al. Management and care of women with invasive cervical cancer: American Society of Clinical Oncology resource-stratified clinical practice guidelines. J Glob Oncol. 2016;2:311–340. doi: 10.1200/JGO.2016.003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eifel PJ, Winter K, Morris M, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: An update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol. 2004;22:872–880. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- 15.Nyongesa C, Ruff P, Donde B, et al. A phase I study of concurrent cisplatin chemotherapy in patients with carcinoma of the cervix receiving pelvic radiotherapy. Int J Gynecol Cancer. 2006;16:1614–1619. doi: 10.1111/j.1525-1438.2006.00635.x. [DOI] [PubMed] [Google Scholar]
- 16.Coghill AE, Shiels MS, Suneja G, et al. Elevated cancer-specific mortality among HIV-infected patients in the United States. J Clin Oncol. 2015;33:2376–2383. doi: 10.1200/JCO.2014.59.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dryden-Peterson S, Bvochora-Nsingo M, Suneja G, et al. HIV Infection and Survival Among Women With Cervical Cancer. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.67.9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Brachytherapy Society. Brachytherapy Guidelines and Consensus Statements. Reston, VA: American Brachytherapy Society; 2016. [Google Scholar]
- 19.Benedet JL, Bender H, Jones H, 3rd, et al. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2000;70:209–262. [PubMed] [Google Scholar]
- 20.Karnofsky DA, Abelmann WH, Craver LF, et al. The use of nitrogen mustards in the palliative treatment of carcinoma. With particular reference to bronchogenic carcinoma Cancer. 1948;1:634–656. [Google Scholar]
- 21.Bvochara-Nsingo M, Grover S, Gierga DP, et al. Cervical brachytherapy exchange: steps toward oncology capacity building in Botswana. Oncologist. 2014;19:e1–2. doi: 10.1634/theoncologist.2013-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Interim WHO Clinical Staging of HIV/AIDS and HIV/AIDS Case Definitions for Surveillance African Region. Geneva: WHO; 2005. [Google Scholar]
- 23.National Institutes of Health National Cancer Institute. Common Terminology Criteria for Adverse Events (v4.03) Bethesda, MD: NIH; 2010. [Google Scholar]
- 24.Dafni U. Landmark analysis at the 25-year landmark point. Circ Cardiovasc Qual Outcomes. 2011;4:363–371. doi: 10.1161/CIRCOUTCOMES.110.957951. [DOI] [PubMed] [Google Scholar]
- 25.Seo Y, Kinsella MT, Reynolds HL, et al. Outcomes of chemoradiotherapy with 5-fluorouracil and mitomycin C for anal cancer in immunocompetent versus immunodeficient patients. Int J Radiat Oncol Biol Phys. 2009;75:143–149. doi: 10.1016/j.ijrobp.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 26.Wieghard N, Hart KD, Kelley K, et al. HIV positivity and anal cancer outcomes: A single-center experience. Am J Surg. 2016;211:886–893. doi: 10.1016/j.amjsurg.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Rengan R, Mitra N, Liao K, et al. Effect of HIVon survival in patients with non-small-cell lung cancer in the era of highly active antiretroviral therapy: A population-based study. Lancet Oncol. 2012;13:1203–1209. doi: 10.1016/S1470-2045(12)70466-7. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira MP, Coghill AE, Chaves CB, et al. Outcomes of cervical cancer among HIV-infected and HIV-uninfected women treated at the Brazilian National Institute of Cancer. AIDS. 2017;31:523–531. doi: 10.1097/QAD.0000000000001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shrivastava SK, Engineer R, Rajadhyaksha S, et al. HIV infection and invasive cervical cancers, treatment with radiation therapy: Toxicity and outcome. Radiother Oncol. 2005;74:31–35. doi: 10.1016/j.radonc.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Simonds HM, Neugut AI, Jacobson JS. HIV status and acute hematologic toxicity among patients with cervix cancer undergoing radical chemoradiation. Int J Gynecol Cancer. 2015;25:884–890. doi: 10.1097/IGC.0000000000000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simonds HM, Wright JD, du Toit N, et al. Completion of and early response to chemoradiation among human immunodeficiency virus (HIV)-positive and HIV-negative patients with locally advanced cervical carcinoma in South Africa. Cancer. 2012;118:2971–2979. doi: 10.1002/cncr.26639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suneja G, Boyer M, Yehia BR, et al. Cancer treatment in patients with HIV infection and non-AIDS-defining cancers: A survey of US oncologists. J Oncol Pract. 2015;11:e380–e387. doi: 10.1200/JOP.2014.002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mdletshe S, Munkupa H, Lishimpi K. Acute toxicity in cervical cancer HIV-positive vs. HIV-negative patients treated by radical chemoradiation in Zambia. South Afr J Gynaecol Oncol. 2016;8:37–41. [Google Scholar]
- 34.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez S, Price P, McKinnon EJ, et al. Low CD4+ T-cell counts in HIV patients receiving effective antiretroviral therapy are associated with CD4+ T-cell activation and senescence but not with lower effector memory T-cell function. Clin Immunol. 2006;120:163–170. doi: 10.1016/j.clim.2006.04.570. [DOI] [PubMed] [Google Scholar]
- 36.Goicoechea M, Smith DM, Liu L, et al. Determinants of CD4+ T cell recovery during suppressive antiretroviral therapy: Association of immune activation, T cell maturation markers, and cellular HIV-1 DNA. J Infect Dis. 2006;194:29–37. doi: 10.1086/504718. [DOI] [PubMed] [Google Scholar]
- 37.Nakanjako D, Ssewanyana I, Mayanja-Kizza H, et al. High T-cell immune activation and immune exhaustion among individuals with suboptimal CD4 recovery after 4 years of antiretroviral therapy in an African cohort. BMC Infect Dis. 2011;11:43. doi: 10.1186/1471-2334-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim A, Sia S. Outcomes of chemoradiotherapy in cervical cancer–the Western Australian experience. Int J Radiat Oncol Biol Phys. 2012;82:1431–1438. doi: 10.1016/j.ijrobp.2011.04.047. [DOI] [PubMed] [Google Scholar]
- 39.Yalman D, Aras AB, Ozkok S, et al. Prognostic factors in definitive radiotherapy of uterine cervical cancer. Eur J Gynaecol Oncol. 2003;24:309–314. [PubMed] [Google Scholar]
- 40.Dunst J, Kuhnt T, Strauss HG, et al. Anemia in cervical cancers: Impact on survival, patterns of relapse, and association with hypoxia and angiogenesis. Int J Radiat Oncol Biol Phys. 2003;56:778–787. doi: 10.1016/s0360-3016(03)00123-8. [DOI] [PubMed] [Google Scholar]
- 41.Hockel M, Schlenger K, Aral B, et al. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–4515. [PubMed] [Google Scholar]
- 42.Barkati M, Fortin I, Mileshkin L, et al. Hemoglobin level in cervical cancer: A surrogate for an infiltrative phenotype. Int J Gynecol Cancer. 2013;23:724–729. doi: 10.1097/IGC.0b013e31828a0623. [DOI] [PubMed] [Google Scholar]
- 43.Han K, Milosevic M, Fyles A, et al. Trends in the utilization of brachytherapy in cervical cancer in the United States. Int J Radiat Oncol Biol Phys. 2013;87:111–119. doi: 10.1016/j.ijrobp.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 44.Ntekim A, Campbell O, Rothenbacher D. Optimal management of cervical cancer in HIV-positive patients: A systematic review. Cancer Med. 2015;4:1381–1393. doi: 10.1002/cam4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dimopoulos JC, Potter R, Lang S, et al. Dose-effect relationship for local control of cervical cancer by magnetic resonance image-guided brachytherapy. Radiother Oncol. 2009;93:311–315. doi: 10.1016/j.radonc.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Dimopoulos JC, Lang S, Kirisits C, et al. Dose-volume histogram parameters and local tumor control in magnetic resonance image-guided cervical cancer brachytherapy. Int J Radiat Oncol Biol Phys. 2009;75:56–63. doi: 10.1016/j.ijrobp.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 47.Ramogola-Masire D, de Klerk R, Monare B, et al. Cervical cancer prevention in HIV-infected women using the “see and treat” approach in Botswana. J Acquir Immune Defic Syndr. 2012;59:308–313. doi: 10.1097/QAI.0b013e3182426227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mingo AM, Panozzo CA, DiAngi YT, et al. Cervical cancer awareness and screening in Botswana. Int J Gynecol Cancer. 2012;22:638–644. doi: 10.1097/IGC.0b013e318249470a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kidd EA, Siegel BA, Dehdashti F, et al. Lymph node staging by positron emission tomography in cervical cancer: Relationship to prognosis. J Clin Oncol. 2010;28:2108–2113. doi: 10.1200/JCO.2009.25.4151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


