Abstract
Introduction
Increased evidence suggests intestinal parasite infections, one of the major causes of morbidity and mortality in sub-Saharan Africa, increase the acquisition and progression of AIDS.
Objective
The aim of this study was to determine the prevalence of HIV and intestinal parasite co-infections, the relationship to the degree of immunosuppression and the effect of antiretroviral treatment (ART) and trimethoprim-sulfamethoxazole (TS) on patients treated at 1° de Maio Health Centre in Maputo, Mozambique.
Methods
A cross sectional study was conducted from December 2015 to August 2016. A total of 517 stool samples from 371 (71.8%) HIV infected and 146 (28.2%) HIV uninfected patients were examined for the presence of parasites using direct wet mount, Ritchie and modified Ziehl Neelsen techniques. A subsample of 201 stools from HIV infected patients was processed for coproantigens for the detection of Cryptosporidium spp.
Results
Overall, 148 (28.6%) of the individuals were infected with at least one parasite. The prevalence of intestinal parasites was 98 (26.4%) and 50 (34.2%) in HIV infected and uninfected patients, respectively. This difference was not statistically significant. We identified 10 different parasites including (most frequently) Trichuris trichiura 67 (12.9%), Ascaris lumbricoides 27 (5.2%) and Entamoeba coli 40 (7.7%). Giardia intestinalis prevalence was significantly higher in HIV infected patients 12 (3.2%), p = 0.02. Parasitic intensity was higher in HIV infected patients than in HIV uninfected patients. Cryptosporidium spp. prevalence by coproantigen detection was 6% and was associated with degree of immune suppression. A CD4+ T-cell count of < 200 cells/μL was significantly associated with higher prevalence and intensity of parasitism, while ART and TS prophylaxis was associated with lower parasitic prevalence.
Conclusions
Our study revealed that the prevalence and intensity of intestinal parasites in HIV infected patients was related to the degree of immune suppression as assessed by CD4+ cell count, while ART and TS seemed to reduce the parasitic infection.
Keywords: Co-Infection HIV intestinal parasites, Helminthes, Protozoan, Coccidiae
Introduction
Intestinal parasites, including helminthes and protozoa are neglected tropical diseases (NTDs), targeted by the sustainable development goals (SDGs) to be eliminated by 2030. They constitute a major cause of morbidity and mortality throughout the world, particularly in resource limited tropical and subtropical regions, including sub-Saharan Africa (SSA) [1,2]. The most important intestinal helminthes are the soil transmitted helminthes (STHs), such as Ascaris lumbricoides, Trichuris Trichiura, Strongyloides stercoralis and hookworms. It is estimated that approximately 2 billion (24%) of the world’s population is infected with intestinal helminthes [2,3]. Less is known about the burden of protozoal infections such as Entamoeba histolytica, Giardia intestinalis, Cryptosporidium spp. and Cystoisospora belli because of the sensitivity of available diagnostic techniques, the paucity of microscopic skills and a general lack of awareness of protozoan infection [4].
In SSA, the parasitic infection rate is remarkably high with some areas reporting 95% incidence as compared to developed countries were the incidence is 50%. In addition, parasitic infections overlap with the regions of high prevalence of human immune deficiency virus (HIV). In the WHO African Region including Mozambique, 11.4 million are infected with intestinal parasites [1,3,5–7]. The distribution and prevalence of STHs is closely associated with a lack of personal and environmental hygiene; infection can be asymptomatic or symptomatic. When symptomatic and depending on the parasitic agent involved, in addition to degree of immune suppression, individuals may present with chronic diarrhea, malabsorption syndrome, dehydration and anemia [4,8,9].
It has been hypothesized that helminthic infections are an important driver of HIV infection in Africa, due to their effect on the immune system, as demonstrated by the increased susceptibility to HIV infection and the clinical progression to AIDS [9–12]. Thus, control of intestinal parasites is an important tool to combat the HIV pandemic and the vicious cycle of poverty, to reduce inequity in alignment with Universal Health Coverage and, ultimately, to achieve the SDGs [2].
Studies aiming to address the prevalence of intestinal parasite and HIV co-infection and its relationship to antiretroviral treatment (ART) are controversial [7,13,14]. Some indicate that parasitic infections are reduced in HIV infected persons on ART, but the underlying mechanisms are not known. Recovery of the immune system on ART may explain this phenomena, especially in protozoan infection, as noted in the case of Cryptosporidium spp. and Cystoisospora belli infection [15–17]. Other authors speculate that the reduction in prevalence of intestinal parasites in HIV patients on ART might be due to the anti-parasitic effects of ART drugs themselves or of trimethoprimsulfamethoxazole (TS) used as a prophylactic agent for several opportunistic infections such as Toxoplasma gondii, Pneumocystis jirovecii, C. belli, and Cyclospora cayetanensis, [5,15,17].
Mozambique represents one of the most affected countries by HIV with an adult prevalence of 11.5%. No recent or systematic data are available that pertain to co-infection with intestinal parasites and HIV [18].
Of the few published studies, there is a wide range of prevalence and distribution of helminthes and protozoa within the country [4,19–22]. T. trichiura prevalence varied from 36.06% to 93%, A. lumbricoides prevalence varied from 35.69% to 56%, S. stercoralis varied from 5.5% to 48%, and hookworm prevalence varied from 1.86% to 38%. The reported prevalence of some protozoan also varies greatly according to the studied population, geographical region, environmental factors and clinical status of the patient. Microscopic and molecular studies done in Mozambique reported Giardia intestinalis prevalence varying from 5.6% to 37%, E. histolytica/E.dispar from 4.83% to 10%, Entamoeba coli prevalence varying from 10.41% to 34%, and for Cryptosporidium spp. prevalence from 2.5% to and 9%.
The present study was undertaken to determine the prevalence of intestinal parasites in HIV infected and uninfected individuals treated at the 1° de Maio Health Center in Maputo city, Mozambique. In addition, this study aims to explore the relationship between the degree of immune suppression as measured by CD4+ cell counts, as well as the effect of TS and ART on the prevalence of intestinal parasites in the HIV-infected patient population.
Materials and Methods
Study Design and Population
We conducted a cross sectional study at the 1° de Maio Health Centre, located in Maputo city from December 2015 to August 2016. The National Bioethics Committee of Mozambique approved the study.
Patients that sought or were in care for HIV infection and other illnesses were approached consecutively about their participation in the study. Prior to enrollment, either the principal investigator, or a research nurse explained the aim of the study and invited their participation.
Volunteers who agreed to participate and provided a written informed consent were enrolled into the study. In the case of children, a consent to include them in the study was obtained from their parents or guardians.
Demographic and clinical data including gender, age, source of drinking water, type of sewage, anorexia, diarrhea, HIV status, CD4+ cell counts and ART (in the case of HIV infected patients) were recorded in a questionnaire designed for this study. The use of selected concomitant medications such as albendazole, mebendazole, metronidazole and TS were also recorded.
Consenting patients were asked to provide one stool sample in a coded sterile bottle that was send to Parasitology Laboratory at the Faculty of Medicine - UEM, in Maputo city, for further processing. Once in the Parasitology laboratory the stool was divided into two aliquots for microscopic examination and Cryptosporidium spp. coproantigen detection.
Parasitological Analysis Methods
One aliquot for each stool sample was examined the same day using light microscopy by simple wet preparation to search for trophozoites of protozoan and within 48 hours for the detection of helminthes and protozoan using formalin-ether sedimentation (10%) concentration technique procedures and iodine staining [23]. For the detection of Cryptosporidium spp., C.belli and Cyclospora cayetanensis. oocysts, the stool samples were also analyzed by modified Ziehl-Neelsen staining [24] (Figure 1).
Figure 1.
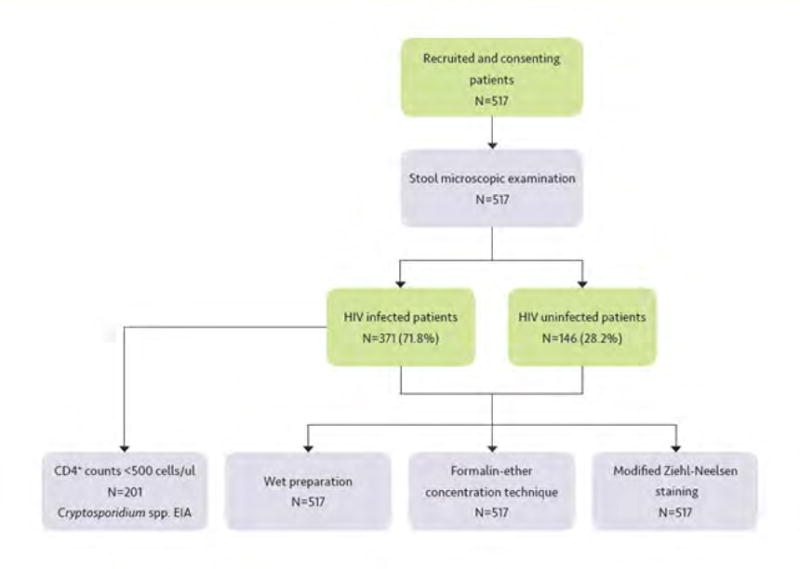
Workflow chart of participants recruitment, enrolment strategy and laboratory diagnostics.
Parasitological intensity was assessed using a semi-quantitative method according to Kato-Katzo technique [25].
A second aliquot comprising a subsample of 201 stools drawn from the HIV infected patients with CD4+ cell counts less than 500 cells/μl was kept refrigerated at −20°C and processed within 3 months to detect coproantigens of Cryptosporidium spp. using the commercial Kit Ridascreen® Cryptosporidium – R-Biopharm AG (Darmstadt, Germany) according manufacturer’s instructions (Figure 1).
Each stool sample was examined both by two laboratory technicians as well as by one of the authors (BZC), reaching 94% concordance among the three observations.
Data Analysis
Epi-info™ version 7.2.1 was used to establish a database and for double-entry data input by two different individuals. After validation of the database, two identical datasets were obtained, of which one was used for all subsequent analyses with Statistical Package for Social Science (SPSS) version 20.0 statistical software. Differences in proportion of HIV positive patients with CD4+ values within specific ranges and stool examination results showing characteristic findings were tested using chi-square test. Multivariate logistic regression modeling was employed to analyze the relationship of socio-demographic, and immunological variables with parasite infection and HIV sero status. We also tested the parasitic infection intensity, according to the HIV sero-status using the Mann-Whitney test. A p-value < 0.05 was considered statistically significant.
Results and Discussion
Socio- Demographic and general characteristics of the study population
We recruited 517 patients over 2 years old of whom 371 (71.8%) were HIV infected and 146 (28.2%) were HIV uninfected. The mean age was 40.8 (range 5 – 70 years) for the HIV infected patients and 37.6 (range 3 – 76 years) for the HIV uninfected population. The majority of study population were female 273 (65%) and only 20 (3.9%) were children up to 14 years of age (see Table 1).
Table 1.
Socio-demographic profile of the study population.
| Demographic data | HIV infected n = 371 (71.8%) |
HIV uninfected n = 146 (28.2%) |
Total n = 517 (100%) |
|
|---|---|---|---|---|
| Sex | Male | 134 (34.9%) | 51 (36.1%) | 185 (35.8%) |
| Female | 237 (65.1%) | 95 (63.9%) | 332 (64.2%) | |
| Age | Children ≤ 14 years | 5 (1.3%) | 15 (10.3%) | 20 (3.9%) |
| Adults | 366 (98.7%) | 131 (89.7%) | 497 (96.1%) | |
| Education | Illiterate | 17 (4.6%) | 19 (13.0%) | 36 (7.0%) |
| Primary school | 243 (65.5%) | 77 (52.7%) | 320 (61.9%) | |
| Secondary school | 86 (23.2%) | 28 (19.2%) | 114 (22.1%) | |
| Pre-University | 18 (4.9%) | 16 (11.0%) | 34 (6.6%) | |
| University | 7 (1.9%) | 6 (4.1%) | 13 (2.5%) | |
| Water source | ||||
| Tap | 362 (97.6%) | 141 (96.6%) | 503 (97.3%) | |
| Mineral | 5 (1.3%) | 1 (0.7%) | 6 (1.2%) | |
| Well | 4 (1.1%) | 4 (2.7%) | 8 (1.5%) | |
| Wash hands after: | ||||
| Defecation | Yes | 369 (99.5%) | 146 (100.0%) | 515 (99.6%) |
| No | 2 (0.5%) | 0 (0.0%) | 2 (0.4%) | |
| Contact with people | Yes | 369 (95.5%) | 143 (97.9%) | 512 (99.0%) |
| No | 2 (0.5%) | 3 (2.1%) | 5 (1.0%) | |
| Contact with animals | Yes | 370 (99.7%) | 146 (100.0%) | 516 (99.8%) |
| No | 1 (0.3%) | 0 (0.0%) | 1 (0.2%) | |
| Animals at home | Yes | 181 (48.8%) | 77 (52.7%) | 258 (49.9%) |
| No | 190 (51.2%) | 69 (47.3%) | 259 (50.1%) |
In total, 360 (97%) of the HIV infected patients were receiving antiretroviral therapy (ART) for a median duration of 48 (IQR: 1–149) months. Their median overall CD4+ cell count at study enrollment was 437.4 cells/μl [IQR: 10 – 1810], and 72 (19.4%) were taking TS. The majority of the study participants were literate 418 (93%), 503 (97.3%) had piped water, 258 (49.9%) had animals at home and 515 (99.6%) reported washing hands after defecation. In our study, we found no association between risk factors such as education level, sources of drinking water, hand washing after defecation, pets in the peridomicile and parasite infection. These findings are similar to those of other studies conducted both within and outside of Mozambique, which suggests that all patients are equally exposed to the risk of infection regardless of their literacy, water source, hygienic behavior and HIV sero status [16,19]. This is further supported by the fact that in our study Entamoeba coli, although not pathogenic, was the second most frequently detected parasite, with 40 (7.7%) prevalence. This is an indication of oral fecal contamination and therefore supports the existence of poor environment and sanitation [19] (see Table 1).
Prevalence of intestinal parasites in the HIV infected and uninfected patients
The microscopic examination of the stools demonstrated that 148 (28.6%) of all patients were infected with at least one parasitic species, and overall, 10 different species comprising helminthes and protozoa, including coccidia were identified in the stool samples (Table 2 and Figure 2).
Table 2.
Prevalence of intestinal parasites in the study population.
| Parasites | HIV infected N = 98/371 (26.4 %) |
HIV uninfected N = 50/146 (34.2 %) |
Total N = 148/517(28.6%) |
|---|---|---|---|
| Helminthes | |||
| T. trichiura | 41 (11.1%) | 26 (17.8%) | 67 (12.9%) |
| A. lumbricoides | 15 (4.0%) | 12 (8.2%) | 27 (5.2%) |
| S. mansoni | 5 (1.3%) | 2 (1.4%) | 7 (1.3%) |
| S. stercoralis | 5 (1.3%) | 3 (2.1%) | 8 (1.5%) |
| A. duodenalis | 1 (0.3%) | 2 (1.4%) | 3 (0.6%) |
| Protozoan | |||
| E. coli | 24 (6.5%) | 16 (11%) | 40 (7.7%) |
| *G. intestinalis | 12 (3.2%) | 1 (0.7%) | 13 (2.5%) |
| E. histolytica | 7 (1.9%) | 2 (1.4%) | 9 (1.7%) |
| Coccidian | |||
| Cryptosporidium spp | 1 (0.3%) | 0 (0.0%) | 1 (0.2%) |
| C. belli | 0 (0.0%) | 1 (0.7%) | 1 (0.2%) |
p = 0.02
Figure 2.
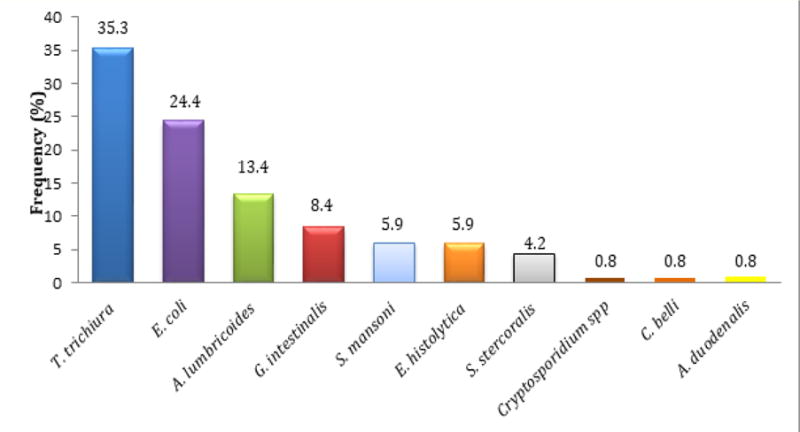
Profile of parasites detected in the study population.
This study demonstrated that the prevalence of intestinal parasites is high in both HIV infected patients 98 (26.4%) and HIV uninfected persons 50 (34.2%), although there was not a statistically significant difference. These findings are similar to other studies conducted in Mozambique and other sub-Saharan countries including Nigeria, Ethiopia and Uganda [5,7,10,16,19,22,26]. One possible explanation for having higher prevalence of parasites in uninfected population when compared with HIV infected population may result from the fact that HIV infected patients are more often likely to be in contact with the health care system and thus more exposed to anti-parasitic drugs. In adition, since syndromic treatment of fever or diarrhea with TS is common in those with AIDS and other conditions such as malaria in Mozambique. Given the effect of TS on folic acid metabolism of the worms, we were not surprised to find a lower prevalence of parasitic infection in HIV infected patients when compared to HIV uninfected patients [15,16]. Other studies document similar results and it is thought that the lower prevalence of intestinal parasites in HIV infected patients compared with HIV uninfected is associated with the use of ART. It is thought that ART drugs may have an effect on the parasite clearance, even when the patients respond poorly to ART in terms of the CD4+ T-cell count, possibly due to the mitochondrial toxicity of the ART on the worms [16,17]. In our study, almost all HIV infected patients 360 (97%) were on ART.
Paradoxically, the overall parasitic intensity in HIV infected patients was 2.4 times that of HIV uninfected. Although these differences were not statistically significant, it might be explained based on the impairment of the immune system in those patients, which is not allowing the clearance of the infection. HIV infection induces cellular depletion and early abnormalities of CD4+ T cells, decreases CD8+ T-cell and function, causes deterioration of specific antigen responses and leads to alteration of innate immunity through impairment of cytolytic activity and cytokine production by natural killer cells, all important pathways to necessary resolve infection [17].
The most frequently detected helminthes in both populations were Trichuris trichiura 67 (12.9%), Ascaris lumbricoides 27 (5.2%), while the most prevalent protozoa was Entamoeba coli 40 (7.7%). G. intestinalis prevalence was significantly higher in HIV infected patients 12 (3.2%) compared with HIV uninfected patients 1 (0.7%) for p = 0.02. This finding is consistent with other studies, such as in Ethiopia, though it is not considered opportunistic parasite [27].
Cryptosporidum spp. and C. belli were detected each in one stool sample of HIV infected (0.3%) and HIV uninfected patients (0.2%), respectively (see Table 2). The relatively low prevalence of Cryptosporidium spp. detected by microscopy is difficult to interpret when compared with other studies conducted within the country and other countries such as in Ethiopia, Cameron, Nigeria and India [4,6,20–22,26,28]. Among HIV infected patients, 360 (97%) were on ART. These findings could also explain the low parasitic prevalence in this group of patients when compared to HIV uninfected patients, if we consider improvement of the immune system function due to ART or to the drugs activity directly against parasites, as observed in other studies [16,27]. In addition, our HIV infected study population was not severally immunosuppressed as shown by the median CD4+ cell count. This could be possibly another reason to explain the lower prevalence of Cryptosporidium spp. or C. belli detected in our study. Further controlled randomized studies with HIV infected patients on ART and without ART need to be carried out.
A single parasite was present in 119 (80.4%) of the study population while the remaining 29 (19,6%) were noted to have two parasite species. Unlike ours, other studies have reported mixed parasite infections of up to 5 parasites [4,19]. This could be related to the better living conditions of our study population or to the possibility that our HIV-1 infected patient population was less immunologically advanced. HIV infected patients had fewer mixed infections, 15 (15.3%) than HIV uninfected patients 14 (28%), but this difference was not statistically significant. The most frequent mixed parasites association was between T. trichiura + A. lumbricoides with 12 (8.1%), followed by the association between T. trichiura + E. coli with 7 (4.7%). Other combination comprised T. trichiura + E. histolytica (1.4%), T. trichiura + S. mansoni (0.7%), T. trichiura + S. stercoralis (1.4%), G. intestinalis + E. coli (1.4%). A. duodenalis +E. coli, A. duodenalis +A. lumbricoides, T. trichiura +G. intestinalis were present in 0.7% each (see Table 3).
Table 3.
Mixed Parasitism in HIV infected and HIV uninfected patients.
| Parasitism | HIV infected patients N = 98/371 (26.4 %) |
HIV uninfected patients N = 50/146 (34.2 %) |
Total N = 148/517(28.6%) |
|---|---|---|---|
| One Parasite | 83 (84,7%) | 36 (72%) | 119 (80,4%) |
| T. trichiura + A. lumbricoides | 5 (5.1%) | 7 (14.0%) | 12 (8.1%) |
| T. trichiura + E. coli | 3 (3.1%) | 4 (8.0%) | 7 (4.7%) |
| T. trichiura + E. hystolitica | 2 (2.0%) | 0 (0.0%) | 2 (1.4%) |
| T. trichiura + S. mansoni | 0 (0.0%) | 1 (2.0%) | 1 (0.7%) |
| T. trichiura + S. stercolaris | 1 (1.0%) | 1 (2.0%) | 2 (1.4%) |
| G. Intestinalis + E. coli | 2 (2.0%) | 0 (0.0%) | 2 (1.4%) |
| A. lumbricoides + A. duodenalis | 1 (1.0%) | 0 (0.0%) | 1 (0.7%) |
| E. coli + A. duodenalis | 0 (0.0%) | 1 (2.0%) | 1 (0.7%) |
| T. trichiura + G. Intestinalis | 1(1.0%) | 0 (0.0%) | 1 (0.7%) |
| Total mixed parasitism | 15 (15.3) | 14 (28%) | 29 (19.6%) |
Given that HIV infected patients are the group most exposed to the health system where anti-helminthic drugs and TS are routinely used for almost all patients seeking care, these results are not surprising. In addition, TS is known to have anthelminthic effect and is used for HIV positive patients for prophylaxis of opportunistic infections such as C. belli. Although C. belli is an opportunistic parasite, it was not identified in stool from any HIV infected patients, but we did identify it in 1 (0.2%) HIV uninfected patient. Similar results were found in other studies where HIV infected patients did not have or presented with a lower prevalence of C. belli. This could be also attributed to the use of TS in those patients as a prophylaxis for opportunistic infections, in which C. belli is included [16]
Overall Cryptosporidium spp. coproantigen prevalence was 8 (6%) in patients with CD4+ cell counts of less than 500. There was no statistically significant differences between patients with CD4+ Cell count less than 200 and CD4+ cell count between 200 – 500 cells/μL. Prior studies have demonstrated that the prevalence of Cryptosporidium spp. may be under-estimated by microscopy and by coproantigens compared to PCR that is much more sensitive [29]. Other studies revealed relatively higher sensitivity of immune enzymatic assays for detection of Cryptosporidium spp. when compared with microscopic detection [30]. In our study, the prevalence of Cryptosporidium spp. determined by coproantigen detection, an immune enzymatic assay (EIA), was higher (6%) than that detected by microscopy (0.3%). Though there is no known effective treatment to this parasite, given the life-threatening diarrhea and associated vomiting in patients with immunologically advanced HIV disease and in malnourished children, the ability to detect, prevent and treat this pathogen must be prioritized [22,27,29].
Parasitic infection in HIV infected patients stratified according to CD4+ cell counts and TS intake
In our study CD4+ T-cell counts of < 200 cells/μl were associated with higher parasitic prevalence and parasitic intensity. We found that patients with CD4+ T-cell counts of < 200 cells/μl had a higher prevalence of parasite infection (16,8%) than those with CD4+ cell counts of more than 200 cells/μl. (p = 0.05). Similarly the mean parasitic intensity was significantly higher in HIV infected patients with CD4+ cell counts below 200 cells/μl (p = 0.04). This difference may also reflect the impairment of both cell-mediated and humoral immunity by HIV with consequent inability of the host to completely clear up the infection [17,29].
HIV infected patients who reported treatment with TS demonstrated 3.5 times less prevalent intestinal parasite co-infection 6 (22.2%) vs 56 (77.8%) than patients not taking TS. Although these differences were not statistically significant, TS might be advised whenever possible if we take into consideration the effect of parasitism on the progression of AIDS and detrimental impact on the nutritional state of the patients [10,12,16,27]. Similarly, parasitic intensity was less in patients taking TS versus patients not on TS.
Several limitations in our study should be highlighted. First, our population was recruited by convenience in a time limited frame from patients attending a single health facility who were willing to informed consent. Thus, the data obtained from HIV uninfected patients cannot be assumed to be representative of the general population not actively receiving health care. Secondly, we used only one stool sample for each patient and it is possible that the parasite prevalence we determined is under-estimated due to the intermittent release of eggs and cysts from helminthes and protozoa and the low sensitivity of microscopic techniques [31]. It is usually recommended to perform serial stool tests with at least 3 samples per patient to increase the sensitivity of the techniques employed [27,32]. Finally, the results for coproantigen detection of Cryptosporidium spp. may be under-estimated due to limitations of the sensitivity of our assay, which is around 92%. Thus, a negative result in this assay does not rule out the possibility of Cryptosporidium spp. infection. Such a result may be due to intermittent excretion of the parasite, or the amount of antigen in the sample may be below the level of detection of our assay.
Conclusion
Given the high prevalence of intestinal parasites detected in HIV infected and HIV uninfected patients and their negative impact on the progression of AIDS and nutritional status of the patients, routine examinations of stool samples for parasite diagnosis and treatment is highly recommended in settings like ours. This might result in significant reductions in morbidity, improvement of the efficacy of antiretroviral therapy as well as the nutritional status of patients and might contribute to the SDGs achievement. In our study a CD4+ T-cell count of < 200 cells/μL was a risk factor for parasitic prevalence and parasitic intensity, while TS intake seemed to have a mitigating effect on prevalence and parasitic intensity.
Acknowledgments
We express our gratitude to all patients who contributed to this study. We also acknowledge to the staff of the 1° de Maio Health Centre where the study was done.
This work was done thanks to the Medical Education Partnership Initiative (MEPI) support which provided financial resources to create a master program courses at Universidade Lúrio, Mozambique. MEPI was supported by Grant Number R24TW008908 and R24TW008910 from the Fogarty International Center. The manuscript writing and publication was supported by Fogarty International Center, Office of the Director, Eunice Kennedy Shriver National Institute of Child Health & Human Development and National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number D43TW010135. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Fogarty International Center or the National Institutes of Health.
The research project was financed by the Fundo Nacional de Investigação from Ministry of Science and Technology of Mozambique, Grant number Msc77-SAÚDE.
Abbreviations
- AIDS
Acquired Immunodeficiency Syndrome
- ART
Anti-Retroviral Therapy
- CD4+
Cluster of Differentiation 4
- EIA
Enzymatic Immuno-Assay
- HIV
Human Immunodeficiency Virus
- NTDs
Neglected Tropical Diseases
- SSA
Sub-Saharan Africa
- SDGs
Sustainable Development Goals
- STHs
Soil Transmitted Helminthes
- TS
Trimethoprim-Sulphamethoxazole
- UEM
University Eduardo Mondlane
- WHO
World Health Organization
- ZNM
Ziehl Neelsen
Footnotes
Conflict of Interest
The authors declare no conflict of interests.
Bibliography
- 1.Veas Francisco, Rey Jean-Loup. Infection à VIH et parasitoses en zone Tropicale. Cahiers Santé. 1991;1:189–201. [Google Scholar]
- 2.Bangert M, et al. The cross-cutting contribution of the end of neglected tropical diseases to the sustainable development goals. Infectious Diseases of Poverty. 2017;6(1):73. doi: 10.1186/s40249-017-0288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Organization WH; World Health Organization. Soil Transmitted Helminth Infections: fact sheet. 2016 [Google Scholar]
- 4.Meurs L, et al. Diagnosing Polyparasitism in a High-Prevalence Setting in Beira, Mozambique: Detection of Intestinal Parasites in Fecal Samples by Microscopy and Real-Time PCR. PLOS Neglected Tropical Diseases. 2017;11(1):e0005310. doi: 10.1371/journal.pntd.0005310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adamu H, et al. High prevalence of diarrhoegenic intestinal parasite infections among non-ART HIV patients in Fitche Hospital, Ethiopia. PLoS One. 2013;8(8):e72634. doi: 10.1371/journal.pone.0072634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nkenfou CN, et al. Intestinal parasitic infections in HIV infected and non-infected patients in a low HIV prevalence region, West- Cameroon. PLoS One. 2013;8(2):e57914. doi: 10.1371/journal.pone.0057914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morawski BM, et al. Hookworm infection is associated with decreased CD4+ T cell counts in HIV-infected adult Ugandans. PLOS Neglected Tropical Diseases. 2017;11(5):e0005634. doi: 10.1371/journal.pntd.0005634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stark D, et al. Clinical significance of enteric protozoa in the immunosuppressed human population. Clinical Microbiology Reviews. 2009;22(4):634–650. doi: 10.1128/CMR.00017-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Assefa Y, et al. Performance of the Antiretroviral Treatment Program in Ethiopia, 2005–2015: strengths and weaknesses toward ending AIDS. International Journal of Infectious Diseases. 2017;60:70–76. doi: 10.1016/j.ijid.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Babatunde SK, et al. Prevalence of intestinal parasitic infestation in HIV seropositive and seronegative patients in Ilorin, Nigeria. Annals of African Medicine. 2010;9(3):123–128. doi: 10.4103/1596-3519.68356. [DOI] [PubMed] [Google Scholar]
- 11.Kaniyarakkal V, et al. Intestinal Parasite Profile in the Stool of HIV Positive Patients in relation to Immune Status and Comparison of Various Diagnostic Techniques with Special Reference to Cryptosporidium at a Tertiary Care Hospital in South India. Advances in Medicine. 2016:3564359. doi: 10.1155/2016/3564359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulu A, et al. Deworming of intestinal helminths reduces HIV-1 subtype C viremia in chronically co-infected individuals. International Journal of Infectious Diseases. 2013;17(10):e897–e901. doi: 10.1016/j.ijid.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Morgan U, et al. Population genetics and population biology: what did they bring to the epidemiology of transmissible diseases? An e-debate. Infection, Genetics and Evolution. 2001;1(2):161–166. doi: 10.1016/s1567-1348(01)00023-5. [DOI] [PubMed] [Google Scholar]
- 14.Wolday D, et al. Treatment of intestinal worms is associated with decreased HIV plasma viral load. Journal of Acquired Immune Deficiency Syndromes. 2002;31(1):56–62. doi: 10.1097/00126334-200209010-00008. [DOI] [PubMed] [Google Scholar]
- 15.Bachur TP, et al. Enteric parasitic infections in HIV/AIDS patients before and after the highly active antiretroviral therapy. Brazilian Journal of Infectious Diseases. 2008;12(2):115–122. doi: 10.1590/s1413-86702008000200004. [DOI] [PubMed] [Google Scholar]
- 16.Kiros H, et al. Prevalence of intestinal protozoan infections among individuals living with HIV/AIDS at Felegehiwot Referral Hospital, Bahir Dar, Ethiopia. International Journal of Infectious Diseases. 2015;35:80–86. doi: 10.1016/j.ijid.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Janssen S, et al. Impact of Anti-Retroviral Treatment and Cotrimoxazole Prophylaxis on Helminth Infections in HIV-Infected Patients in Lambarene, Gabon. PLOS Neglected Tropical Diseases. 2015;9(5):e0003769. doi: 10.1371/journal.pntd.0003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MISAU, Inquérito Nacional de prevalência, riscos, comportamentos e informaçâo sobre o HIV/SIDA em Moçambique. Instituto Nacional de Saúde. 2009 [Google Scholar]
- 19.Noormahomed EV, et al. Seroprevalence of anti-cysticercus antibodies among the children living in the urban environs of Maputo, Mozambique. Annals of Tropical Medicine and Parasitology. 2003;97(1):31–35. doi: 10.1179/000349803125002742. [DOI] [PubMed] [Google Scholar]
- 20.Nhampossa T, et al. Diarrheal Disease in Rural Mozambique: Burden, Risk Factors and Etiology of Diarrheal Disease among Children Aged 0–59 Months Seeking Care at Health Facilities. PLoS One. 2015;10(5):e0119824. doi: 10.1371/journal.pone.0119824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irisarri-Gutierrez MJ, et al. Association between enteric protozoan parasites and gastrointestinal illness among HIV- and tuberculosis-infected individuals in the Chowke district, southern Mozambique. Acta Tropica. 2017;170:197–203. doi: 10.1016/j.actatropica.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Noormahomed EV. Cisticercosis y otros parasitismos en la población de Maputo (Mozambique) Granada University; Granada, Spain: 2005. [Google Scholar]
- 23.Ritchie LS. An ether sedimentation technique for routine stool examinations. Bulletin of the US Army Medical Department. 1948;8(4):326. [PubMed] [Google Scholar]
- 24.Henriksen SA, Pohlenz JF. Staining of cryptosporidia by a modified Ziehl-Neelsen technique. Acta Veterinaria Scandinavica. 1981;22(3–4):594–596. doi: 10.1186/BF03548684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rey L. In: Parasitologia Parasitos e doenças parasitárias do homem nos trópicos ocidentais. Redbstyle, editor. Rio de Janeiro: Guanabara-Koogan; 2008. [Google Scholar]
- 26.Adamu H, Petros B. Intestinal protozoan infections among HIV positive persons with and without Antiretroviral Treatment (ART) in selected ART centers in Adama, Afar and Dire-Dawa, Ethiopia. Ethiopian Journal of Health Development. 2009;23(2):133–140. [Google Scholar]
- 27.Shimelis T, et al. Cryptosporidium and other intestinal parasitic infections among HIV patients in southern Ethiopia: significance of improved HIV-related care. Parasites and Vectors. 2016;9(1):270. doi: 10.1186/s13071-016-1554-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kulkarni SV, et al. Opportunistic parasitic infections in HIV/AIDS patients presenting with diarrhoea by the level of immunesuppression. Indian Journal of Medical Research. 2009;130(1):63–66. [PubMed] [Google Scholar]
- 29.Majewska AC, et al. [Cryptosporidiosis in HIV-positive patients] Wiadomości Parazytologiczne. 1999;45(2):125–128. [PubMed] [Google Scholar]
- 30.Silva CV, et al. Detection of Cryptosporidium–specific coproantigen in human immunodeficiency virus/acquired immunodeficiency syndrome patients by using a commercially available immunoenzymatic assay. Memórias do Instituto Oswaldo Cruz. 2003;98(8):1097–1099. doi: 10.1590/s0074-02762003000800022. [DOI] [PubMed] [Google Scholar]
- 31.van Lieshout L, Roestenberg M. Clinical consequences of new diagnostic tools for intestinal parasites. Clinical Microbiology and Infection. 2015;21(6):520–528. doi: 10.1016/j.cmi.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 32.Katz DE, Taylor DN. Parasitic infections of the gastrointestinal tract. Gastroenterology Clinics of North America. 2001;30(3):797–815. doi: 10.1016/s0889-8553(05)70211-9. [DOI] [PubMed] [Google Scholar]


