Abstract
Objective
This study evaluated whether reduced vestibular function in aging adults is associated with lower hippocampal volume.
Study Design
Cross-sectional study.
Setting
Baltimore Longitudinal Study of Aging (BLSA), a long-running longitudinal cohort study of healthy aging.
Patients
Eligible participants were age ≥ 60 years and had both vestibular physiological testing and brain MRI at the same visit.
Intervention
Vestibular function testing consisted of the cervical vestibular-evoked myogenic potential (cVEMP) to assess saccular function, ocular VEMP (oVEMP) to assess utricular function, and video head-impulse testing (VHIT) to assess the horizontal semicircular canal vestibulo-ocular reflex (VOR).
Main Outcome Measure
Hippocampal volume calculated using diffeomorphometry.
Results
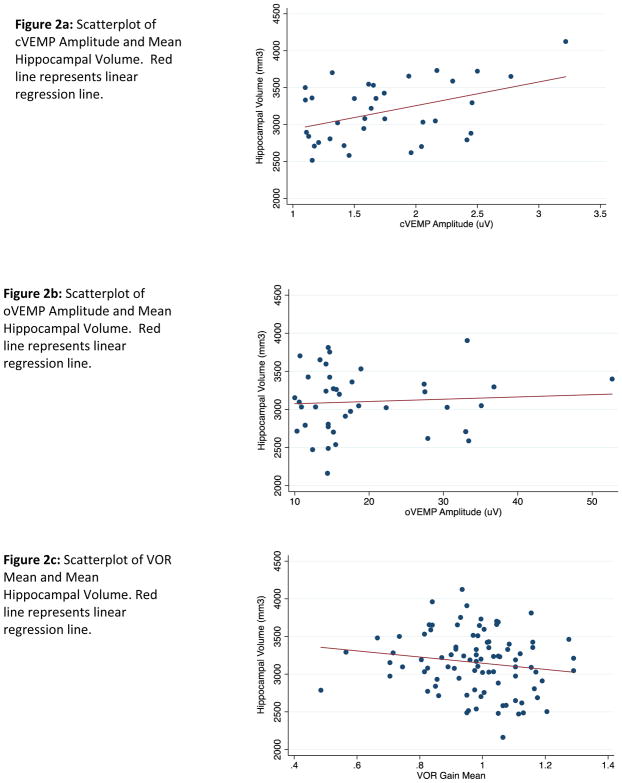
The study sample included 103 participants (range of 35–90 participants in sub-analyses) with mean (±SD) age 77.2 years (±8.71). Multivariate linear models including age, intracranial volume, sex, and race showed that 1μV amplitude increase of cVEMP was associated with an increase of 319.1 mm3 (p=0.003) in mean hippocampal volume. We did not observe a significant relationship between oVEMP amplitude or VOR gain and mean hippocampal volume.
Conclusions
Lower cVEMP amplitude (i.e. reduced saccular function) was significantly associated with lower mean hippocampal volume. This is in line with prior work demonstrating a link between saccular function and spatial cognition. Hippocampal atrophy may be a mechanism by which vestibular loss contributes to impaired spatial cognition in older adults. Future work using longitudinal data will be needed to evaluate the causal nature of the association between vestibular loss and hippocampal atrophy.
Keywords: Vestibular function, hippocampal volume, BLSA, older adults, saccule, cVEMP
INTRODUCTION
Loss of peripheral vestibular sensitivity is common with advanced age, with nearly 85% of older adults over age 80 having measurable vestibular hypofunction. 1–9 Age-related vestibular loss contributes to the elevated balance impairment and falls risk observed in older adults. 2,10–13 Additionally, recent evidence suggests that age-related vestibular loss is also associated with impaired cognition. 13,14 Spatial cognition, which encompasses skills such as spatial memory and navigation, appears to be specifically affected by reduced vestibular function. 15–20
The hippocampus is critical for spatial memory and navigation, 21 and the peripheral vestibular system is known to provide critical inputs to hippocampal neurons. 22–28 Animal studies have shown that bilateral vestibular ablation results in spatial disorientation and hippocampal dysfunction. 19,22,23,28,29 Several studies in humans have shown that patients with bilateral vestibulopathy (e.g. due to Neurofibromatosis 2) have impaired spatial navigation and memory, 15,18 as well as hippocampal atrophy relative to age-matched controls. 25,30 However, it is unknown whether the more common vestibular losses that occur with aging impact hippocampal volume.
In this study, we investigated the relationship between vestibular function and hippocampal volume in a cohort of healthy older adults from the Baltimore Longitudinal Study of Aging (BLSA). In this cross-sectional analysis, we hypothesized that reduced vestibular function would be independently associated with lower hippocampal volume.
MATERIALS AND METHODS
Participants
Participants were selected from the Baltimore Longitudinal Study of Aging (BLSA), a longitudinal study of the aging process in community-dwelling adults that was initiated in 1958. 31 There are currently over 1100 participants enrolled aged 20 to over 100. Eligible participants were ≥60 years old and underwent both vestibular physiologic testing and Brain MRI scans during the same study visit between 2013 (when vestibular testing was started in the BLSA) and 2015. Brain MRI scans were conducted as part of a neuroimaging sub-study of the BLSA. All participants provided written informed consent, and the BLSA study protocol was approved by the National Institute of Environmental Health Sciences Institutional Review Board.
Vestibular Function Testing
Vestibular physiologic testing included assessment of saccular function using the cervical vestibular-evoked myogenic potential (cVEMP) test and utricular function using the ocular vestibular-evoked myogenic potential (oVEMP) test. Video head-impulse testing (VHIT) was used to assess semicircular canal function and determine a vestibular-ocular reflex (VOR) gain. Each vestibular test is briefly described below.
Vestibular-Evoked Myogenic Potentials (VEMP)
A commercial electromyographic system (software version 14.1, Carefusion Synergy, Dublin, OH) was used to record cVEMP and oVEMP. 3,32 Electromyogram signals were recorded with disposable, pre-gelled Ag/AgCl electrodes with 40-inch safety lead wires from GN Otometrics (Schaumburg, IL). Signals were amplified and band-pass filtered using 20–2000 Hz for cVEMP and 3–500 Hz for oVEMP.
Cervical Vestibular-Evoked Myogenic Potentials (cVEMP)
cVEMP uses sound to evoke cervical myogenic potentials and measures saccular function. Following an established protocol, participants sat on a chair inclined to 30 degrees and qualified examiners placed electromyographic (EMG) electrodes on the sternocleidomastoid (SCM) muscle and sternoclavicular junction bilaterally. 1,3,14,32 A ground electrode was placed on the manubrium. Sound stimuli involved 500 Hz and 125 dB tone bursts delivered monaurally through headphones (VIASYS Healthcare, Madison, WI). Amplitudes of myogenic potential response were recorded. These amplitudes were normalized for background EMG activity collected 10 ms before the onset of sound stimulus. An absent response was defined by a response below a threshold level per published guidelines. 1,3 If this occurred, the assessment was repeated to confirm an absent response. For participants with a present response, the cVEMP amplitude of the better ear was used in the analysis. 80 participants (78% of the cohort) had cVEMPs measured during the study period.
Ocular Vestibular-Evoked Myogenic Potentials (oVEMP)
oVEMP testing uses vibration to evoke ocular myogenic potentials and measure utricular function. Following an established protocol, participants sat on a chair inclined to 30 degrees and qualified examiners placed a non-inverting electrode on the cheek inferior to the pupil approximately 3mm below the orbit. 3,14,32 Another inverting electrode was placed 2cm below the non-inverting electrode and lastly, a grounding electrode was placed on the manubrium. Before testing, participants were asked to perform several 20-degree vertical saccades to confirm bilateral signals were symmetric. New electrodes were applied if signals revealed more than 25% asymmetry. During oVEMP testing, participants were asked to continue a 20-degree upgaze. Head taps were performed using a reflex hammer (Aesculap model ACO12C, Center Valley, PA) in the midline of the face at the hairline and approximately one third of the space between the inion and nasion. If the response was below threshold levels, an absent response was recorded. 1,3 If this occurred, the assessment was repeated to confirm an absent response. For participants with a present response, the oVEMP amplitude of the better ear was used for analysis. 76 participants (74% of the cohort) had oVEMPs measured during the study period.
Video Head Impulse Testing (VHIT)
Video head impulse testing (VHIT) was used to measure the horizontal vestibular-ocular reflex (VOR). 14 The EyeSeeCam system (Interacoustics, Eden Prarie, MN) was used in the same plane as the right and left horizontal semicircular canals to determine VOR gain. 33 The participant’s head was slanted down 30 degrees from the horizontal axis to place the horizontal canals in the correct plane of stimulation. Participants were directed to fix their gaze on a wall target 1.5 meters away. The participant’s head was moved 5–15 degrees with high speed (approximately 150–250 degrees per second) in the horizontal plane at least 10 times toward the right side and at least 10 times toward left side. The direction of head movement was randomized so would be unpredictable. The EyeSeeCam system measured eye and head velocity, and the resultant VOR gain was calculated by dividing the eye velocity by the head velocity. A normal eye and head velocity should be equal therefore a normal VOR gain should equal 1.0. A VOR gain less than 0.8 with clear refixation saccades suggests peripheral vestibular hypofunction. 34,35 90 participants (87% of the cohort) had VOR gain measured during the study period.
MRI Brain Acquisition and Processing
In the BLSA, MRI scans were performed using a 3T Philips Achieva scanner at the National Institute on Aging (NIA) Clinical Research Unit. Sequences included a T-1 volumetric scan magnetization prepared rapid acquisition with gradient echo (MPRAGE; TR=6.5ms, TE 3.1ms, flip angle = 8 degrees, 256×256 image matrix, 170 slices, voxel size = 1.0×1.0mm, slice thickness=1.2mm, FOV=256×240mm).
The images were parcellated by MRICloud which is an automated pipeline (https://www.mricloud.org/) that uses large diffeomorphic deformation metric mapping (LDDMM) and multi-atlas likelihood fusion (MALF) algorithms. 36–38 Ten atlases (BIOCARD3T_297labels_10atlases_am_hi_erc_M2_252_V1) were used, in which 297 brain structures were defined with a multi-level hierarchical ontology. 39 In the pipeline, the raw images were pre-processed (skull-stripped, and where necessary orientation adjusted, intensity matched, and inhomogeneity corrected). From the parcellation, the left and right hippocampi were obtained. Following anatomical definitions (http://caportal.cis.jhu/protocols/), the segmented hippocampi were inspected and corrected via Seg3D (http://www.sci.utah.edu/software/seg3D.html). The reliability of hippocampal segmentation has been established for similar scans. 40
MRI Brain Volume
The mean of the right and left hippocampal volume was used in analyses. Intracranial volume (ICV) was defined by the volume within the skull and included the left and right cerebral hemispheres, brainstem, cerebellum, and cerebrospinal fluid (CSF).
Statistical Analysis
Multivariate linear regression adjusted for participant age, intracranial volume, sex, and race was used to investigate the relationship between mean hippocampal volume and vestibular function. All statistical analyses were performed using Stata 12.1 (College Station, TX).
RESULTS
There were 103 participants who had at least one vestibular physiologic test and MRI brain scan completed on the same visit. Among the entire cohort, the mean (±SD) age was 77.2 years (±8.71). Participant demographic characteristics are provided in Table 1. Among participants with a present cVEMP response in either ear (44.3% of participants with cVEMP testing completed), the mean cVEMP amplitude was 1.75 μV (±0.54) (Table 2). Among participants with a present oVEMP response in either ear (52.6% of participants with oVEMP testing completed), the mean oVEMP amplitude was 19.3 μV (±9.50). For the participants with measured VOR gain (n=90), the mean VOR gain was 0.98 (±0.15). Among all participants, the mean intracranial volume was 1,235,318 mm3 (±118,375) and the mean hippocampal volume (i.e. overall mean of each participant’s mean of right and left hippocampal volume) was 3178.9 mm3 (±411.6).
Table 1. Demographic Characteristics of Cohort.
Baltimore Longitudinal Study of Aging (BLSA), 2013–2015.
| Demographic Characteristics | Total Cohort (n=103) |
|---|---|
|
| |
| Age, n (%) | |
| 60–69 | 25 (24.3) |
| 70–79 | 28 (27.2) |
| 80–89 | 44 (42.7) |
| ≥90 | 6 (5.83) |
|
| |
| Sex, n (%) | |
| Female | 29 (28.2) |
| Male | 74 (71.8) |
|
| |
| Race, n (%) | |
| White | 74 (71.8) |
| Black | 23 (22.3) |
| Other | 6 (5.83) |
Table 2. Vestibular Testing and Brain Volume.
Baltimore Longitudinal Study of Aging (BLSA), 2013–2015.
| Vestibular Testing and Brain Volume | n | Mean (±SD) |
|---|---|---|
| cVEMP amplitude1 in μV | 35 | 1.75 (±0.54) |
| oVEMP amplitude2 in μV | 40 | 19.3 (±9.50) |
| VOR gain | 90 | 0.98 (±0.15) |
| Intracranial volume in mm3 | 103 | 1,235,318 (±118,375) |
| Mean3 hippocampal volume in mm3 | 103 | 3178.9 (±411.6) |
For those who had a response, the cVEMP amplitude of the better ear was used for the analysis.
For those who had a response, the oVEMP amplitude of the better ear was used for the analysis.
The mean of the right and left hippocampal volume for each participant was calculated. The mean of this value for the entire cohort is displayed in the table.
The raw data and linear trend of the relationships between vestibular physiologic function and mean (i.e. mean of right and left) hippocampal volume across all participants are provided in Figure 2. Univariate linear regression showed that with each increase in year of age, there was a decrease of 19.8 mm3 mean hippocampal volume (p<0.001) among all participants.
Figure 2. Scatterplots of Vestibular Test Results and Mean Hippocampal Volume.
The mean of the right and left hippocampal volume (mm3) was used for analyses. The data presented in the following plots are unadjusted.
In a multivariate linear model adjusted for age, intracranial volume, sex, and race, each 1 μV increase of cVEMP amplitude was associated with 319.1 mm3 (p=0.003) increase in mean (i.e. mean of right and left) hippocampal volume (Table 3). Neither oVEMP nor VOR gain were significantly associated with mean hippocampal volume. We further evaluated the significant relationship between cVEMP and hippocampal volume by considering right and left cVEMP stimuli and also right and left hippocampal volumes to assess for laterality of associations (Table 4). We found that relationships between cVEMP amplitude and hippocampal volume were significant for left-sided cVEMPs but not right-sided cVEMPs, although the right vs. left cVEMP coefficients were not statistically significantly different (Table 4). We also did not find any significant differences in the coefficients comparing the associations between cVEMP and right vs. left hippocampal volume (Table 4).
Table 3. Linear Models of Mean Hippocampal Volume and Vestibular Tests.
Baltimore Longitudinal Study of Aging (BLSA), 2013–2015. The dependent variable was the mean of right and left hippocampal volume (mm3). Models were adjusted for age, intracranial volume, sex, and race.
| Vestibular Variable | β (95% CI) | Standard Error | p-value |
|---|---|---|---|
| cVEMP1 | 319.1 (110.6, 527.7) | 106.4 | 0.003* |
| oVEMP2 | −10.9 (−23.6, 1.77) | 6.48 | 0.09 |
| VOR Gain | −257.1 (−749.5, 235.3) | 251.2 | 0.31 |
For those who had a response, the cVEMP amplitude of the better ear was used for the analysis.
For those who had a response, the oVEMP amplitude of the better ear was used for the analysis.
p-value <0.05 and statistically significant.
Table 4. Mean Hippocampal Volume and cVEMP by Laterality.
Baltimore Longitudinal Study of Aging (BLSA), 2013–2015. The dependent variables include the mean of right and left hippocampal volume, the right hippocampal volume, and the left hippocampal volume (mm3). All models were adjusted for age, intracranial volume, sex, and race.
| cVEMP Amplitude | Mean Hippocampal Volume | Right Hippocampal Volume | Left Hippocampal Volume | p-value1 |
|---|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | ||
|
| ||||
| Best cVEMP Amplitude | 319.1 (110.6, 527.7) | 342.3 (128.9, 555.6) | 296.0 (78.0, 514.0) | 0.31 |
| n=35 | (p=0.003) | (p=0.002) | (p=0.008) | |
|
| ||||
| Right cVEMP Amplitude | 230.4 (−53.4, 514.2) | 249.5 (−31.4, 530.4) | 211.2 (−104.8, 527.2) | 0.58 |
| n=19 | (p=0.11) | (p=0.08) | (p=0.19) | |
|
| ||||
| Left cVEMP Amplitude | 516.9 (227.6, 806.2) | 541.8 (233.1, 850.6) | 492.0 (210.2, 773.7) | 0.46 |
| n=25 | (p<0.001) | (p=0.001) | (p=0.001) | |
|
| ||||
| p-value2 | 0.06 | 0.06 | 0.08 | |
Comparing regression coefficients for right hippocampal volume to left hippocampal volume.
Comparing regression coefficients for right cVEMP to left cVEMP.
DISCUSSION
In this cross-sectional study of healthy older adults, we observed a significant association between higher cVEMP amplitude and greater mean hippocampal volume. The cVEMP measures the function of the saccule, which is the vestibular end-organ that detects linear accelerations and head tilts in the vertical axis and is thought to be critical to maintaining spatial orientation. 3,32 In prior work also in healthy older adults, we observed that higher cVEMP amplitudes were associated with better performance on measures of spatial cognitive function. 18 The current study provides a potential neuroanatomic mechanism by which reduced vestibular function may influence spatial cognition – via hippocampal atrophy.
Our study builds on an emerging body of evidence that the vestibular system is critical to spatial cognitive function. 24,30,41 The hippocampus is thought to play a critical role in spatial memory and navigation via transmission through hippocampal place cells that encode a cognitive map. 42,43 The peripheral vestibular organs make substantial projections to the hippocampus. 44–48 Moreover, a study in rats demonstrated that vestibular signaling is critical for the normal functioning of hippocampal place cells. 49 These studies offer compelling evidence for an association between vestibular loss and spatial cognitive decline. Indeed, some have hypothesized that vestibular loss contributes to the onset of AD more broadly, 50 given the large population of cholinergic fibers that project from the vestibular system to the hippocampus.
In our study, we specifically found an association between saccular function and hippocampal volume. The saccule is the vestibular end-organ involved in detecting the orientation of the head with respect to gravity, and is thought to play a pre-eminent role in the orientation and encoding of space. 3,32 Mice with congenitally absent saccular function showed poorer ability to navigate their environment and poorer homing skills than normal controls. 51,52 The saccule detects gravitational forces which are directed along the vertical axis, and one study observed that rats placed in hypergravity had subsequent impaired spatial memory and up-regulation of a growth factor gene in the hippocampus, consistent with a saccular influence on hippocampal physiology. 53 Additionally, a recent study using functional MRI (fMRI) found that the hippocampus (specifically the posterior hippocampus) was sensitive only to movements in the vertical axis, which are encoded by the saccule. 54 In prior work, we observed associations between saccular function and spatial cognitive skills in healthy older adults. 13,18,55 Additionally, we found that patients with AD specifically had poorer saccular and utricular function relative to age-matched controls. 14 The saccule appears to have particular relevance for spatial cognitive function, and may make specific connections to regions of the hippocampus that are involved in spatial cognition. Moreover, we observed that left saccular function had a greater association with both right and left hippocampal volumes relative to right saccular function, although the difference between left and right saccular function was not statistically significant. Lateralization of vestibular processing has been described at the cortical level. 56 Whether lateralization also exists of peripheral vestibular inputs which function in a push-pull manner remains to be established.
In this study, we only considered total hippocampal volume rather than the volume of functionally-specific hippocampal subfields. Numerous studies have considered which sub-regions of the hippocampus are involved in spatial cognition. A study comparing taxi drivers to normal controls showed taxi drivers with navigational experience had significantly larger posterior hippocampi and smaller anterior hippocampi relative to controls. 57 This pattern of increased posterior hippocampal volume and decreased anterior hippocampal volume was also seen in professional dancers and slackliners, who are presumed to have high levels of spatial cognitive ability. 58 With respect to vestibular loss and hippocampal structure, patients with vestibular neuritis were observed in one study to have relative atrophy of the left posterior hippocampus, regardless of laterality of disease. 59 Additionally, a study comparing patients with bilateral vestibular failure to normal controls showed no overall difference in gray matter hippocampal volume, but saw a significant relationship between decreased CA3 region volume and vestibular symptom severity. 44 Evaluating which specific regions of the hippocampus are atrophic in the setting of vestibular loss could provide further mechanistic insight into how vestibular loss might impact hippocampal structure.
Limitations of this study include the cross-sectional design, such that causal inferences about the influence of vestibular loss on hippocampal volume cannot be made. Future longitudinal studies will be needed to determine the causal nature of this association. Nevertheless, these findings provide preliminary evidence that the common phenomenon of age-related vestibular loss is associated with CNS structural change (specifically of the hippocampus), which may manifest phenotypically as reduced spatial cognitive ability and consequently spatial disorientation, gait abnormality, and falls.
Figure 1. Volume Analysis Using MRI Cloud.
Images are processed via automated parcellation through MRI Cloud and volumes are determined by binary segmentation.
Acknowledgments
SOURCE OF FUNDING: This research was supported in part by the Intramural Research Program, National Institute on Aging, NIH. The Baltimore Longitudinal Study of Aging (BLSA) was supported by NIA award (1ZIAAG000015-57). Dr. Agrawal was supported by NIH NIDCD K23-DC013056 and NIH NIDCD R03 DC015583. Dr. Ratnanather was supported by NIH NIBIB EB P41-EB015909.
Footnotes
CONFLICTS OF INTEREST: The authors have no conflicts of interest to disclose.
References
- 1.Li C, Layman AJ, Carey JP, Agrawal Y. Epidemiology of vestibular evoked myogenic potentials: Data from the Baltimore Longitudinal Study of Aging. Clin Neurophysiol. 2015;126(11):2207–2215. doi: 10.1016/j.clinph.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001–2004. Arch Intern Med. 2009;169(10):938–944. doi: 10.1001/archinternmed.2009.66. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen KD, Welgampola MS, Carey JP. Test-retest reliability and age-related characteristics of the ocular and cervical vestibular evoked myogenic potential tests. Otol Neurotol. 2010;31(5):793–802. doi: 10.1097/MAO.0b013e3181e3d60e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paige GD. Senescence of human visual-vestibular interactions. 1. Vestibulo-ocular reflex and adaptive plasticity with aging. J Vestib Res. 1992;2(2):133–151. [PubMed] [Google Scholar]
- 5.Peterka RJ, Black FO, Schoenhoff MB. Age-related changes in human vestibulo-ocular reflexes: sinusoidal rotation and caloric tests. J Vestib Res. 1990;1(1):49–59. [PubMed] [Google Scholar]
- 6.Anson E, Jeka J. Perspectives on Aging Vestibular Function. Front Neurol. 2015;6 doi: 10.3389/fneur.2015.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernández L, Breinbauer HA, Delano PH. Vertigo and Dizziness in the Elderly. Front Neurol. 2015;6 doi: 10.3389/fneur.2015.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agrawal Y, Ward BK, Minor LB. Vestibular dysfunction: Prevalence, impact and need for targeted treatment. J Vestib Res. 2013;23(3):113–117. doi: 10.3233/VES-130498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brosel S, Laub C, Averdam A, Bender A, Elstner M. Molecular aging of the mammalian vestibular system. Ageing Res Rev. 2016;26:72–80. doi: 10.1016/j.arr.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Liston MB, Bamiou DE, Martin F, et al. Peripheral vestibular dysfunction is prevalent in older adults experiencing multiple non-syncopal falls versus age-matched non-fallers: a pilot study. Age Ageing. 2014;43(1):38–43. doi: 10.1093/ageing/aft129. [DOI] [PubMed] [Google Scholar]
- 11.Arshad Q, Seemungal BM. Age-Related Vestibular Loss: Current Understanding and Future Research Directions. Front Neurol. 2016;7:231. doi: 10.3389/fneur.2016.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiarovano E, Wang W, Rogers SJ, MacDougall HG, Curthoys IS, de Waele C. Balance in Virtual Reality: Effect of Age and Bilateral Vestibular Loss. Front Neurol. 2017;8:5. doi: 10.3389/fneur.2017.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semenov YR, Bigelow RT, Xue QL, du Lac S, Agrawal Y. Association Between Vestibular and Cognitive Function in U.S. Adults: Data From the National Health and Nutrition Examination Survey. J Gerontol A Biol Sci Med Sci. 2016;71(2):243–250. doi: 10.1093/gerona/glv069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harun A, Oh E, Bigelow R, Studenski S, Agrawal Y. Vestibular Impairment in Dementia. Otology & Neurotology. 2016;37(8):1137–1142. doi: 10.1097/MAO.0000000000001157. http://www.ncbi.nlm.nih.gov/pubmed/27466890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schautzer F, Hamilton D, Kalla R, Strupp M, Brandt T. Spatial memory deficits in patients with chronic bilateral vestibular failure. Ann N Y Acad Sci. 2003;1004:316–324. doi: 10.1196/annals.1303.029. [DOI] [PubMed] [Google Scholar]
- 16.Besnard S, Lopez C, Brandt T, Denise P, Smith PF. Editorial: The Vestibular System in Cognitive and Memory Processes in Mammalians. Front Integr Neurosci. 2015;9:55. doi: 10.3389/fnint.2015.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bigelow RT, Agrawal Y. Vestibular involvement in cognition: Visuospatial ability, attention, executive function, and memory. Journal of vestibular research: equilibrium & orientation. 2015;25(2):73. doi: 10.3233/VES-150544. http://www.ncbi.nlm.nih.gov/pubmed/26410672. [DOI] [PubMed] [Google Scholar]
- 18.Bigelow RT, Semenov YR, Trevino C, et al. Association Between Visuospatial Ability and Vestibular Function in the Baltimore Longitudinal Study of Aging. J Am Geriatr Soc. 2015;63(9):1837–1844. doi: 10.1111/jgs.13609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baek JH, Zheng Y, Darlington CL, Smith PF. Evidence that spatial memory deficits following bilateral vestibular deafferentation in rats are probably permanent. Neurobiol Learn Mem. 2010;94(3):402–413. doi: 10.1016/j.nlm.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Blazer DG, Yaffe K, Karlawish J. Cognitive aging: a report from the Institute of Medicine. JAMA. 2015;313(21):2121–2122. doi: 10.1001/jama.2015.4380. [DOI] [PubMed] [Google Scholar]
- 21.Bilkey DK. Space and context in the temporal cortex. Hippocampus. 2007;17(9):813–825. doi: 10.1002/hipo.20318. [DOI] [PubMed] [Google Scholar]
- 22.Aitken P, Zheng Y, Smith PF. Effects of bilateral vestibular deafferentation in rat on hippocampal theta response to somatosensory stimulation, acetylcholine release, and cholinergic neurons in the pedunculopontine tegmental nucleus. Brain Struct Funct. 2017 doi: 10.1007/s00429-017-1407-1. [DOI] [PubMed] [Google Scholar]
- 23.Russell NA, Horii A, Smith PF, Darlington CL, Bilkey DK. Lesions of the vestibular system disrupt hippocampal theta rhythm in the rat. J Neurophysiol. 2006;96(1):4–14. doi: 10.1152/jn.00953.2005. [DOI] [PubMed] [Google Scholar]
- 24.Besnard S, Machado ML, Vignaux G, et al. Influence of vestibular input on spatial and nonspatial memory and on hippocampal NMDA receptors. Hippocampus. 2012;22(4):814–826. doi: 10.1002/hipo.20942. [DOI] [PubMed] [Google Scholar]
- 25.Brandt T, Schautzer F, Hamilton DA, et al. Vestibular loss causes hippocampal atrophy and impaired spatial memory in humans. Brain. 2005;128(Pt 11):2732–2741. doi: 10.1093/brain/awh617. [DOI] [PubMed] [Google Scholar]
- 26.Lopez C, Blanke O. The thalamocortical vestibular system in animals and humans. Brain Res Rev. 2011;67(1–2):119–146. doi: 10.1016/j.brainresrev.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Smith PF. Vestibular-hippocampal interactions. Hippocampus. 1997;7(5):465–471. doi: 10.1002/(SICI)1098-1063(1997)7:5<465::AID-HIPO3>3.0.CO;2-G. AID-HIPO3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 28.Cuthbert PC, Gilchrist DP, Hicks SL, MacDougall HG, Curthoys IS. Electrophysiological evidence for vestibular activation of the guinea pig hippocampus. Neuroreport. 2000;11(7):1443–1447. doi: 10.1097/00001756-200005150-00018. [DOI] [PubMed] [Google Scholar]
- 29.Zheng Y, Goddard M, Darlington CL, Smith PF. Long-term deficits on a foraging task after bilateral vestibular deafferentation in rats. Hippocampus. 2009;19(5):480–486. doi: 10.1002/hipo.20533. [DOI] [PubMed] [Google Scholar]
- 30.Kremmyda O, Hufner K, Flanagin VL, et al. Beyond Dizziness: Virtual Navigation, Spatial Anxiety and Hippocampal Volume in Bilateral Vestibulopathy. Front Hum Neurosci. 2016;10:139. doi: 10.3389/fnhum.2016.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. [Accessed 12/8/, 2017];BLSA History. https://www.blsa.nih.gov/about/history/
- 32.Li C, Zuniga MG, Nguyen KD, Carey JP, Agrawal Y. How to interpret latencies of cervical and ocular vestibular-evoked myogenic potentials: Our experience in fifty-three participants. Clin Otolaryngol. 2014;39(5):297–301. doi: 10.1111/coa.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider E, Villgrattner T, Vockeroth J, et al. EyeSeeCam: an eye movement-driven head camera for the examination of natural visual exploration. Ann N Y Acad Sci. 2009;1164:461–467. doi: 10.1111/j.1749-6632.2009.03858.x. [DOI] [PubMed] [Google Scholar]
- 34.Weber KP, MacDougall HG, Halmagyi GM, Curthoys IS. Impulsive testing of semicircular-canal function using video-oculography. Ann N Y Acad Sci. 2009;1164:486–491. doi: 10.1111/j.1749-6632.2008.03730.x. [DOI] [PubMed] [Google Scholar]
- 35.Li C, Layman AJ, Geary R, et al. Epidemiology of vestibulo-ocular reflex function: data from the Baltimore Longitudinal Study of Aging. Otol Neurotol. 2015;36(2):267–272. doi: 10.1097/MAO.0000000000000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beg MF, Miller MI, Trouvé A, Younes L. Computing Large Deformation Metric Mappings via Geodesic Flows of Diffeomorphisms. International Journal of Computer Vision. 2005;61(2):139–157. https://doi.org/10.1023/B:VISI.0000043755.93987.aa. VISI.0000043755.93987.aa. [Google Scholar]
- 37.Tang X, Crocetti D, Kutten K, et al. Segmentation of brain magnetic resonance images based on multi-atlas likelihood fusion: testing using data with a broad range of anatomical and photometric profiles. Frontiers in Neuroscience. 2015;9:61. doi: 10.3389/fnins.2015.00061. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4347448/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang X, Oishi K, Faria AV, et al. Bayesian Parameter Estimation and Segmentation in the Multi-Atlas Random Orbit Model. PLoS One. 2013;8(6):e65591. doi: 10.1371/journal.pone.0065591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu D, Ma T, Ceritoglu C, et al. Resource Atlases for Multi-Atlas Brain Segmentations with Multiple Ontology Levels Based on T1-Weighted MRI. Neuroimage. 2015;125:120–130. doi: 10.1016/j.neuroimage.2015.10.042. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4691373/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller MI, Younes L, Ratnanather JT, et al. The diffeomorphometry of temporal lobe structures in preclinical Alzheimer’s disease. Neuroimage Clin. 2013;3:352–360. doi: 10.1016/j.nicl.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popp P, Wulff M, Finke K, Ruhl M, Brandt T, Dieterich M. Cognitive deficits in patients with a chronic vestibular failure. J Neurol. 2017;264(3):554–563. doi: 10.1007/s00415-016-8386-7. [DOI] [PubMed] [Google Scholar]
- 42.O’Keefe J, Black AH. Single unit and lesion experiments on the sensory inputs to the hippocampal cognitive map. Ciba Found Symp. 1977;(58):179–198. doi: 10.1002/9780470720394.ch9. [DOI] [PubMed] [Google Scholar]
- 43.O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Clarendon Press; 1978. http://arizona.openrepository.com/arizona/handle/10150/620894. [Google Scholar]
- 44.Gottlich M, Jandl NM, Sprenger A, et al. Hippocampal gray matter volume in bilateral vestibular failure. Hum Brain Mapp. 2016;37(5):1998–2006. doi: 10.1002/hbm.23152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hitier M, Besnard S, Smith PF. Vestibular pathways involved in cognition. Front Integr Neurosci. 2014;8:59. doi: 10.3389/fnint.2014.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vitte E, Derosier C, Caritu Y, Berthoz A, Hasboun D, Soulie D. Activation of the hippocampal formation by vestibular stimulation: a functional magnetic resonance imaging study. Exp Brain Res. 1996;112(3):523–526. doi: 10.1007/BF00227958. [DOI] [PubMed] [Google Scholar]
- 47.Seo YJ, Kim J, Kim SH. The change of hippocampal volume and its relevance with inner ear function in Meniere’s disease patients. Auris Nasus Larynx. 2016;43(6):620–625. doi: 10.1016/j.anl.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 48.Indovina I, Riccelli R, Chiarella G, et al. Role of the Insula and Vestibular System in Patients with Chronic Subjective Dizziness: An fMRI Study Using Sound-Evoked Vestibular Stimulation. Front Behav Neurosci. 2015;9:334. doi: 10.3389/fnbeh.2015.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stackman RW, Clark AS, Taube JS. Hippocampal spatial representations require vestibular input. Hippocampus. 2002;12(3):291–303. doi: 10.1002/hipo.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Previc FH. Vestibular loss as a contributor to Alzheimer’s disease. Med Hypotheses. 2013;80(4):360–367. doi: 10.1016/j.mehy.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 51.Yoder RM, Kirby SL. Otoconia-deficient mice show selective spatial deficits. Hippocampus. 2014;24(10):1169–1177. doi: 10.1002/hipo.22300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoder RM, Goebel EA, Koppen JR, Blankenship PA, Blackwell AA, Wallace DG. Otolithic information is required for homing in the mouse. Hippocampus. 2015;25(8):890–899. doi: 10.1002/hipo.22410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Horii A, Mitani K, Masumura C, et al. Hippocampal gene expression, serum cortisol level, and spatial memory in rats exposed to hypergravity. J Vestib Res. 2017;27(4):209–215. doi: 10.3233/VES-170521. [DOI] [PubMed] [Google Scholar]
- 54.Kim M, Jeffery KJ, Maguire EA. Multivoxel Pattern Analysis Reveals 3D Place Information in the Human Hippocampus. J Neurosci. 2017;37(16):4270–4279. doi: 10.1523/JNEUROSCI.2703-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xie Y, Bigelow RT, Frankenthaler SF, Studenski SA, Moffat SD, Agrawal Y. Vestibular Loss in Older Adults Is Associated with Impaired Spatial Navigation: Data from the Triangle Completion Task. Front Neurol. 2017;8:173. doi: 10.3389/fneur.2017.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dieterich M, Brandt T. Global orientation in space and the lateralization of brain functions. Curr Opin Neurol. 2018;31(1):96–104. doi: 10.1097/WCO.0000000000000516. [DOI] [PubMed] [Google Scholar]
- 57.Maguire EA, Gadian DG, Johnsrude IS, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A. 2000;97(8):4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hufner K, Binetti C, Hamilton DA, et al. Structural and functional plasticity of the hippocampal formation in professional dancers and slackliners. Hippocampus. 2011;21(8):855–865. doi: 10.1002/hipo.20801. [DOI] [PubMed] [Google Scholar]
- 59.zu Eulenburg P, Stoeter P, Dieterich M. Voxel-based morphometry depicts central compensation after vestibular neuritis. Ann Neurol. 2010;68(2):241–249. doi: 10.1002/ana.22063. [DOI] [PubMed] [Google Scholar]