Abstract
Background
Rho kinases (ROCKs) are the typical downstream effectors of RhoA, which can regulate corneal epithelial wound healing. In this study, the role of ROCK1 in lipopolysaccharide (LPS)-induced cornea inflammation was investigated.
Material/Methods
The expression of ROCK1 in human corneal epithelial cells (HCECs) was bilaterally modulated with ROCK1 expression vector and ROCK1 inhibitor Y-27632. The effects of ROCK1 modulation on the inflammatory response, cell viability, cell apoptosis, and cell cycle distribution were detected by ELISA assay, MTT assay, and flow cytometry, respectively. The pathways involved in the effect of ROCK1 in HCECs was preliminarily explained by detecting changes of TLR4-mediated NF-κB and ERK signaling using western blotting and electrophoretic mobility shift assays.
Results
Overexpression of ROCK1 promoted LPS-induced production of IL-6, IL-8, IL-1β, and TNF-α, and the apoptotic process in HCECs. Augmented inflammation and apoptosis were associated with stronger activation of TLR4-mediated signal transduction; the phosphorylation of IκBα, JNK, ERK1/2, and p38, and nuclear translocation of NF-κB p65 induced by LPS were further increased by overexpression of ROCK1. Inhibition of ROCK1 function by Y-27632 blocked the effect of LPS on HCECs; both LPS-induced inflammation and apoptosis was alleviated by Y-27632, which was associated with suppression of TLR4-mediated NF-κB and ERK signaling.
Conclusions
LPS-induced inflammation and apoptosis in HCECs depended on the function of ROCK1, inhibition of which would attenuate impairments on cornea cells due to LPS.
MeSH Keywords: Corneal Diseases, Lipopolysaccharides, MAP Kinase Signaling System, NF-kappa B, Toll-Like Receptors
Background
Bacterial keratitis is a common ocular infectious disease characterized by corneal epithelial defect and necrosis in the lower corneal matrix in defect areas [1]. The disorder is the major cause of blindness and visual morbidity worldwide [2,3]. Bacterial invasions by Staphylococcus aureus, Pseudomonas aeruginosa, Streptococcus pneumonia, and Serratia species into corneas due to injuries and other pathological factors are the major factor contributing to the onset of keratitis [4]. Thus, antibacterial therapies are currently the most widely used treatment method against bacterial keratitis. Nevertheless, the effectiveness of antibacterial therapies is limited by the abusive use of antibiotics and geographic specificity of microbial profiles. To improved treatment outcomes for bacterial keratitis, the development of therapies based on strategies other than antibacterial treatments is urgently needed.
Previous studies have shown that well-conserved structural motifs secreted by multiple microbes can mediate innate immune responses and cell death processes [5]. Of different compounds, lipopolysaccharide (LPS), which is accumulated in gram-negative bacteria, has been proven to effectively induce inflammation in corneas of corneal injury models [6]. Moreover, the administration of LPS on keratocytes can modulate migration, proliferation, apoptosis, autophagy, and the wound healing process by interacting with toll-like receptor 4 (TLR4) on cell surfaces [7,8]. Based on these results, scientists have proposed a possible strategy for handling bacterial keratitis by blocking the signaling transduction of TLR4.
TLR members play an important role in recognizing pathogen-associated molecular patterns and are crucial components of the innate immunity [9]. Earlier studies have shown that the interaction between TLRs and myeloid differentiation protein 88 (MyD88) further activates mitogen activated protein kinase p38, c-Jun N-terminal kinase (JNK), and nuclear factor (NF)-κB [9], which is essential to produce cytokines such as interleukin (IL)-6, IL-8, and tumor necrosis factor (TNF)-α [10]. Additionally, in the study by Chen et al., the authors indicated that TLR4 also induced the activation of RhoA and the synthesis of pro-inflammatory cytokines in human monocytes [11].
RhoA is a typical member of low molecular weight G proteins of the Rho family and is involved in the regulation of actin cytoskeletons, cell aggregation, and cell motility in corneal epitheliums [12–14]. Rho kinases (ROCKs), activation, which enhances cell proliferation and promotes epithelial differentiation keratocytes [15], are the best characterized downstream effectors of RhoA [15]. Currently, two isoforms of ROCKs have been identified: ROCK1 and ROCK2 (majorly expressed in neural system) [16–18]. The two molecules are the major mediators of RhoA-induced stress fiber and focal adhesion formation [17,19]. Regarding pathological process of corneas, ROCKs participate in the regulation of epithelial differentiation, cell cycle progression, endothelial barrier integrity, and corneal epithelial wound healing [15,20–22]. Based on this information, we hypothesized that targeted regulation of the RhoA/ROCK pathway would promote inflammatory signal transduction mediated by TLR4 during bacterial keratitis.
To validate our hypothesis, the expression of ROCK1 in LPS-treated human corneal epithelial cells was bilaterally modulated with expression vector and ROCK1 inhibitor. Then the effect of different treatments on the inflammation initiation, proliferation, and apoptosis in corneal epithelial cells was detected. For a preliminary explanation of role of ROCK1 in the onset of keratitis, the activities of TLR4-mediated NF-κB and ERK pathways were also assessed in the current study.
Material and Methods
Materials and agents
Pseudomonas aeruginosa LPS (cat. no. L9143) and MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide] (cat. no. M-2128) were purchased from Sigma (USA). RIPA lysis buffer (cat. no. P0013B) and protein concentration detection kit (cat. no. P0009) with BCA method were obtained from Beyotime Biotechnology (China). Plasma and nuclear protein extraction kit (cat. no. P0027) was purchased from Beyotime Biotechnology (China). ROCK inhibitor (Y-27632) (cat. no. HY-10071) was purchased from MedChem Express (USA). Lipofectamine2000 (cat. no. 1024993) was purchased from Invitrogen (USA). Cell apoptosis detection kit (cat. no. KGA106) were purchased from KeyGEN BioTECH (China). Cell cycle detection kit (cat. no. C1052) was purchased from Beyotime Biotechnology (China). Electrophoretic mobility shift assay (EMSA) kit (cat. no. BITF001) was purchased from Viagene (China). Detecting kits for IL-6 (cat. no. SEA079Hu), IL-8 (cat. no. SEA080Hu), IL-1β (cat. no. SEA563Hu), and TNF-α (cat. no. SEA133Hu) were purchased from USCN Business Co., Ltd. (China).
Antibodies
Antibodies against RhoA (cat. no. A00207), ROCK1 (cat. no. BM4203), and TLR4 (cat. no. BA1717) were purchased from Boster (China). Antibodies against NF-κB p65 (cat. no. D221030), Bax (cat. no. D120073), and Bcl-2 (cat. no. D160117) were purchased from Sangon Biotech (China). Antibodies against IκBα (cat. no. bs-1287R), p-IκBα (cat. no. bs-2513R), p38 (cat. no. bs-0637R), p-p38 (cat. no. bs-5476R), β-actin (cat. no. bsm-33139M), and histone H3 (cat. no. bs-17422R) were purchased from Bioss (China). Antibodies against ERK1/2 (cat. no. #4695), p-ERK1/2 (cat. no. #4370), JNK (cat. no. #9252), and p-JNK (cat. no. #4671) were purchased from CST (USA). Secondary goat anti-rabbit (cat. no. A0208) and goat anti-mouse (cat. no. A0216) IgG-HRP antibodies were purchased from Beyotime Biotechnology (China).
Cell culture
Human corneal epithelial cells (HCECs) (cat. no. 6510) were obtained from Shanghai Zhong Qiao Xin Zhou Biotechnology Co., Ltd. (China) and cultured in CEpiCM (cat. no. 6511, ScienCell, USA). For induction of inflammation, cells were cultured with LPS (10 μg/mL) for 24 hours. For validation of the function of ROCK1 in strengthening the inflammation in HCEC, the cells were subjected to 10 μM Y-27632 for two hours prior to LPS administration.
Plasmid construction
cDNAs encoding the catalytic domain of ROCK1 (amino acid, 1 to 477) were inserted into NheI and XhoI sites of pcDNA3.1 vector to form pcDNA3.1-ROCK1. Empty DNA3.1 vector was used as the negative control (NC). Transfection of pcDNA3.1-ROCK1 and NC plasmid was performed using Lipofectamine2000 according to the manufacturer’s instructions before LPS administration.
Cell viability detection
MTT test was performed to detect the change of cell viability. Briefly, 4×103 cells/mL were seeded into each well of a 96-well plate and incubated at 37°C in an atmosphere consisting of 5% CO2 and 95% air overnight. Cells were then transferred to medium containing 0.5 mg/mL MTT and incubated for four hours at 37°C. Afterwards, 150 μL DMSO was added to the medium to dissolve purple crystal. The OD values at 570 nm in different wells were recorded using a Mircoplate Reader (ELX-800, BIOTEK, USA).
Enzyme-linked immunosorbent assay
The production of IL-6 (cat. no. SEA079Hu, USCN Business Co., Ltd., China), IL-8 (cat. no. cat. no. SEA080Hu, USCN Business Co., Ltd., China), IL-1β (cat. no. SEA080Hu, USCN Business Co., Ltd., China), and TNF-α (cat. no. SEA133Hu, USCN Business Co., Ltd., China) in supernatant of HCECs in different groups was detected using corresponding ELISA kits according the manufacturer’s instructions.
Flow cytometry detection
Cell cycle distribution was determined with a cell cycle detection kit using a FACS flow cytometer (Accuri C6, BD, USA). Apoptotic process in HCECs was determined using a cell apoptosis detection kit according to the manufacturer’s instructions with a FACScan flow cytometer (Accuri C6, BD, USA). The total apoptotic rate was equal to the sum of the late apoptotic rate (UR, upper right quadrant-advanced stage apoptosis) and the early apoptotic rate (LR, lower right quadrant-prophase apoptosis).
Immunohistochemistry (IHC)
HCECs were incubated with 0.1% TritonX-100 for 20 minutes and then were fixed using 3% H2O2 for 15 minutes. Primary antibodies against ROCK1 (1: 200) and RhoA (1: 200) were added into the medium and incubated at 4°C overnight. Secondary antibodies (1: 200) were added to the medium for 30-minute incubation at 37°C, followed by five cycles of PBS wash. Thereafter, HRP-labeled avidin was added into the medium and incubated for 30 minutes at room temperature. DAB was then added and incubated for three to 10 minutes before the reaction was stopped by ddH2O. The sections were re-stained with hematoxylin and dehydrated. The results were detected using a microscope (DP73, Olympus, Japan) at 200× magnification.
Western blotting assay
Total proteins were extracted using the RIPA lysis buffer. Nuclear NF-κB subunit p65 was extracted using a plasma and nuclear protein extraction kit. Concentration of protein sample was determined using the BCA method and western blotting was performed routinely. Primary antibodies against RhoA (1: 300), ROCK1 (1: 1,000), TLR4 (1: 300), NF-κB p65 (1: 500), IκBα (1: 500), p-IκBα (1: 500), ERK1/2 (1: 1,000), p-ERK1/2 (1: 2,000), JNK (1: 1,000), p-JNK (1: 1,000), p38 (1: 500), p-p38 (1: 500), Bax (1: 500), Bcl-2 (1: 500), β-actin (1: 500) or histone H3 (1: 500) was added and the membranes were incubated at 4°C overnight. Following washing with TTBS, the membranes were incubated with HRP-conjugated IgG secondary antibodies (1: 5,000) for 45 minutes at 37°C. After washing, the blots were developed using Beyo ECL Plus reagent and scanned in the Gel Imaging System (WD-9413B, Liuyi Factory, China). The relative expression levels of the target proteins were calculated with Gel-Pro-Analyzer (Media Cybernetics, USA).
Electrophoretic mobility shift assay (EMSA)
Nuclear protein was extracted using a plasma and nuclear protein extraction kit and supershift EMSA was performed using electrophoretic mobility shift assay kit according to the manufacturer’s instructions: briefly, nuclear extraction (5 μL), 10× reaction solution (1.5 μL), and ddH2O (8 μL) were mixed and incubated at room temperature for 30 minutes. Biotin-labeled probe (0.5 μL) was then added into the mixture and incubated from another 20 minutes at room temperature. The EMSA reaction system was then subjected to electrophoresis at 180 V for 80 minutes. The membrane was transferred into 0.5× TBE for 10 minutes under 360 mA and fixed under UV light for 30 minutes. Afterwards, the membrane was sealed and reacted with streptavidin-HRP (1: 750) for 20 minutes at room temperature. The result was detected in the Gel Imaging System (WD-9413B, Liuyi Factory, China) and analyzed using Gel-Pro-Analyzer (Media Cybernetics, USA).
Statistical analysis
One-way ANOVA and post hoc comparisons using Fisher’s Least Significant Difference (LSD) method were conducted with different assays. All the statistical analyses were performed using GraphPad Prism version 6.0 (GraphPad Software, Inc., San Diego, CA, USA). Statistical significance was accepted when p-value was less than 0.05 (two-tailed).
Results
Influence of ROCK1 on the inflammatory response induced by LPS in HCECs
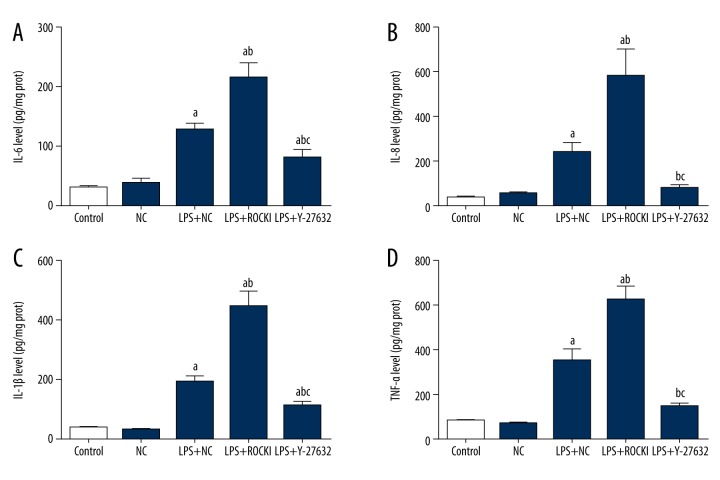
HCECs were first transfected with NC or ROCK1 expression vectors and then incubated with 10 μg/mL LPS for 24 hours to induced inflammation. As shown in Figure 1, the production of pro-inflammation cytokines, including IL-6, IL-8, IL-1β, and TNF-α was all enhanced by LPS compared with the control and NC groups (p<0.05), representing the successful induction of inflammation in HCECs. Overexpression of ROCK1 further increased the production of the four indicators, which inferred the pro-inflammation effect of ROCK1 during the bacterial keratitis (Figure 1). To further demonstrate the possible central role of ROCK1 in LPS-induced inflammation, HCECs was then incubated with ROCK1 specific inhibitor Y-27832 (10 μM) two hours prior to LPS administration. It was found that in the absence of the function of ROCK1, the production of the four cytokines were all suppressed, and the differences between inhibitor and LPS+NC or inhibitor and ROCK1 groups were statistically significant (p<0.05) (Figure 1).
Figure 1.
Influence of ROCK1 on the production of pro-inflammation cytokines in LPS-treated HCECs. a p<0.05 versus NC group; b p<0.05 versus LPS+NC group; c p<0.05 versus LPS+ROCK1 group. Each ELISA assay was represented by three replicates and data were expressed as mean ± standard deviation.
Influence of ROCK1 on cell viability in LPS-treated HCECs
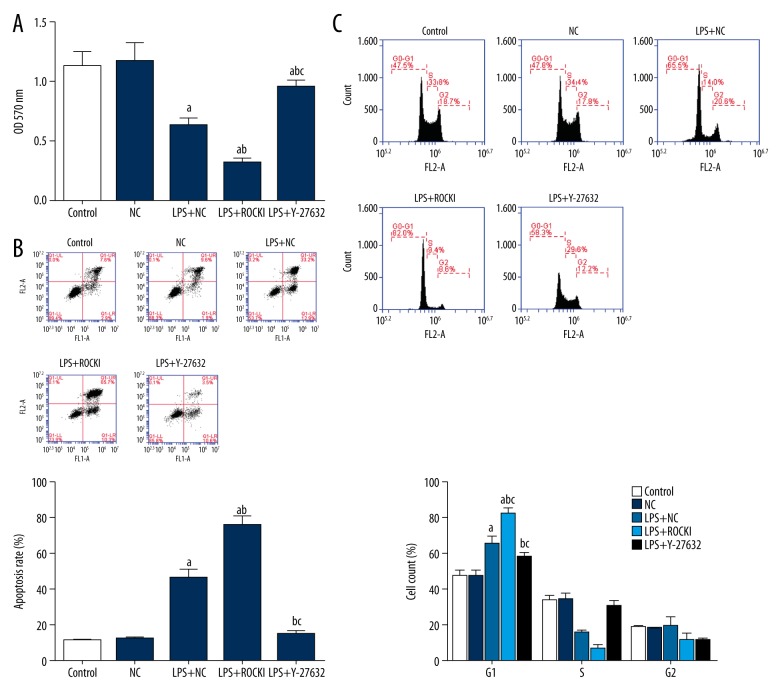
The effect of ROCK1 overexpression on cell viability was detected using MTT assay. LPS administration decreased the OD570 value, representing the suppression of cell viability in HCECs (Figure 2A). Overexpression of ROCK1 further suppressed the HCECs viability (Figure 2A). To the contrary, suppression of ROCK1 function by Y-27832 relieved HCECs from the LPS-induced damages, and the difference between LPS+Y27832 and LPS+NC groups was statistically significant (p<0.05) (Figure 2A).
Figure 2.
Influence of ROCK1 on the cell viability, apoptosis, and cell cycle distribution of LPS-treated HCECs. (A) MTT assay. (B) Flow cytometry: apoptotic rates. (C) Flow cytometry, cell cycle distribution. a p<0.05 versus NC group; b p<0.05 versus LPS+NC group; c p<0.05 versus LPS+ROCK1 group. Each assay was represented by three replicates and data were expressed as mean ± standard deviation.
Influence of ROCK1 on the cell apoptosis and cell cycle distribution in LPS-treated HCECs
Regarding the apoptotic process and cell cycle distribution of HCECs, LPS administration increased the apoptosis apoptotic rate and induced G1 cell cycle arrest in HCECs. ROCK1 overexpression strengthened the effect of LPS by further increasing the apoptotic rate and the proportion of cells in G1 phase (Figure 2B, 2C). Similar to the functioning pattern on production of cytokines and cell viability, pre-administration of Y-27832 inhibited the cell apoptosis increase and the cell cycle arrest induced by LPS.
Influence of ROCK1 on the LPS-induced production of RhoA
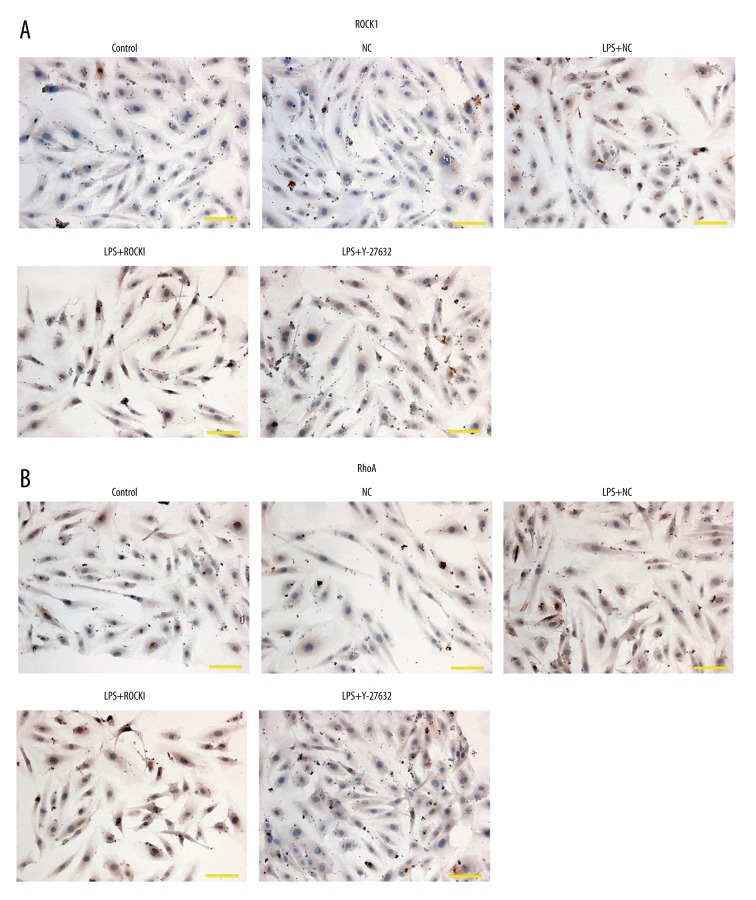
Immunohistochemistry (IHC) results showed that LPS induced the expression of ROCK1 and RhoA. Although ROCK1 was reported to be a downstream effector of RhoA, overexpression further increased the expression and distribution of RhoA (Figure 3) while function inhibition of ROCK1 counteracted the effect of LPS on RhoA expression and distribution (Figure 3).
Figure 3.
Influence of ROCK1 on the expression and distribution of RhoA in LPS-treated HCECs. (A) Expression and distribution of ROCK1. (B) Expression and distribution of RhoA. Scale bar, 100 μm. Each assay was represented by three replicates.
Influence of ROCK1 on the LPS-induced activation of TRL4-mediated NF-κB and ERK signaling
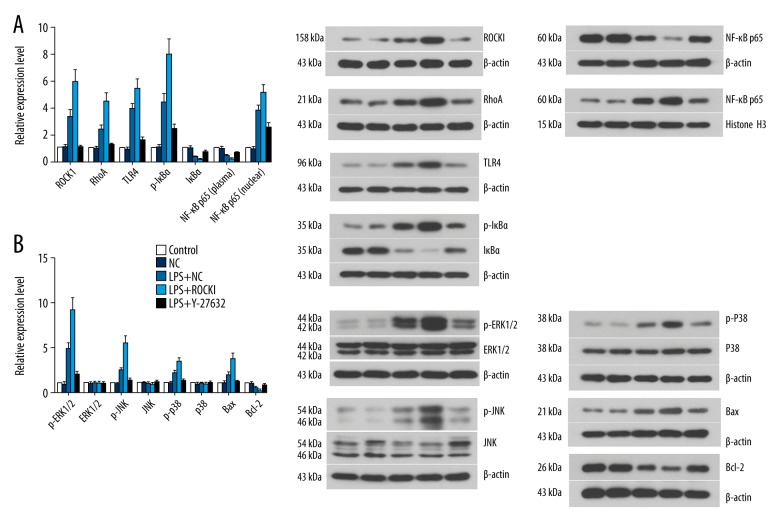
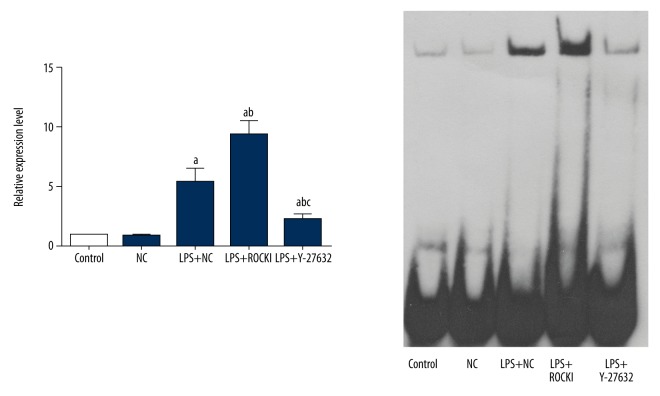
The results of IHC were further verified by western blotting assay: overexpression of ROCK1 induced the expression of RhoA and inhibition of ROCK1 suppressed the expression of RhoA (Figure 4, Supplementary Table 1). Regarding the TLR4-mediated NF-κB and ERK signaling, overexpression of ROCK1 increased the activity (phosphorylation form) of ERK1/2, JNK, and p38 which was firstly induced by LPS (Figure 4, Supplementary Table 1). The activation of ERK signaling led to upregulation of pro-apoptosis factor, Bax, and downregulation of anti-apoptosis factor, Bcl-2 (Figure 4). Moreover, the activity of pro-inflammation NF-κB pathway was also further promoted by ROCK1 overexpression, represented by lower level of plasma IκBα (Figure 4, Supplementary Table 1) and higher nuclear translocation level of NF-κB p65 (Figure 5). The regulating effect of ROCK1 on TLR4-mediated NF-κB and ERK in HCECs were further supported by pre-administration of Y-27832, which decreased the expression and activity of all the pro-inflammation and pro-apoptosis molecules (Figures 4, 5, Supplementary Table 1) while increased the expression of Bcl-2 (Figure 4, Supplementary Table 1).
Figure 4.
Influence of ROCK1 on the activity of TLR4-mediated NF-κB and ERK signaling in LPS-treated HCECs. (A) ROCK1, RhoA, and members of TLR4-mediated NF-κB signaling. (B) Members of TLR4-mediated ERK signaling. Each western blotting assay was represented by three replicate and data were expressed as mean ± standard deviation.
Figure 5.
Influence of ROCK1 on nuclear translocation of NF-κB p65 in LPS-treated HCECs. a p<0.05 versus NC group; b p<0.05 versus LPS+NC group; c p<0.05 versus LPS+ROCK1 group. Each EMSA was represented by three replicates and data were expressed as mean ± standard deviation.
Discussion
Infectious corneal diseases are the major cause of blindness all over the world, of which bacterial keratitis is a common type. Well-conserved structural motifs secreted by gram-negative bacteria will induce innate immune responses leading to the activation of inflammation and eventually cause cell death [23]. Therefore, blockade of the interaction between those motifs and their receptors on corneal cells has been proposed as a potential therapeutic strategy to treat bacterial keratitis. In the current study, the effects of ROCK1 on the LPS-induced inflammation in HCECs were investigated. The results showed that the functions of ROCK1 were necessary for the LPS-induced activation of TLR4-mediated NF-κB and ERK signaling. The lack of ROCK1 function evidently inhibited the LPS-induced inflammatory responses, which was associated with the suppressed activities of TLR4-mediated NF-κB and ERK signaling.
Members of Rho family, including RhoA, Rac1, and Cdc4, have been reported to be involved in the regulation of numerous aspects of cytoskeletal rearrangements [11,24]. Of the many downstream effectors of Rho, two isoforms of ROCKs, ROCK1 and ROCK2, were the first identified and have been proven to control cellular barrier function and to mediate actomyosin contractility in different cells [19]. The activation of ROCK1 has been reported to promote tumor cell invasion [25]; the inhibition of ROCK1 by miR-340 has been reported to be associated with suppressed tumor growth and metastasis of osteosarcoma tumor [26]. Regarding the role of ROCK1 in the cornea, multiple studies suggest regulation on endothelial barrier integrity, cell-matrix interaction, cell-cell adhesion, and epithelial differentiation [12,21,22,27,28]. The inhibition of ROCK1 has also been shown to restore normal behavior of HCECs [29]. Moreover, results for our study provided more information on the effects of ROCK1 on HCECs. The administration of LPS induced inflammatory responses in HCECs, which imitated the conditions in cornea during the processing of bacterial keratitis [23]. The inflammatory responses were associated with the activation of TLR4-mediated NF-κB and ERK signaling [9]. The overexpression of ROCK1 in LPS-treated HCECs strengthened the inflammation by further inducing the activities of TLR4-mediated NF-κB and ERK signaling, indicating a promoting role of ROCK1 in the onset of bacterial keratitis. To explore whether the functions of ROCK1 in LPS-induced inflammation were prerequisite, the activity of ROCK1 was inhibited by specific inhibitor Y-27632. It was found that once the activity of ROCK1 was inhibited, the inflammatory responses were reduced and the activities of TLR4-mediated NF-κB and ERK signaling were suppressed. These results inferred that the functions of ROCK1 were necessary for the LPS-induced inflammation in corneal cells, which was closely related to the TLR4-mediated pro-inflammation pathway.
The interaction between RhoA and TLR4 signaling has been already verified. In the study by Chen et al., the authors indicated that RhoA was a signaling transducer for LPS-induced TLR4-mediated synthesis of pro-inflammation cytokines in monocytes [11]: the activation of TLR4 by LPS rapidly activated RhoA GTPase and increased transcription of IL-8 in human peripheral blood monocytes. However, in the current study, the modulation of the downstream effector of RhoA led to the expression changes of RhoA itself and TLR4-mediated factors. Such results were a supplement to the regulating sequence between RhoA and TLR4 in LPS-induced cornea inflammation; the effects of ROCK1 on the expression of RhoA and TLR4-mediated factors indicated that there existed a regulating loop between RhoA/ROCK and TLR4-mediated NF-κB and ERK signaling. The suppression of any factor in the loop would cause a break in the inflammatory response during bacterial keratitis and other infectious corneal diseases.
Conclusions
Findings outlined in our study demonstrated that the central role of ROCK1 in the LPS-induced inflammation in HCECs which was that the overexpression of ROCK1 strengthened the inflammation while the inhibition of ROCK1 suppressed the inflammation. The effects of ROCK1 were closely associated with the activity changes of RhoA and TLR-mediated NF-κB and ERK signaling. Our results were supplementary to the interaction between RhoA/ROCK and TLR4 signaling in LPS-induced inflammation in corneas: a regulating loop existed among TLR4-RhoA sequence, which might be mediated by ROCK1. However, due to the small number of repeats, the present findings should be interpreted with care and more comprehensive work is needed.
Supplementary Table
Supplementary Table 1.
Relative expression levels of proteins on western blotting assays.
| Protein | Group | ||||
|---|---|---|---|---|---|
| Control | NC | LPS+NC | LPS+ROCK1 | LPS+Y-27632 | |
| ROCK1 | 1.00±0.00 | 1.07±0.15 | 3.33±0.48a | 5.98±0.86ab | 1.07±0.15bc |
| RhoA | 1.00±0.00 | 0.98±0.14 | 2.36±0.34a | 4.47±0.64ab | 1.21±0.17bc |
| TLR4 | 1.00±0.00 | 0.90±0.13 | 3.91±0.41a | 5.46±0.65ab | 1.59±0.23abc |
| p-IκBα | 1.00±0.00 | 1.05±0.15 | 4.39±0.63a | 7.96±1.14ab | 2.40±0.35abc |
| IκBα | 1.00±0.00 | 0.98±0.14 | 0.35±0.02a | 0.17±0.01ab | 0.68±0.08abc |
| NF-κB p65 (plasma) | 1.00±0.00 | 0.99±0.14 | 0.41±0.06a | 0.19±0.03ab | 0.65±0.05abc |
| NF-κB p65 (nuclear) | 1.00±0.00 | 0.94±0.14 | 3.78±0.40a | 5.13±0.62ab | 2.50±0.40abc |
| p-ERK1/2 | 1.00±0.00 | 0.90±0.13 | 4.83±0.69a | 9.21±1.32ab | 2.00±0.29abc |
| ERK1/2 | 1.00±0.00 | 0.94±0.14 | 1.00±0.15 | 0.97±0.14 | 0.95±0.14 |
| p-JNK | 1.00±0.00 | 0.89±0.13 | 2.44±0.27a | 5.48±0.79ab | 1.29±0.19abc |
| JNK | 1.00±0.00 | 1.04±0.15 | 0.94±0.13 | 0.87±0.13 | 1.11±0.16 |
| p-p38 | 1.00±0.00 | 0.97±0.14 | 2.14±0.31a | 3.34±0.48ab | 1.30±0.19bc |
| p38 | 1.00±0.00 | 0.95±0.14 | 1.02±0.15 | 0.95±0.14 | 1.08±0.15 |
| Bax | 1.00±0.00 | 1.06±0.15 | 1.98±0.27a | 3.77±0.54ab | 1.13±0.11bc |
| Bcl-2 | 1.00±0.00 | 0.96±0.14 | 0.50±0.06a | 0.25±0.02ab | 0.77±0.11abc |
p<0.05 versus NC group;
p<0.05 versus LPS+NC group;
p<0.05 versus LPS+ROCK1 group.
Data were expressed as mean ± standard deviation. ROCK1, Rho kinase type 1. TLR4, toll-like receptor 4. IκBα, I κ B proteins α. NF-κB p65, nuclear factor of κ B subunit p65. ERK1/2, extracellular signal-regulated kinase 1/2. JNK, c-Jun N-terminal kinase.
Footnotes
Conflict of interest
None.
Source of support: This study was supported by grants from the National Natural Science Foundation of China (No. 81400438) and the Science Foundation for Youths of Heilongjiang Province (No. QC2013C099)
References
- 1.Lakhundi S, Siddiqui R, Khan NA. Pathogenesis of microbial keratitis. Microb Pathogenes. 2017;104:97–109. doi: 10.1016/j.micpath.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Oldenburg CE, Lalitha P, Srinivasan M, et al. Moxifloxacin susceptibility mediates the relationship between causative organism and clinical outcome in bacterial keratitis moxifloxacin susceptibility. Invest Ophth Vis Sci. 2013;54(2):1522–26. doi: 10.1167/iovs.12-11246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katara RS, Patel ND, Sinha M. A clinical microbiological study of corneal ulcer patients at western Gujarat, India. Acta Medica Iranica. 2013;51(6):399–403. [PubMed] [Google Scholar]
- 4.Wong VWY, Lai TYY, Chi SCC, Lam DSC. Pediatric ocular surface infections: A 5-year review of demographics, clinical features, risk factors, microbiological results, and treatment. Cornea. 2011;30(9):995–1002. doi: 10.1097/ICO.0b013e31820770f4. [DOI] [PubMed] [Google Scholar]
- 5.Hull C, McLean G, Wong F, et al. Lipopolysaccharide signals an endothelial apoptosis pathway through TNF receptor-associated factor 6-mediated activation of c-Jun NH2-terminal kinase. J Immunol. 2002;169(5):2611–18. doi: 10.4049/jimmunol.169.5.2611. [DOI] [PubMed] [Google Scholar]
- 6.Liang H, Brignole-Baudouin F, Labbé A, et al. LPS-stimulated inflammation and apoptosis in corneal injury models. Mol Vis. 2007;13:1169–80. [PubMed] [Google Scholar]
- 7.Uchida K, Unuma K, Funakoshi T, et al. Activation of master autophagy regulator TFEB during systemic LPS administration in the cornea. J Toxicol Pathol. 2014;27(2):153–58. doi: 10.1293/tox.2014-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eslani M, Movahedan A, Afsharkhamseh N, et al. The role of Toll-like receptor 4 in corneal epithelial wound healing TLR4 in corneal epithelial wound healing. Invest Ophth Vis Sci. 2014;55(9):6108–15. doi: 10.1167/iovs.14-14736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren M, Gao L, Wu X. TLR4: the receptor bridging Acanthamoeba challenge and intracellular inflammatory responses in human corneal cell lines. Immunol Cell Biol. 2010;88(5):529–36. doi: 10.1038/icb.2010.6. [DOI] [PubMed] [Google Scholar]
- 10.Proost P, Vynckier AK, Mahieu F, et al. Microbial Toll-like receptor ligands differentially regulate CXCL10/IP-10 expression in fibroblasts and mononuclear leukocytes in synergy with IFN-γ and provide a mechanism for enhanced synovial chemokine levels in septic arthritis. Eur J Immunol. 2003;33(11):3146–53. doi: 10.1002/eji.200324136. [DOI] [PubMed] [Google Scholar]
- 11.Chen L-Y, Zuraw BL, Liu F-T, et al. IL-1 receptor-associated kinase and low molecular weight GTPase RhoA signal molecules are required for bacterial lipopolysaccharide-induced cytokine gene transcription. J Immunol. 2002;169(7):3934–39. doi: 10.4049/jimmunol.169.7.3934. [DOI] [PubMed] [Google Scholar]
- 12.Anderson SC, Stone C, Tkach L, SundarRaj N. Rho and Rho-kinase (ROCK) signaling in adherens and gap junction assembly in corneal epithelium. Invest Ophth Vis Sci. 2002;43(4):978–86. [PubMed] [Google Scholar]
- 13.Nakamura M, Nagano T, Chikama T-i, Nishida T. Role of the small GTP-binding protein rho in epithelial cell migration in the rabbit cornea. Invest Ophth Vis Sci. 2001;42(5):941–47. [PubMed] [Google Scholar]
- 14.Saito J, Morishige N, Chikama T-i, et al. Differential regulation of focal adhesion kinase and paxillin phosphorylation by the small GTP-binding protein Rho in human corneal epithelial cells. Jpn J Ophthalmol. 2004;48(3):199–207. doi: 10.1007/s10384-003-0059-2. [DOI] [PubMed] [Google Scholar]
- 15.Yin J, Fu-Shin XY. Rho kinases regulate corneal epithelial wound healing. Am J Physiol Cell Physiol. 2008;295(2):C378–87. doi: 10.1152/ajpcell.90624.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishizaki T, Maekawa M, Fujisawa K, et al. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 1996;15(8):1885–93. [PMC free article] [PubMed] [Google Scholar]
- 17.Leung T, Chen X-Q, Manser E, Lim L. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16(10):5313–27. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung T, Manser E, Tan L, Lim L. A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J Biol Chem. 1995;270(49):29051–54. doi: 10.1074/jbc.270.49.29051. [DOI] [PubMed] [Google Scholar]
- 19.Riento K, Ridley AJ. Rocks: Multifunctional kinases in cell behaviour. Nat Rev Mol Cell Bio. 2003;4(6):446–56. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 20.Anderson SC, SundarRaj N. Regulation of a Rho-associated kinase expression during the corneal epithelial cell cycle. Invest Ophth Vis Sci. 2001;42(5):933–40. [PubMed] [Google Scholar]
- 21.D’hondt C, Ponsaerts R, Srinivas SP, et al. Thrombin inhibits intercellular calcium wave propagation in corneal endothelial cells by modulation of hemichannels and gap junctions. Invest Ophth Vis Sci. 2007;48(1):120–33. doi: 10.1167/iovs.06-0770. [DOI] [PubMed] [Google Scholar]
- 22.SundarRaj N, Kinchington PR, Wessel H, et al. A Rho-associated protein kinase: Differentially distributed in limbal and corneal epithelia. Invest Ophth Vis Sci. 1998;39(7):1266–72. [PubMed] [Google Scholar]
- 23.Vantaku VR, Gupta G, Rapalli KC, Karnati R. Lacritin Salvages human corneal epithelial cells from lipopolysaccharide induced cell death. Sci Rep. 2015;5:18362. doi: 10.1038/srep18362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ortega MC, Santander-García D, Marcos-Ramiro B, et al. Activation of Rac1 and RhoA Preserve Corneal Endothelial Barrier function homeostatic RhoA and Rac1 activation upon stress. Invest Ophth Vis Sci. 2016;57(14):6210–22. doi: 10.1167/iovs.16-20031. [DOI] [PubMed] [Google Scholar]
- 25.Itoh K, Yoshioka K, Akedo H, et al. An essential part for Rho? associated kinase in the transcellular invasion of tumor cells. Nat Med. 1999;5(2):221–25. doi: 10.1038/5587. [DOI] [PubMed] [Google Scholar]
- 26.Zhou X, Wei M, Wang W. MicroRNA-340 suppresses osteosarcoma tumor growth and metastasis by directly targeting ROCK1. Biochem Bio Res Co. 2013;437(4):653–58. doi: 10.1016/j.bbrc.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 27.Kim A, Lakshman N, Petroll WM. Quantitative assessment of local collagen matrix remodeling in 3-D culture: the role of Rho kinase. Exp Cell Res. 2006;312(18):3683–92. doi: 10.1016/j.yexcr.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Xu Q, Wang Y, Chen F, He J. Development of a new miniaturized bioreactor for axon stretch growth. J Integr Neurosci. 2016;15(3):1–16. doi: 10.1142/S0219635216500230. [DOI] [PubMed] [Google Scholar]
- 29.Okumura N, Sakamoto Y, Fujii K, et al. Rho kinase inhibitor enables cell-based therapy for corneal endothelial dysfunction. Sci Rep. 2016;6:26113. doi: 10.1038/srep26113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Relative expression levels of proteins on western blotting assays.
| Protein | Group | ||||
|---|---|---|---|---|---|
| Control | NC | LPS+NC | LPS+ROCK1 | LPS+Y-27632 | |
| ROCK1 | 1.00±0.00 | 1.07±0.15 | 3.33±0.48a | 5.98±0.86ab | 1.07±0.15bc |
| RhoA | 1.00±0.00 | 0.98±0.14 | 2.36±0.34a | 4.47±0.64ab | 1.21±0.17bc |
| TLR4 | 1.00±0.00 | 0.90±0.13 | 3.91±0.41a | 5.46±0.65ab | 1.59±0.23abc |
| p-IκBα | 1.00±0.00 | 1.05±0.15 | 4.39±0.63a | 7.96±1.14ab | 2.40±0.35abc |
| IκBα | 1.00±0.00 | 0.98±0.14 | 0.35±0.02a | 0.17±0.01ab | 0.68±0.08abc |
| NF-κB p65 (plasma) | 1.00±0.00 | 0.99±0.14 | 0.41±0.06a | 0.19±0.03ab | 0.65±0.05abc |
| NF-κB p65 (nuclear) | 1.00±0.00 | 0.94±0.14 | 3.78±0.40a | 5.13±0.62ab | 2.50±0.40abc |
| p-ERK1/2 | 1.00±0.00 | 0.90±0.13 | 4.83±0.69a | 9.21±1.32ab | 2.00±0.29abc |
| ERK1/2 | 1.00±0.00 | 0.94±0.14 | 1.00±0.15 | 0.97±0.14 | 0.95±0.14 |
| p-JNK | 1.00±0.00 | 0.89±0.13 | 2.44±0.27a | 5.48±0.79ab | 1.29±0.19abc |
| JNK | 1.00±0.00 | 1.04±0.15 | 0.94±0.13 | 0.87±0.13 | 1.11±0.16 |
| p-p38 | 1.00±0.00 | 0.97±0.14 | 2.14±0.31a | 3.34±0.48ab | 1.30±0.19bc |
| p38 | 1.00±0.00 | 0.95±0.14 | 1.02±0.15 | 0.95±0.14 | 1.08±0.15 |
| Bax | 1.00±0.00 | 1.06±0.15 | 1.98±0.27a | 3.77±0.54ab | 1.13±0.11bc |
| Bcl-2 | 1.00±0.00 | 0.96±0.14 | 0.50±0.06a | 0.25±0.02ab | 0.77±0.11abc |
p<0.05 versus NC group;
p<0.05 versus LPS+NC group;
p<0.05 versus LPS+ROCK1 group.
Data were expressed as mean ± standard deviation. ROCK1, Rho kinase type 1. TLR4, toll-like receptor 4. IκBα, I κ B proteins α. NF-κB p65, nuclear factor of κ B subunit p65. ERK1/2, extracellular signal-regulated kinase 1/2. JNK, c-Jun N-terminal kinase.