Abstract
Objective
The purpose of this study was to conduct a proof-of-concept study to evaluate remote recruitment and assessment of individuals (“virtual research visits”) with Parkinson's disease who have pursued direct-to-consumer genetic testing.
Methods
Participants in 23andMe’s “Parkinson’s Research Community” were contacted by 23andMe. Fifty willing participants living in 23 states underwent a remote, standardized assessment including cognitive and motor tests by a neurologist via video conferencing and then completed a survey. Primary outcomes assessed were (a) proportion of participants who completed the remote assessments; (b) level of agreement (using Cohen’s kappa coefficient) of patient-reported data with that of a neurologist; and (c) interest in future virtual research visits.
Results
The self-reported diagnosis of Parkinson’s disease was confirmed in all cases (k = 1.00). The level of agreement for age of symptom onset (k = 0.97) and family history (k = 0.85) was very good but worse for falling (k = 0.59), tremor (k = 0.56), light-headedness (k = 0.31), and urine control (k = 0.15). Thirty-eight (76%) of the 50 participants completed a post-assessment survey, and 87% of respondents said they would be more or much more willing to participate in future clinical trials if they could do research visits remotely.
Conclusion
Remote clinical assessments of individuals with known genotypes were conducted nationally and rapidly from a single site, confirmed self-reported diagnosis, and were received favorably. Direct-to-consumer genetic testing and virtual research visits together may enable characterization of genotype and phenotype for geographically diverse populations.
Keywords: Direct-to-consumer genetic testing, telemedicine, Parkinson's disease, genetics, technology
Introduction
Technology is rapidly reshaping genetics and research studies, as new techniques identify the genetic underpinnings of many diseases.1 Aided by the falling cost of DNA sequencing,2 consumers can now assess their relative genetic risk for many disorders through direct-to-consumer genetic testing.3 For example, in Parkinson’s disease 16 genetic risk loci have been identified over the past 15 years.4,5 Technology and rising drug development costs6 are fueling novel approaches to conducting clinical research and clinical trials,7 including enrichment for targeted genotypes, the use of social networks to conduct observational drug studies8 and virtual clinical trials.9
New collaborations continue to arise from these trends in genetics and clinical research. For example, the partnership between the direct-to-consumer (DTC) genetics company 23andMe and the Michael J. Fox Foundation for Parkinson’s disease research10,11 has led to genotyping and collection of self-reported outcomes of over 10,000 individuals and currently represents the largest cohort of genotyped individuals with Parkinson’s disease.12 Preliminary analyses of this data have identified new phenotype-genotype associations, risk factors, and differences in disease progression and symptoms associated with factors such as gender and body mass index.13 This research model has enabled broad and rapid research participation by individuals in 49 states and over 30 countries and allowed for the discovery of new genetic loci associated with Parkinson’s disease and replicated previously known associations.14 The ability to conduct more in-depth assessments and confirm self-reported data in this population could allow larger-scale participation in clinical trials and observational studies which could capture clinician-assessed phenotypic data at a single time point or longitudinally. Therapeutic trials targeted at specific genetic subpopulations could also use remote assessments to facilitate participation among geographically diverse cohorts of genotypically similar individuals. These genetic subpopulations may have different phenotypes,15 responses to current treatments,16 and almost certainly will be the preferred study population for future gene-targeted therapies, as has been the case for cancer,17 and is increasingly the case for other central nervous system disorders.18
One means of connecting to remote populations is virtual visits. These video visits, conducted via secure video conferencing, are increasingly used for clinical care,19–22 including for Parkinson's disease,23,24 but their application to research has been limited to date.
We conducted a proof of concept study aimed at (a) evaluating remote recruitment and assessment of individuals who have undergone DTC genetic testing, (b) assessing the level of agreement of self-reported phenotypic data with that of a specialist, and (c) soliciting feedback on virtual research visits.
Methods
Study design and participants
Members of 23andMe’s “Parkinson’s Research Community”25 were invited to participate. Eligible members had contributed a DNA sample to 23andMe, reported a diagnosis of Parkinson’s disease, completed 23andMe’s Parkinson’s disease symptom survey developed in collaboration with expert Parkinson’s disease clinicians (Appendix 1), and had been active in the 23andMe community in the previous three months. To obtain a convenience sample of 50 participants, a random sample of 166 of these individuals received an email from 23andMe inviting them to participate, and to review and electronically sign the consent document and a data release authorization form. Participants had to have access to a non-public internet-enabled device. Researchers at Johns Hopkins (KCD, ERD) received contact information for interested participants and reviewed consent over the phone. Consenting participants received a web camera (Logitech C110 or C210 model) if needed, and an email link to download secure, Health Insurance Portability and Accountability Act-compliant videoconferencing software from Vidyo (Hackensack, New Jersey, USA), hosted by ID Solutions (Indianapolis, Indiana, USA). The study team (KCD, SD) also provided technical assistance by phone if necessary. Researchers performed a test connection between the remote research site and the participant’s home prior to the one-time assessment. The Institutional Review Board at Johns Hopkins Medicine approved the research protocol and consent form.
Assessments
A neurologist (ERD) conducted the 30–60 min remote assessment, which was structured to elicit information confirming the participants’ responses to the survey participants had completed upon enrollment in the 23andMe project (Appendix 1). Visits, which were modeled similar to a remote clinic visit,24 included a review of participants’ histories, including symptoms of atypical parkinsonian disorders, onset of specific symptoms (age-at-onset of parkinsonian symptoms, falling, urine control problems, light-headedness, and tremor), and family history (first or second degree relative with Parkinson’s disease). The specialist completed part IA, non-motor aspects of experiences of daily living, part III, motor examination (excluding rigidity and postural stability tests which require in-person assessment), and part IV, motor complications, of the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS).26 For the motor portion of the examination, individuals were asked to perform the tasks (e.g. tapping thumb and index finger, walking) in front of the camera, in line with a previous remote study.23 Visits also included the Montreal Cognitive Assessment, a cognitive assessment tool commonly used in Parkinson’s disease.27,28 Participants were emailed a copy of the visuospatial/executive and naming portions, and completed and demonstrated these tasks in the video call before the examiner completed the remainder of the test. The neurologist determined the most likely diagnosis based on the history and examination focused on the cardinal features of parkinsonism that can be assessed remotely (e.g. rest tremor, bradykinesia, and gait difficulties). Parkinson's disease was identified as the diagnosis if it was the most likely explanation for the individual’s condition as opposed to other causes of parkinsonism (e.g. multiple system atrophy).
Following completion of the clinical assessments, participants were emailed a survey developed for this study asking about their satisfaction with the virtual research visit, comfort in discussing their condition with the specialist, and willingness to participate in future trials remotely (Appendix 2). Completed surveys were returned via US mail, email, or fax and labeled with participant names. Responses were entered manually into an Excel database.
Analysis
Most of the analyses performed were descriptive. Cohen’s kappa coefficient29 was calculated to determine the level of agreement between the participants’ self-reported outcomes on the survey that they initially completed as part of the 23andMe project (Appendix 1) and those assessed by the specialists. The analyses were conducted by NE using Excel.
Results
Study participants and feasibility
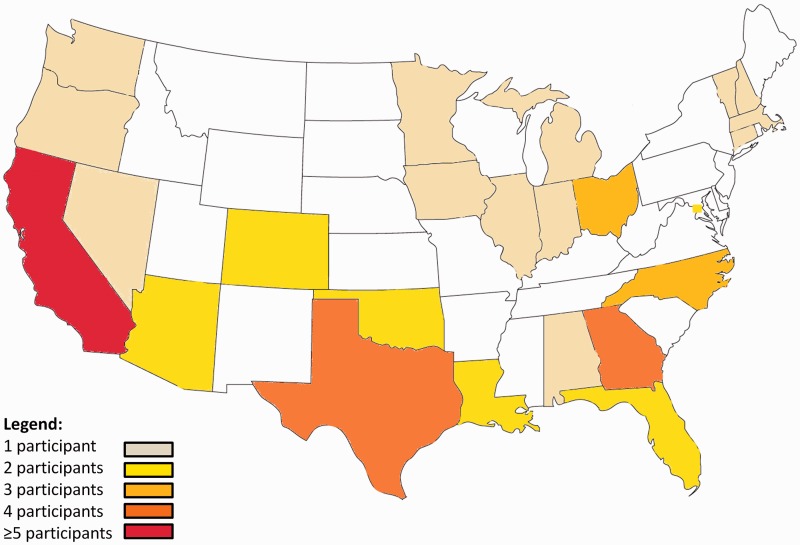
A total of 166 individuals were invited to participate, and 103 opened the email invitation. Fifty-four (32%) agreed to participate and 50 (30%) completed the one-time assessment as scheduled between 25 March 2013–5 June 2013. The reasons for not completing the assessment were: unreachable by phone (n = 2); withdrawal of consent (n = 1); and a scheduling conflict (n = 1). The baseline characteristics of those invited to participate and those that did were generally similar (Table 1). The severity of Parkinson’s disease among participants varied widely, including duration (mean 9.2 years, range from 2–28 years), motor signs (mean motor score 31, range from 18–52; higher scores indicate greater impairment), and cognitive impairment (mean score on the Montreal Cognitive Assessment was 27, range from 18–30; higher scores indicate less impairment). Overall, the characteristics of the participants in this study were similar to those in clinical trials aimed at individuals with moderate Parkinson's disease.30 Six (12%) participants had genotypes associated with at least 50% increased relative risk of developing Parkinson’s disease (Table 1). The participants came from 23 states and the District of Columbia (Figure 1).
Table 1.
Baseline characteristics of study participants.
| Invited to participate (n = 166) | Completed remote assessment (n = 50) | Completed survey (n = 38) | |
|---|---|---|---|
| Demographics | |||
| Age in years (SE) | 63.6 (0.8) | 62.4 (1.5) | 63.3 (1.8) |
| Number (%) women | 64 (38.6) | 16 (32.0) | 13 (34.2) |
| Number (%) Caucasian | 153 (92.2) | 48 (95.7) | 34 (89.5) |
| Education (number (%) completing college) | 101 (60.9)a | 37 (73.5) | 28 (73.7) |
| Parkinson’s disease characteristics | |||
| Number (%) with Parkinson’s disease | 166 (100.0) | 50 (100.0) | 38 (100.0) |
| Disease duration in years (SE) | 9.6 (0.5) | 9.2 (0.9) | 9.2 (0.8) |
| Movement Disorder Society-Unified Parkinson’s Disease Rating Scale, part Ia: Non-Motor Aspects of Experiences of Daily Living score (SE)b | NA | 3.0 (0.4) | 3.4 (0.6) |
| Movement Disorder Society - Unified Parkinson’s Disease Rating Scale, Part III: Modified Motor Score (SE)c | NA | 31.3 (1.1) | 30.6 (1.5) |
| Movement Disorder Society - Unified Parkinson’s Disease Rating Scale, Part IV: Motor Complications Score (SE)b | NA | 3.5 (0.5) | 3.8 (0.7) |
| Montreal Cognitive Assessmentd (SE) | NA | 26.9 (0.4) | 26.5 (0.5) |
| Number (%) with a family history of Parkinson’s disease | 56 (33.7) | 19 (38.0) | 14 (36.8) |
| Number (%) with GBA or LRRK2 mutations | 6 (3.6) | 3 (6.0) | 1 (2.6) |
| Number (%) with substantially increased risk due to other common genetic variantse | 12 (7.2) | 3 (6.0) | 3 (7.9) |
GBA: glucocerebrosidase gene; LRRK2: leucine-rich repeat kinase 2; SE: standard error.
Responses on education are only available for the individuals who were invited to participate.
Scale ranges from with 0–24 with higher scores indicating greater impairment.
Excludes assessments of rigidity and balance. Scale ranges from 0–116 with higher scores indicating greater impairment.
Scale ranges from 0–30 with lower scores indicating greater cognitive impairment.
Over 1.5 times average risk due to common genetic variants associated with Parkinson's disease in populations of European descent (single nucleotide polymorphisms near SNCA, MAPT, PARK16, BST1, DGKQ, and STK39).
Figure 1.
Map of locations of research participants by state.
Level of agreement
Based on the history and examination, all individuals with self-reported Parkinson’s disease were judged by the clinician to have Parkinson’s disease (k = 1.00). The level of agreement between the self-reported data and that assessed by the remote clinician was also very high for age of onset (k = 0.97) and presence of family history (k = 0.85) but much lower for the presence of falling (k = 0.59), tremor (k = 0.56), and non-motor symptoms of light-headedness (k = 0.31), and problems with urine control (k = 0.15).
Post-assessment survey
Thirty-eight of the 50 participants (76% response rate) completed the post-assessment survey. Respondents were satisfied or highly satisfied with the virtual research visit and with the specialist’s ability to assess their symptoms (95%), and comfortable or very comfortable discussing their condition with the specialist (100%). Nearly all respondents were comfortable or very comfortable (97%) with the software. Satisfaction with connection quality was lower (84% satisfied or very satisfied). Nearly all (95%) reported interest in future virtual research visits, and 87% reported that they would be willing or much more willing to participate in clinical trials if they could do visits remotely from their home.
Discussion
Remote recruitment and conduct of research visits in individuals who have undergone DTC genetic testing is feasible, shows relatively good agreement with self-reported data, and is well received by participants. While the benefits of DTC genetic testing continue to be debated,1,2,11,31–39 this proof of concept study demonstrates its potential to identify, follow, and engage geographically dispersed populations with a given disorder and an underlying genotype in research.40 Our 32% response rate to a single recruitment email suggests that targeted electronic recruitment in populations unrestricted by geography may improve participation in research studies. Traditional approaches requiring genetic testing of large populations at multiple medical centers require substantial investment in time and resources to satisfy regulatory needs, execute contracts, and conduct in-person assessments at each institution. By contrast, this study took less than three months and utilized a single investigator to assess individuals throughout the country. This approach lays the foundation for larger scale, broader scope studies that can target genetic sub-populations of different disorders and foster partnerships between consumer genetics companies, pharmaceutical firms, academic institutions, and patient communities. We envision the possibility of conducting remote phenotypic assessments in very large cohorts, marrying data from clinical rating scales such as the MDS-UPDRS with genetic information to better characterize and elucidate variability in Parkinson’s disease symptoms in relation to genetic markers.
The Institute of Medicine has recently called for such collaborations. In its 2012 workshop on genome-based therapies, the Institute highlighted the need to mobilize patient communities to spur research.41 In its Rare diseases and orphan products report, one of the key elements of its national strategy to promote rare diseases research and development is “[timely] application of advances in science and technology that can make rare diseases research and product development faster, easier, and less expensive.[3]”42
Remote assessments are likely to play an increasingly large role in clinical studies of Parkinson's disease43 and other chronic conditions.44 Several clinical trials have been conducted over the internet45–49 with many forgoing in-person visits altogether.45,47–49 Previous clinical studies have found self-reported data to be valid,50 including for self-reported diagnosis.51 This study extends these disruptive models for clinical research by laying the foundation for the inclusion of increasingly available genotypic data into remotely conducted studies.
This study has three key limitations. First, the study population was highly selected – only the most recent and active participants in the 10,000-member “Parkinson’s Community” were asked to participate. These individuals who are highly educated are also more likely to have the internet and technology savvy required to be able to install and operate videoconferencing software, as well as access to high-speed broadband connections, without which remote assessment would be much more difficult. However, many individuals show interest in Parkinson’s disease clinical trial participation, including more than 30,000 individuals registered (all remotely) in the Michael J. Fox Foundation’s “Fox Trial Finder” as of September 2014.52 Because less than a third (n = 50) of the invited population (n = 166) participated in the study, the results should be interpreted with caution as the low response rate may have resulted in selection bias. Similarly, while the survey response was good (76%), the respondents may have been more satisfied than non-respondents.
Second, some questions used in the background survey on falls, tremor, lightheadedness, and urine control were subjective and non-specific and may have contributed to the low level of agreement with the remote assessment. In addition, while a previous study demonstrated good agreement between remote and in-person assessments of the original UPDRS,53 remote administrations of the newer MDS-UPDRS and the Montreal Cognitive Assessment scales remain to be validated.
Third, this study only included a one-time assessment. Future studies could be used to evaluate remote research visits in assessing the natural history of different genetic subpopulations, or as a screening or interim assessment in interventional studies.
Notwithstanding these limitations, this study demonstrates the feasibility and potential value of combining phenotypic and genotypic data for research participants across half the country using remote videoconferencing assessments. These novel technology applications and partnerships can accelerate research at low cost and enhance our understanding of the natural history of genetically linked disorders.
Acknowledgements
The authors wish to thank Tim Harris and Ajay Verma from Biogen for their thoughtful review and critique.
Appendix 1. 23andMe Parkinson’s disease baseline survey
Has a doctor ever diagnosed you with Parkinson’s disease?
Yes
No
I’m not sure/I don’t remember
[if yes, diagnosed with Parkinson's disease]
Which of the following best describes who diagnosed you with Parkinson’s disease? Please check all that apply.
Family physician
Internist
Neurologist
Parkinson's specialist
Another physician
Someone other than a physician
None of the above
[if another physician or someone other than a physician]
Please describe who diagnosed you with Parkinson’s disease.
[free text]
[if no or not sure]
Has a doctor ever diagnosed you with any of the following? Please check all that apply.
Another form of parkinsonism (other than Parkinson's disease)
Dementia, cognitive impairment, or senility
A tremor disorder
None of the above
[if another form of parkinsonism]
Which of the following have you been diagnosed with? Please check all that apply.
Progressive supranuclear palsy (PSP)
Multiple system atrophy (MSA), Shy-Drager syndrome, or striatonigral degeneration
Spinal cerebellar ataxia (SCA), cerebellar degeneration, olivopontocerebellar atrophy (OPCA)
Cortical basal ganglionic degeneration
Atypical parkinsonism or Parkinson's plus
Genetic or familial parkinsonism
Parkinsonism due to medications, drug-induced parkinsonism
Alzheimer's disease with parkinsonism
Frontotemporal dementia with parkinsonism
Parkinson's disease dementia (PDD)
Dementia with Lewy bodies (DLB), Lewy body dementia or cortical Lewy body disease
Another form of dementia
Vascular parkinsonism
None of the above
[if dementia, cognitive impairment, senility]
Which of the following have you been diagnosed with? Please check all that apply.
Alzheimer's disease
Dementia with Lewy bodies (DLB), Lewy body dementia or cortical Lewy body disease
Frontotemporal dementia (FTD)
Parkinson's disease dementia (PDD)
Vascular dementia
Picks disease
Mild cognitive impairment (MCI)
Another form of dementia
None of the above
[if a tremor disorder]
Which of the following have you been diagnosed with? Please check all that apply.
Essential tremor (ET), benign tremor, or senile tremor
Dystonic tremor
Rubral tremor
Primary writing tremor
Tremor due to a stroke
Cerebellar tremor
Multiple sclerosis (MS)
Another tremor disorder
None of the above
How old were you when you were first diagnosed with Parkinson’s disease?
[free text]
How old were you when you first experienced symptoms of Parkinson’s disease?
[free text]
What were the first symptoms of Parkinson’s disease you experienced? Please check all that apply.
Tremor or shaking
Changes in handwriting
Soft voice or speech
Changes in walking
Slowness of movement
Balance problems or falling
Other symptoms
How did your symptoms start?
Gradually
Suddenly
I’m not sure/I don’t remember
When they first started, were your symptoms more noticeable on one side of your body than the other?
Yes
No
I’m not sure/I don’t remember
Are your symptoms currently more noticeable on one side of your body than the other?
Yes
No
I’m not sure/I don’t remember
Overall, how do your symptoms compare to when they first began?
They are worse
They are about the same
They have improved
I’m not sure/I don’t remember
Did your symptoms improve because of medications you are taking?
Yes
No
I’m not sure/I don’t remember
Is your diagnosis still Parkinson's disease or has your diagnosis changed?
My diagnosis is still Parkinson’s disease
My diagnosis changed
I’m not sure/I don’t remember
[if diagnosis changed]
Which of the following best describes how your diagnosis change?
I no longer have the symptoms that led to the Parkinson's disease diagnosis
I still have the symptoms, but the cause is unknown
I was diagnosed with a different disease
[if diagnosed with different disease]
Did your diagnosis change to any of the following?
Another form of parkinsonism
Dementia, cognitive impairment, or senility
A tremor disorder
Something else
[if another form of parkinsonism]
Did your diagnosis change to any of the following? Please check all that apply.
Progressive supranuclear palsy (PSP)
Multiple system atrophy (MSA), Shy-Drager syndrome, or striatonigral degeneration
Spinal cerebellar ataxia (SCA), cerebellar degeneration, olivopontocerebellar atrophy (OPCA)
Cortical basal ganglionic degeneration
Atypical parkinsonism or Parkinson's plus
Genetic or familial parkinsonism
Parkinsonism due to medications, drug-induced parkinsonism
Alzheimer's disease with parkinsonism
Frontotemporal dementia with parkinsonism
Parkinson's disease dementia (PDD)
Dementia with Lewy bodies (DLB), Lewy body dementia or cortical Lewy body disease
Another form of dementia
Vascular parkinsonism
None of the above
[if dementia]
Did your diagnosis change to any of the following? Please check all that apply.
Alzheimer's disease
Dementia with Lewy bodies (DLB), Lewy body dementia or cortical Lewy body disease
Frontotemporal dementia (FTD)
Parkinson's disease dementia (PDD)
Vascular dementia
Picks disease
Mild cognitive impairment (MCI)
Another form of dementia
None of the above
[if a tremor disorder]
Did your diagnosis change to any of the following? Please check all that apply.
Essential tremor (ET), benign tremor, or senile tremor
Dystonic tremor
Rubral tremor
Primary writing tremor
Tremor due to a stroke
Cerebellar tremor
Multiple sclerosis (MS)
Another tremor disorder
None of the above
[if something else]
Did your diagnosis change to any of the following? Please check all that apply.
Motor neuron disease (MND), amyotrophic lateral sclerosis (ALS), Lou Gehrig's disease
Spinal muscular atrophy (SMA), Progressive muscular atrophy (PMA), primary lateral sclerosis (PLS)
Spinal cerebellar ataxia (SCA), cerebellar degeneration, olivopontocerebellar atrophy (OPCA)
Multiple sclerosis (MS)
Stroke or cerebrovascular accident (CVA)
Epilepsy, seizure disorder
Myoclonus
Dystonia
Tourette syndrome, tic disorder
Another brain or neurological disorder
Parkinsonism due to medications, drug-induced parkinsonism
Depression
Thyroid disease
Arthritis
Benign positional vertigo, vertigo, balance problems due to the inner ear, or vestibular disease
Another medical problem
None of the above
We are now going to ask you a few questions about the characteristics of your movement.
Do you have trembling or shaking of any part of your body?
Yes
No
I’m not sure/I don’t remember
[if yes]
What parts of your body tremble or shake?
Head
Jaw or chin
Tongue
Voice
Hands or arms
Feet or legs
Entire body
None of the above
Is the trembling or shaking more noticeable on one side of your body than the other?
Yes
No
I’m not sure/I don’t remember
Does this problem occur every day?
Yes
No
I’m not sure/I don’t remember
About how old were you when you first noticed the trembling or shaking?
[free text]
How did the trembling or shaking begin?
Gradually
Suddenly
I’m not sure/I don’t remember
Since you first noticed it, has the trembling or shaking…
Become worse
Stayed about the same
Improved
I’m not sure/I don’t remember
Have you noticed any of the following changes in your handwriting? Please check all that apply.
Handwriting became slower
Handwriting became smaller
Handwriting became shakier
None of the above
[if changes in handwriting]
Does this problem occur every time you write?
Yes
No
I’m not sure/I don’t remember
About how old were you when you first noticed the changes in your handwriting?
[free text]
How did the changes in your handwriting begin?
Gradually
Suddenly
I’m not sure/I don’t remember
Since you first noticed a change, has your handwriting…
Become worse
Stayed about the same
Improved
I’m not sure/I don’t remember
Have you noticed or have people told you that your speech or voice has become softer than before?
Yes
No
I’m not sure/I don’t remember
[if speech is softer]
Does this problem occur every day?
Yes
No
I’m not sure/I don’t remember
About how old were you when you first noticed changes in your speech or voice?
[free text]
How did the changes in your speech or voice begin?
Gradually
Suddenly
I’m not sure/I don’t remember
Since you first noticed a change, has the problem with your speech/voice…
Become worse
Stayed about the same
Improved
I’m not sure/I don’t remember
Have you noticed any of the following changes in the way you walk? Please check all that apply.
Dragging one or both feet
Feet shuffling
Walking more slowly
Taking smaller steps than before
Steps becoming faster and faster
Feet getting stuck as if glued to the floor
None of the above
[if changes in walk]
Does this problem occur every day?
Yes
No
I’m not sure/I don’t remember
About how old were you when you first noticed changes in the way you walk?
[free text]
How did the changes in the way you walk begin?
Gradually
Suddenly
I’m not sure/I don’t remember
Since you first noticed a change, has the way you walk…
Become worse
Stayed about the same
Improved
I’m not sure/I don’t remember
When walking, do you swing your arms less than you used to?
Yes
No
I’m not sure
[if yes]
Which arm do you swing less?
Left
Right
Both
I’m not sure/I don’t remember
Does this problem occur every day?
Yes
No
I’m not sure
About how old were you when you noticed that you swing your arms less?
[free text]
How did the changes in the way you swing your arms begin?
Gradually
Suddenly
I’m not sure/I don’t remember
Since you first noticed that you swing your arms less, has this problem…
Become worse
Stayed about the same
Improved
I’m not sure/I don’t remember
When standing or walking, do you stoop or bend forward more than you used to?
Yes
No
I’m not sure/I don’t remember
Does this problem occur every day?
Yes
No
I’m not sure/I don’t remember
About how old were you when you noticed changes in your posture?
[free text]
How did the changes in your posture begin?
Gradually
Suddenly
I’m not sure/I don’t remember
Since you first noticed a change, has your posture…
Become worse
Stayed about the same
Improved
I’m not sure/I don’t remember
Do you have trouble with your balance or fall sometimes?
Yes
No
I’m not sure/I don’t remember
Does this problem occur every day?
Yes
No
I’m not sure/I don’t remember
About how old were you when you noticed <symptom>?
[free text]
How did the changes in your <symptom> begin?
Gradually
Suddenly
I’m not sure/I don’t remember
Since you first noticed a change, has your <symptom>…
Become worse
Stayed about the same
Improved
I’m not sure/I don’t remember
[if diagnosis is not Parkinson's disease]
Are you currently taking any prescription medication for the following reasons? Please check all that apply.
Shaking, tremor, balance problems, or problems walking
Indigestion
Depression, nervous breakdown, hallucinations, psychosis, or another psychiatric problem
None of the above
[if shaking, tremor, balance problems or problems walking]
Are you currently taking any of the following medications? Please check all that apply.
Azilect (rasagiline)
Eldepryl, Carbex, Atapryl, or Emsam patch (selegiline or deprenyl)
Mirapex (pramipexole)
Parcopa (carbidopa/levodopa orally disintegrating tablet)
Parlodel (bromocriptine)
Permax (pergolide)
Requip (ropinerole)
Sinemet or Atamet (carbidopa/levodopa)
Stalevo (carbidopa, levodopa and entacapone)
Symmetrel (amantadine)
None of the above
[if indigestion or depression]
Are you taking any of the following medications? Please check all that apply.
Abilify (aripiprazole)
Eldepryl, Carbex, Atapryl, or Emsam patch (selegiline or deprenyl)
Eskalith, Lithobid (lithium)
Haldol (haloperidol)
Prolixin (flupenthixal)
Reglan (metoclopramide)
Risperdal (risperidone)
Seroquel (quetiaprine)
Zyprexa (olanzepine)
None of the above
Have you experienced any of the following symptoms at any time since you developed Parkinson’s disease? Please check all that apply.
Falling
Trouble with urine control
Frequently feeling light-headed on standing
Shaking or tremor
None of the above
[if yes to any of above symptoms]
How old were you when you first experienced [symptom]?
[free text]
Now we would like to find out more about your average or usual function over the past week, including today. Some patients can do things better at one time of the day than at others. However, only one answer is allowed for each question, so please mark the answer that best describes what you can do most of the time.
Over the past week, have you had trouble going to sleep at night or staying asleep through the night? Consider how rested you felt after waking up in the morning.
Normal (No problems.)
Slight (Sleep problems are present but usually do not cause trouble getting a full night of sleep.)
Mild (Sleep problems usually cause some difficulties getting a full night of sleep.)
Moderate (Sleep problems cause a lot of difficulties getting a full night of sleep, but I still usually sleep for more than half the night.)
Severe (I usually do not sleep for most of the night.)
Over the past week, have you had trouble staying awake during the daytime?
Normal (No daytime sleepiness.)
Slight (Daytime sleepiness occurs but I can resist and I stay awake.)
Mild (Sometimes I fall asleep when alone and relaxing. For example, while reading or watching TV.)
Moderate (I sometimes fall asleep when I should not. For example, while eating or talking with other people.)
Severe (I often fall asleep when I should not. For example, while eating or talking with other people.)
Over the past week, have you had uncomfortable feelings in your body like pain, aches tingling or cramps?
Normal (No uncomfortable feelings.).
Slight (I have these feelings. However, I can do things and be with other people without difficulty.)
Mild (These feelings cause some problems when I do things or am with other people.)
Moderate (These feelings cause a lot of problems, but they do not stop me from doing things or being with other people.)
Severe (These feelings stop me from doing things or being with other people.)
Over the past week, have you had trouble with urine control? For example, an urgent need to urinate, a need to urinate too often, or urine accidents?
Normal (No urine control problems.)
Slight (I need to urinate often or urgently. However, these problems do not cause difficulties with my daily activities.)
Mild (Urine problems cause some difficulties with my daily activities. However, I do not have urine accidents.)
Moderate (Urine problems cause a lot of difficulties with my daily activities, including urine accidents.)
Severe (I cannot control my urine and use a protective garment or have a bladder tube.)
Over the past week have you had constipation troubles that cause you difficulty moving your bowels?
Normal (No constipation.)
Slight (I have been constipated. I use extra effort to move my bowels. However, this problem does not disturb my activities or my being comfortable.)
Mild (Constipation causes me to have some troubles doing things or being comfortable.)
Moderate (Constipation causes me to have a lot of trouble doing things or being comfortable. However, it does not stop me from doing anything.)
Severe (I usually need physical help from someone else to empty my bowels.)
Over the past week, have you felt faint, dizzy or foggy when you stand up after sitting or lying down?
Normal (No dizzy or foggy feelings.)
Slight (Dizzy or foggy feelings occur. However, they do not cause me troubles doing things.)
Mild (Dizzy or foggy feelings cause me to hold on to something, but I do not need to sit or lie back down.)
Moderate (Dizzy or foggy feelings cause me to sit or lie down to avoid fainting or falling.)
Severe (Dizzy or foggy feelings cause me to fall or faint.)
Over the past week, have you usually felt fatigued? This feeling is not part of being sleepy or sad.
Normal (No fatigue.)
Slight (Fatigue occurs. However it does not cause me troubles doing things or being with people.)
Mild (Fatigue causes me some troubles doing things or being with people.)
Moderate (Fatigue causes me a lot of troubles doing things or being with people. However, it does not stop me from doing anything.)
Severe (Fatigue stops me from doing things or being with people.)
Over the past week, have you had problems with your speech?
Normal (Not at all.)
Slight (My speech is soft, slurred or uneven, but it does not cause others to ask me to repeat myself.)
Mild (My speech causes people to ask me to occasionally repeat myself, but not everyday.)
Moderate (My speech is unclear enough that others ask me to repeat myself every day even though most of my speech is understood.)
Severe (Most or all of my speech cannot be understood.)
Over the past week, have you usually had too much saliva while you are awake or while you sleep?
Normal (Not at all.)
Slight (I have too much saliva, but do not drool.)
Mild (I have some drooling during sleep, but none when I am awake.)
Moderate (I have some drooling when I am awake, but I usually do not need tissues or a handkerchief.)
Severe (I have so much drooling that I regularly need to use tissues or a handkerchief to protect my clothes.)
Over the past week, have you usually had problems swallowing pills or eating meals? Do you need your pills cut or crushed or your meals to be made soft, chopped or blended to avoid choking?
Normal (No problems.)
Slight (I am aware of slowness in my chewing or increased effort at swallowing, but I do not choke or need to have my food specially prepared.)
Mild (I need to have my pills cut or my food specially prepared because of chewing or swallowing problems, but I have not choked over the past week.)
Moderate (I choked at least once in the past week.)
Severe (Because of chewing and swallowing problems, I need a feeding tube.)
Over the past week, have you usually had troubles handling your food and using eating utensils? For example, do you have trouble handling finger foods or using forks, knifes, spoons, chopsticks?
Normal (Not at all.)
Slight (I am slow, but I do not need any help handling my food and have not had food spills while eating.)
Mild (I am slow with my eating and have occasional food spills. I may need help with a few tasks such as cutting meat.)
Moderate (I need help with many eating tasks but can manage some alone.)
Severe (I need help for most or all eating tasks.)
Over the past week, have you usually had problems dressing? For example, are you slow or do you need help with buttoning, using zippers, putting on or taking off your clothes or jewelry?
Normal (Not at all.)
Slight (I am slow but I do not need help.)
Mild (I am slow and need help for a few dressing tasks, such as with buttons, bracelets.)
Moderate (I need help for many dressing tasks.)
Severe (I need help for most or all dressing tasks.)
Over the past week, have you usually been slow or do you need help with washing, bathing, shaving, brushing teeth, combing your hair or with other personal hygiene?
Normal (Not at all.)
Slight (I am slow but I do not need any help.)
Mild (I need someone else to help me with some hygiene tasks.)
Moderate (I need help for many hygiene tasks.)
Severe (I need help for most or all of my hygiene tasks.)
Over the past week, have people usually had trouble reading your handwriting?
Normal (Not at all.)
Slight (My writing is slow, clumsy or uneven, but all words are clear.)
Mild (Some words are unclear and difficult to read.)
Moderate (Many words are unclear and difficult to read.)
Severe (Most or all words cannot be read.)
Over the past week, have you usually had trouble doing your hobbies or other things that you like to do?
Normal (Not at all.)
Slight (I am a bit slow but do these activities easily.)
Mild (I have some difficulty doing these activities.)
Moderate (I have major problems doing these activities, but still do most.)
Severe (I am unable to do most or all of these activities.)
Over the past week, have you usually had trouble turning over in bed?
Normal (Not at all.)
Slight (I have a bit of trouble turning, but I do not need any help.)
Mild (I have a lot of trouble turning and need occasional help from someone else.)
Moderate (To turn over, I often need help from someone else.)
Severe (I am unable to turn over without help from someone else.)
Over the past week, have you usually had shaking or tremor?
Normal (Not at all.)
Slight (Shaking or tremor occurs but does not cause problems with any activities.)
Mild (Shaking or tremor causes problems with only a few activities.)
Moderate (Shaking or tremor causes problems with many of my daily activities.)
Severe (Shaking or tremor causes problems with most or all activities.)
Over the past week, have you usually had trouble getting out of bed, a car seat, or a deep chair?
Normal (Not at all.)
Slight (I am slow or awkward, but I usually can do it on my first try.)
Mild (I need more than one try to get up or need occasional help.)
Moderate (I sometimes need help to get up, but most times I can still do it on my own.)
Severe (I need help most or all of the time.)
Over the past week, have you usually had problems with balance and walking?
Normal (Not at all.)
Slight (I am slightly slow or may drag a leg. I never use a walking aid.)
Mild (I occasionally use a walking aid, but I do not need any help from another person.)
Moderate (I usually use a walking aid to walk safely without falling. However, I do not usually need the support of another person.)
Severe (I usually use the support of another persons to walk safely without falling.)
Over the past week, on your usual day when walking, do you suddenly stop or freeze as if your feet are stuck to the floor?
Normal (Not at all.)
Slight (I briefly freeze but I can easily start walking again. I do not need help from someone else or a walking aid because of freezing.)
Mild (I freeze and have trouble starting to walk again, but I do not need someone's help or a walking aid because of freezing.)
Moderate (When I freeze I have a lot of trouble starting to walk again and, because of freezing, I sometimes need to use a walking aid or need someone else's help.)
Severe (Because of freezing, most or all of the time, I need to use a walking aid or someone's help.)
The questions in this section will ask about the medications you are taking and have taken in the past.
Which of the following Parkinson's disease medications have you ever taken? Please check all that apply.
Aricept (donepezil)
Artane (trihexyphenidyl)
Apokyn injection (apomorphine)
Azilect (rasagiline)
Cogentin (benztropine)
Comtan (entacapone)
Eldepryl, Carbex, Atapryl, or Emsam patch (selegiline or deprenyl)
Exelon patch (rivastigimine transdermal system)
Mirapex (pramipexole)
Neupro patch (rotigotine patch)
Parcopa (carbidopa/levodopa orally disintegrating tablet)
Parlodel (bromocriptine)
Permax (pergolide)
Razadyne (galantamine)
Requip (ropinerole)
Sinemet or Atamet (carbidopa/levodopa)
Sinemet CR (controlled release carbidopa/levodopa)
Stalevo (carbidopa, levodopa and entacapone)
Symmetrel (amantadine)
Tasmar (tolcapone)
Some other Parkinson's disease medication
None of the above
Which of the following Parkinson's disease medications are you currently taking? Please check all that apply.
Aricept (donepezil)
Artane (trihexyphenidyl)
Apokyn injection (apomorphine)
Azilect (rasagiline)
Cogentin (benztropine)
Comtan (entacapone)
Eldepryl, Carbex, Atapryl, or Emsam patch (selegiline or deprenyl)
Exelon patch (rivastigimine transdermal system)
Mirapex (pramipexole)
Neupro patch (rotigotine patch)
Parcopa (carbidopa/levodopa orally disintegrating tablet)
Parlodel (bromocriptine)
Permax (pergolide)
Razadyne (galantamine)
Requip (ropinerole)
Sinemet or Atamet (carbidopa/levodopa)
Sinemet CR (controlled release carbidopa/levodopa)
Stalevo (carbidopa, levodopa and entacapone)
Symmetrel (amantadine)
Tasmar (tolcapone)
Some other Parkinson's disease medication
I'm not taking any Parkinson's disease medication
During a typical day, are you better after you take your first dose of Parkinson's disease medication in the morning?
Yes
No
I’m not sure/I don’t remember
During a typical day, is it very clear to you when your Parkinson's disease medication stops working?
Yes, the effect of the medication wears off and doesn't come back until I take my next dose
No, my function is the same all day long
I'm not sure/I don't remember
On average, during a typical day, how much of the time are you having a good response to the medications you take for Parkinson's disease? In other words, how much of the time do you think the medications are working well?
1/4 of the day or less
More than 1/4 of the day up to 1/2 the day
More than 1/2 the day up to 3/4 of the day
More than 3/4 of the day up to all day
I'm not sure/I don't remember
The questions in this final section will help us better understand your background and family characteristics.
Has a doctor ever diagnosed any of your biological family members with Parkinson's disease? Please check all that apply.
Mother
Father
Sister
Brother
Daughter
Son
Maternal grandparent
Paternal grandparent
Maternal aunt or uncle
Paternal aunt or uncle
Maternal first cousin
Paternal first cousin
None of the above
In your lifetime, have you ever smoked cigarettes regularly (at least one cigarette per day for six months or longer)?
Yes
No
I’m not sure/I don’t remember
Have you ever had a job where you were regularly exposed to pesticides? Please include herbicides (to kill weeds), fungicides (to kill fungus or mold), insecticides (to kill insects), rodenticides (to kill rodents), or fumigants (a gas used to kill fungus, mold, or insects).
Yes
No
I’m not sure/I don’t remember
Have you regularly used pesticides in your home, garden or on your pets?
Yes
No
I’m not sure/I don’t remember
Have you ever had a head injury or concussion? Please include injuries that may have occurred during sporting activities, from falls, violence, car accidents, or other accidents, both during your childhood and adulthood.
Yes
No
I’m not sure/I don’t remember
[if yes, head injury]
How old were you the first time you had a head injury or concussion?
[free text]
Did you lose consciousness after this injury?
Yes
No
I’m not sure/I don’t remember
Now think about anti-inflammatory medications you have ever taken. These medications are commonly used to treat mild to moderate pain, fever, inflammation or swelling, and sometimes to thin the blood or to protect the heart.
Have you used any of the following types of medications regularly (two or more tablets a week for six months or longer)? Please check all that apply.
Ibuprofen-based non-aspirin medications (These medications are sometimes called non-steroidal anti-inflammatory drugs, or NSAIDS, and include Ibuprofen, Advil, Genpril, Haltran, IBU, Ibu-Tab, Menadol, Midol, Motrin, and Nuprin.)
Other non-steroidal anti-inflammatory medications (For pain, inflammation, or swelling, such as Naprosyn, Aleve, Naproxen, Indocin, Indomethacin, Voltaren, Feldene, Clinoril, Relafen, Lodine, Orudis, and Ansaid. DO NOT include aspirin, Tylenol or acetaminophen, or narcotic pain relievers such as Vicodin or Codeine here.)
Aspirin
None of the above
In what year were you born?
[free text]
Which of the following best describes who completed this questionnaire?
The person diagnosed with Parkinson's disease, without assistance from someone else.
The person diagnosed with Parkinson's disease, with help from someone else.
Someone else on behalf of the person diagnosed with Parkinson's disease
Which of the following best describes the relationship between the person diagnosed with Parkinson's disease and the person who completed or assisted with completing the questionnaire?
Spouse
Adult child
Adult grandchild
Sister or brother
Friend
Someone else
Appendix 2: Telemedicine satisfaction survey
Participant follow-up questionnaire
Parkinson’s telemedicine study
Instructions: Please complete this remote assessment follow-up survey. Return to Johns Hopkins University via email, paper mail, or fax (contact information is listed on the last page).
Participant name: ____________________________
Date completed by participant (mm/dd/yy): ___________________
I. Remote assessment
A. Experience
Were you able to connect with the Parkinson’s disease specialist and complete your web-based (video) appointment as scheduled?
□ Yes
□ No
How much time did it take you to connect with the specialist?
□ <15 minutes
□ 15–30 minutes
□ 30–45 minutes
□ 45–60 minutes
□ ≥60 minutes
How comfortable were you using the web-based software?
□ Very comfortable
□ Comfortable
□ Neither comfortable nor uncomfortable
□ Uncomfortable
□ Very uncomfortable
How comfortable were you discussing your Parkinson’s disease with the specialist?
□ Very comfortable
□ Comfortable
□ Neutral
□ Uncomfortable
□ Very uncomfortable
Do you believe that the specialist was able to learn important information about your Parkinson’s disease during the web-based visit?
□ Yes
□ No
B. Satisfaction
How satisfied were you with the technical quality of the connection during the web-based visit?
□ Very satisfied
□ Satisfied
□ Neutral
□ Unsatisfied
□ Very unsatisfied
How satisfied were you with the specialist’s ability to acquire information about your Parkinson’s disease and overall health?
□ Very satisfied
□ Satisfied
□ Neutral
□ Unsatisfied
□ Very unsatisfied
How satisfied were you with the overall experience of using videoconferencing for this research visit?
□ Very satisfied
□ Satisfied
□ Neutral
□ Unsatisfied
□ Very unsatisfied
II. Clinical trials
A. Awareness
Have you ever sought information on any Parkinson’s disease clinical trial or observational study for yourself?
□ Yes
□ No
Have you ever participated in a research study or clinical trial?
□ Yes
□ No
Would you like more information about clinical trials?
□ Yes
□ No
B. Willingness
How important to you is participating in a drug trial or observational study for Parkinson’s disease?
□ Highly important
□ Important
□ Not important
□ Undecided
Would you be interested in conducting clinical trial visits with a specialist or nurse via web-based appointments in the future?
□ Yes
□ No
How would the option to participate in remote visits (from your home) as part of a clinical trial affect your willingness to participate in a clinical trial?
□ Much more willing
□ More willing
□ Neutral
□ Less willing
□ Much less willing
Would you be comfortable being contacted by someone other than your physician (i.e. a pharmaceutical firm) about becoming involved in a clinical trial?
□ Very comfortable
□ Comfortable
□ Neutral
□ Uncomfortable
□ Very uncomfortable
C. Ability
How close is the nearest Parkinson’s disease research center to your home?
□ <30 minutes
□ 30 minutes–1 hour
□ 1–3 hours
□ ≥3 hours
□ I don’t know
Would remote visits make you more able to participate in a clinical trial?
□ Yes
□ No
□ I don’t know
Please provide suggestions for how we may improve this research study:
–––––––––-
–––––––––-
–––––––––-
Funding
This study was supported by a research grant from Biogen Idec (Cambridge, Massachusetts, USA) and by the efforts of 23andMe (Mountain View, California, USA).
Conflict of interest
ERD is an advisor to, and has stock options in, Grand Rounds; is a compensated consultant to Clintrex, mc10, Shire, and National Institute of Neurological Disorders and Stroke; is an unpaid advisor to SBR Health and Vidyo; receives grant support from Auspex Pharmaceuticals, Prana Biotechnology, the Patient-Centered Outcomes Research Institute, Davis Phinney Foundation, Michael J. Fox Foundation, Huntington Study Group, and Great Lakes Neurotechnologies; and has filed a patent application related to neurology and telemedicine. KD, SD, AT and MA have no conflicting interests. EC is an employee of Biogen Idec. SM and EC are employees of 23andMe. NE was an employee of 23andMe. BR and SW were previously employees of Biogen Idec.
Ethical approval
The Institutional Review Board at Johns Hopkins Medicine approved the research protocol and consent form (IRB Application Number NA_00081180).
Guarantor
ERD
Contributorship
Contributors to the study were as follows: ERD contributed to the design, organization, and execution of the research, participated in designing and reviewing the statistical analysis, and wrote the first draft of the manuscript with KCD. KCD, SM, SD, AT contributed to the execution of the research. EDC contributed to the design, organization, and execution of the research. NE led the design and execution of the statistical analysis. BR contributed to the design of the research and participated in designing and reviewing the statistical analysis. All authors contributed to the analysis and interpretation of the data and review and edited the manuscript and approved the final version of the manuscript.
Peer review
This manuscript was reviewed by Paul Wicks (PatientsLikeMe), and two other reviewers who wish to remain anonymous.
References
- 1.Topol E. The creative destruction of medicine: How the digital revolution will create better health care, New York: Basic Books, 2012. [Google Scholar]
- 2.Altman RB. Direct-to-consumer genetic testing: Failure is not an option. Clin Pharmacol Ther 2009; 86: 15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su P. Direct-to-consumer genetic testing: A comprehensive view. Yale J Biol Med 2013; 86: 359–365. [PMC free article] [PubMed] [Google Scholar]
- 4.Singleton AB, Farrer MJ, Bonifati V. The genetics of Parkinson's disease: Progress and therapeutic implications. Mov Disord 2013; 28: 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nalls MA, Plagnol V, Hernandez DG, et al. International Parkinson Disease Genomics Consortium. Imputation of sequence variants for identification of genetic risks for Parkinson's disease: A meta-analysis of genome-wide association studies. Lancet 2011; 377: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: New estimates of drug development costs. J Health Econ 2003; 22: 151–185. [DOI] [PubMed] [Google Scholar]
- 7.Lipset C. Telethinking with Craig Lipset. Interview by Jamie Devereaux. Telemed J E Health 2010; 16: 857–859. [DOI] [PubMed] [Google Scholar]
- 8.Wicks P, Vaughan TE, Massagli MP, et al. Accelerated clinical discovery using self-reported patient data collected online and a patient-matching algorithm. Nat Biotech 2011; 29: 411–414. [DOI] [PubMed] [Google Scholar]
- 9.US National Institutes of Health. Web-based methodology trial to evaluate the efficacy and safety of tolterodine ER in subjects with overactive bladder (REMOTE). Sponsor: Pfizer, http://clinicaltrials.gov/ct2/show/NCT01302938?term=NCT01302938&rank=1 (2013, accessed 7 October 2013).
- 10.Bonetta L. New citizens for the life sciences. Cell 2009; 138: 1043–1045. [DOI] [PubMed] [Google Scholar]
- 11.Fox JL. What price personal genome exploration? Nat Biotech 2008; 26: 1105–1108. [DOI] [PubMed] [Google Scholar]
- 12.Teichholtz H. Foxfeed blog: 23andMe Parkinson's community hits 10,000 members, https://www.michaeljfox.org/foundation/news-detail.php?23andme-parkinson-community-hits-10-000-members-enrollment-still-free-of-charge-to-parkinson-patients (2013, accessed 3 October 2013).
- 13.Hadly S. Foxfeed blog: 23andMe reports differences in Parkinson’s disease symptoms between men and women, https://www.michaeljfox.org/foundation/news-detail.php?23andme-reports-differences-in-parkinson-symptoms-between-men-and-women (2014, accessed 9 September 2014).
- 14.Do CB, Tung JY, Dorfman E, et al. Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson's disease. PLoS Genet 2011; 7: e1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Healy DG, Falchi M, O'Sullivan SS, et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson's disease: A case-control study. Lancet Neurol 2008; 7: 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan NL, Graham E, Critchley P, et al. Parkin disease: A phenotypic study of a large case series. Brain 2003; 126: 1279–1292. [DOI] [PubMed] [Google Scholar]
- 17.Dachs GU, Dougherty GJ, Stratford IJ, et al. Targeting gene therapy to cancer: A review. Oncol Res 1997; 9: 313–325. [PubMed] [Google Scholar]
- 18.Miller TM, Smith RA, Kordasiewicz H, et al. Gene-targeted therapies for the central nervous system. Arch Neurol 2008; 65: 447–451. [DOI] [PubMed] [Google Scholar]
- 19.Verhoeven F, van Gemert-Pijnen L, Dijkstra K, et al. The contribution of teleconsultation and videoconferencing to diabetes care: A systematic literature review. J Med Internet Res 2007; 9: e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fortney JC, Pyne JM, Mouden SB, et al. Practice-based versus telemedicine-based collaborative care for depression in rural federally qualified health centers: A pragmatic randomized comparative effectiveness trial. Am J Psychiatry 2013; 170: 414–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrer-Roca O, Garcia-Nogales A, Pelaez C. The impact of telemedicine on quality of life in rural areas: The Extremadura model of specialized care delivery. Telemed J E Health 2010; 16: 233–243. [DOI] [PubMed] [Google Scholar]
- 22.Stahl JE, Dixon RF. Acceptability and willingness to pay for primary care videoconferencing: A randomized controlled trial. J Telemed Telecare 2010; 16: 147–151. [DOI] [PubMed] [Google Scholar]
- 23.Dorsey ER, Deuel LM, Voss TS, et al. Increasing access to specialty care: A pilot, randomized controlled trial of telemedicine for Parkinson's disease. Mov Disord 2010; 25: 1652–1659. [DOI] [PubMed] [Google Scholar]
- 24.Dorsey ER, Venkataraman V, Grana MJ, et al. Randomized controlled clinical trial of “virtual house calls” for Parkinson disease. JAMA Neurol 2013; 70: 565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. 23andMe. 23andMe Parkinson's research community online, https://www.23andme.com/pd/ (accessed 19 September 2014).
- 26.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society–sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov Disord 2008; 23: 2129–2170. [DOI] [PubMed] [Google Scholar]
- 27.Gill DJ, Freshman A, Blender JA, et al. The Montreal Cognitive Assessment as a screening tool for cognitive impairment in Parkinson's disease. Mov Disord 2008; 23: 1043–1046. [DOI] [PubMed] [Google Scholar]
- 28.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–699. [DOI] [PubMed] [Google Scholar]
- 29.Carletta J. Assessing agreement on classification tasks: The kappa statistic. Comput Linguist 1996; 22: 249–254. [Google Scholar]
- 30.Rascol O, Brooks DJ, Melamed E, et al. Rasagiline as an adjunct to levodopa in patients with Parkinson's disease and motor fluctuations (LARGO, Lasting effect in Adjunct therapy with Rasagiline Given Once daily, study): A randomised, double-blind, parallel-group trial. Lancet 365: 947–954. [DOI] [PubMed]
- 31.Bloss CS, Schork NJ, Topol EJ. Effect of direct-to-consumer genomewide profiling to assess disease risk. N Engl J Med 2011; 364: 524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gollust SE, Hull SC, Wilfond BS. Limitations of direct-to-consumer advertising for clinical genetic testing. JAMA 2002; 288: 1762–1767. [DOI] [PubMed] [Google Scholar]
- 33.Hogarth S, Javitt G, Melzer D. The current landscape for direct-to-consumer genetic testing: Legal, ethical, and policy issues. Annu Rev Genomics Hum Genet 2008; 9: 161–182. [DOI] [PubMed] [Google Scholar]
- 34.Hudson K, Javitt G, Burke W, et al. ASHG statement on direct-to-consumer genetic testing in the United States. Am J Hum Genet 2007; 81: 635–637. [DOI] [PubMed] [Google Scholar]
- 35.Kuehn BM. Risks and benefits of direct-to-consumer genetic testing remain unclear. JAMA 2008; 300: 1503–1505. [DOI] [PubMed] [Google Scholar]
- 36.McGuire AL, Burke W. An unwelcome side effect of direct-to-consumer personal genome testing: Raiding the medical commons. JAMA 2008; 300: 2669–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts JS, Ostergren J. Direct-to-consumer genetic testing and personal genomics services: A review of recent empirical studies. Curr Genet Med Rep 2013; 1: 182–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hauskeller C. Direct to consumer genetic testing. BMJ 2011; 342: d2317. [DOI] [PubMed]
- 39.US Food and Drug Administration. Warning Letter [to 23andMe, Inc.], http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2013/ucm376296.htm (2013, accessed 3 December 2013).
- 40.Couzin J. Parkinson's disease. Streamlined clinical trials, from a home computer. Science (New York, NY). 2008; 320: 1143. [DOI] [PubMed] [Google Scholar]
- 41.Rapporteurs: Berger AC, Olson S. Genome-based therapeutics: Targeted drug discovery and development: Workshop summary. Washington, DC: The National Academies Press, 2012. [PubMed]
- 42.Field MJ and Boat TF (eds). Rare diseases and orphan products: Accelerating research and development. Washington, DC: The National Academies Press, 2011. [PubMed]
- 43.Achey M, Aldred JL, Aljehani N, et al. The past, present, and future of telemedicine for Parkinson's disease. Mov Disord 2014; 29: 871–883. [DOI] [PubMed] [Google Scholar]
- 44.Dorsey ER, Venuto C, Venkataraman V, et al. Novel methods and technologies for 21st-century clinical trials: A review. JAMA Neurol 2015; 72(5): 582–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McAlindon T. Why are clinical trials of glucosamine no longer uniformly positive? Rheum Dis Clin North Am 2003; 29: 789–801. [DOI] [PubMed] [Google Scholar]
- 46.Eilenberg KL, Hoover AM, Rutherford ML, et al. From informed consent through database lock: An interactive clinical trial conducted using the internet. Therapeutic Innovation & Regulatory Science 2004; 38(3): 239–251. [Google Scholar]
- 47.Jacobs BP, Bent S, Tice JA, et al. An internet-based randomized, placebo-controlled trial of kava and valerian for anxiety and insomnia. Medicine (Baltimore) 2005; 84: 197–207. [DOI] [PubMed] [Google Scholar]
- 48.Wicks P, Vaughan TE, Massagli MP, et al. Accelerated clinical discovery using self-reported patient data collected online and a patient-matching algorithm. Nat Biotechnol 2011; 29: 411–414. [DOI] [PubMed] [Google Scholar]
- 49.Orri M, Lipset CH, Jacobs BP, et al. Web-based trial to evaluate the efficacy and safety of tolterodine ER 4 mg in participants with overactive bladder: REMOTE trial. Contemp Clin Trials 2014; 38: 190–197. [DOI] [PubMed] [Google Scholar]
- 50.Cascade E, Marr P, Winslow M, et al. Conducting research on the Internet: Medical record data integration with patient-reported outcomes. J Med Internet Res 2012; 14: e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharkey EK, Zoellner NL, Abadin S, et al. Validity of participant-reported diagnoses in an online patient registry: A report from the NF1 Patient Registry Initiative. Contemp Clin Trials 2015; 40: 212–217. [DOI] [PubMed] [Google Scholar]
- 52.The Michael J. Fox Foundation for Parkinson's Research. Fox trial finder 2013, https://foxtrialfinder.michaeljfox.org/ (2013, accessed 8 October 2013).
- 53.Cubo E, Gabriel-Galan JM, Martinez JS, et al. Comparison of office-based versus home web-based clinical assessments for Parkinson's disease. Mov Disord 2012; 27: 308–311. [DOI] [PubMed] [Google Scholar]