Abstract
Purpose
This study explored the superiority of temozolomide (TMZ) + interferonβ (IFNβ) to standard TMZ as treatment for newly diagnosed glioblastoma (GBM) via randomized phase II screening design.
Experimental design
Eligibility criteria included histologically proven GBM, with 50% of the tumor located in supratentorial areas, without involvement of the optic, olfactory nerves, and pituitary gland and without multiple lesions and dissemination. Patients in the TMZ + radiotherapy (RT) arm received RT (2.0 Gy/fr/day, 30 fr) with TMZ (75 mg/m2, daily) followed by TMZ maintenance (100–200 mg/m2/day, days 1–5, every 4 weeks) for 2 years. Patients in the TMZ + IFNβ + RT arm intravenously received IFNβ (3 MU/body, alternative days during RT and day 1, every 4 weeks during maintenance period) and TMZ + RT. The primary endpoint was overall survival (OS). The planned sample size was 120 (one-sided alpha 0.2; power 0.8).
Results
Between Apr 2010 and Jan 2012, 122 patients were randomized. The median OS with TMZ + RT and TMZ + IFNβ + RT was 20.3 and 24.0 months (HR 1.00, 95% CI 0.65–1.55; one-sided log rank P = 0.51). The median progression-free survival times were 10.1 and 8.5 months (HR 1.25, 95% CI 0.85–1.84). The incidence of neutropenia with the TMZ + RT and the TMZ + IFNβ + RT (grade 3–4, CTCAE version 3.0) was 12.7 versus 20.7% during concomitant period and was 3.6 versus 9.3% during maintenance period. The incidence of lymphopenia was 54.0 versus 63.8% and 34.5 versus 41.9%.
Conclusions
TMZ + IFNβ + RT is not considered as a candidate for the following phase III trial, and TMZ + RT remained to be a most promising treatment. This trial was registered with the UMIN Clinical Trials Registry: UMIN000003466.
Keywords: Glioblastoma, Interferon-beta, Temozolomide, MGMT, RCT
Introduction
Gliomas account for approximately 40% of all brain tumors and are thus the most common primary tumors of the central nervous system (CNS) [1]. In particular, glioblastoma (GBM) is one of the most frequent brain tumors in the CNS in adults and is highly malignant, with a median survival time of about 1 year from diagnosis [2]. An international randomized trial by the European Organisation for Research and Treatment of Cancer (EORTC)/National Cancer Institute of Canada that compared concomitant radiotherapy (RT) and temozolomide (TMZ) to RT alone clearly demonstrated the benefits of adjuvant TMZ chemotherapy for GBM patients [3]. The median OS in the GBM patients who received RT + TMZ in trials in Europe [3], the United States [4], and an international collaboration (AVAglio) [5] were 14.6, 16.8, and 15.7 months, respectively.
Since then, TMZ has been the current first-line chemotherapeutic agent for GBM. A subgroup analysis in the trial above revealed the effectiveness of epigenetic silencing of the O6-methylguanine-DNA methyltransferase (MGMT) gene via promoter methylation, with longer survival, in patients with primary GBM. It also suggested the benefits of agents targeting MGMT combining with TMZ plus radiotherapy [6]. Interferonβ (IFNβ) exerts pleiotropic biological effects [7, 8] and has been widely used either as a single agent or in combination with other antitumor agents in the treatment of malignant gliomas and melanomas [9]. In the treatment of malignant gliomas, IFNβ can act as a drug sensitizer, and it enhances the toxicity of chemotherapeutic agents against various neoplasms when administered in combination with nitrosourea [10]. Combination therapy with IFNβ and nitrosourea has been used primarily in the treatment of gliomas in Japan [11]. In our previous in vitro study of human glioma cells, we found that IFNβ markedly enhanced chemosensitivity to TMZ [12]. This finding suggested that one of the major mechanisms by which IFNβ enhances chemosensitivity is the downregulation of MGMT transcription via p53 induction. This effect was also observed in an experimental animal model [13]. The results of these 2 studies suggested that chemotherapy with IFNβ and TMZ with concomitant RT might further improve the clinical outcome of patients with malignant gliomas, comparing to chemotherapy with TMZ alone and concomitant RT. Based on these results, we translated the preclinical evidence to clinical studies. A phase I study showed the safety and feasibility of chemotherapy with IFNβ and TMZ combined with concomitant radiotherapy [14, 15]. In addition, a retrospective study demonstrated that addition of IFNβ for newly diagnosed primary GBM achieved a favorable outcome, particularly in patients with an unmethylated MGMT promoter [16].
Based on the rationale shown above, we conducted a randomized screening phase II trial of chemoradiotherapy with TMZ plus IFNβ in comparison with chemoradiotherapy with TMZ alone for newly diagnosed GBM (JCOG0911 INTEGRA study), as the Japan Clinical Oncology Group (JCOG) study to explore the superiority of TMZ + IFNβ therapy to TMZ alone in terms of overall survival (OS) in patients with newly diagnosed GBM.
Materials and methods
Patients
For inclusion in the study, patients had to meet all of the following criteria: histologically proven newly diagnosed GBM based upon WHO 2007 (IARC 4th edition); 50% of the tumor located in supratentorial areas, without involvement of the optic, olfactory nerves, and pituitary gland and without multiple, disseminated, or large tumors in which the planned irradiated target volume exceeds one-third of the whole brain volume; enrollment 3–20 days after surgery; age between 20 and 75 years; Eastern Cooperative Oncology Group (ECOG) performance status of 0–2 or 3 (only if caused by the tumor); no history of previous chemotherapy or radiotherapy; appropriate organ function and written informed consent.
Treatment
Patients in the TMZ + RT arm received RT (2.0 Gy/fr/day, 30 fr) with TMZ (75 mg/m2, daily) followed by maintenance of TMZ (100–200 mg/m2/day, days 1–5, every 4 weeks) for 2 years because (1) optimal duration of maintenance temozolomide had not been determined, and (2) the majority of the investigators in this study agreed that the maintenance temozolomide period was > 12 months.
Patients in the TMZ + IFNβ + RT arm intravenously received IFNβ (3 MU/body on day 1, day 3, and day 5 during RT concomitant period and day 1, every 4 weeks during the maintenance period) in addition to TMZ + RT (Fig. 1). We determined IFN-beta dosage based on previously published trials, including a Phase I trial [11, 14, 17–20].
Fig. 1.
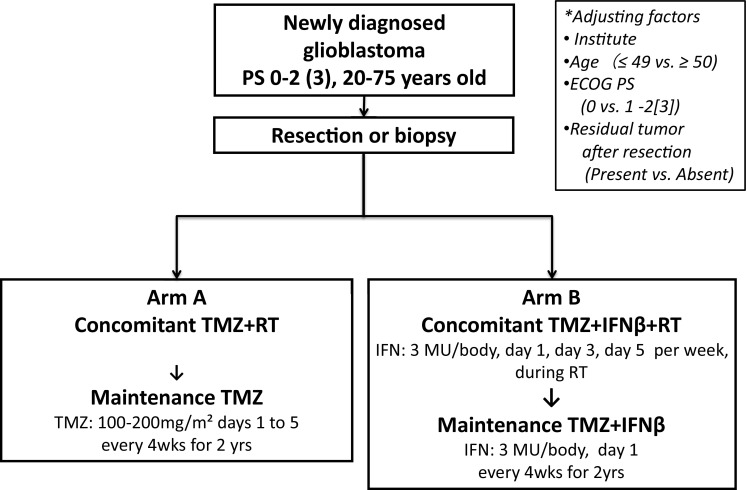
Patient flow diagram of a randomized screening phase II trial of chemoradiotherapy with interferonβ plus temozolomide in comparison with chemoradiotherapy with temozolomide alone for newly diagnosed glioblastoma
RT with concomitant chemotherapy was started within 3 weeks after the surgery. Three-dimensional conformal radiotherapy was planed. Quality assurance reviews were performed at the Radiotherapy Support Center under supervision of JCOG Radiotherapy Committee, with feedback sent to each institution by the RT study coordinator (Minako Sumi). The minimum and maximum doses in the planning target volume (PTV) should comprise between 90 and 110% of the reference point dose of the International Commission on Radiation Units. Gross tumor volume (GTV) was defined as residual tumor, with or without enhancement on computed tomography or magnetic resonance imaging. The clinical target volume 1 (CTV1) included GTV, the resection cavity, and surrounding edema (high-intensity area on T2-weighted or fluid-attenuated inversion recovery image) plus a 1.5-cm margin. The CTV2 included GTV and the resection cavity plus a 1.5-cm margin. PTV was defined as CTV plus a margin of 0.5 cm. The doses for PTV1 and PTV2 were 50 Gy in 25 fractions and 10 Gy in 5 fractions, respectively.
Study design
This trial was designed as a multicenter, prospective, randomized screening phase II study to explore the superiority of TMZ + IFNβ therapy to TMZ alone in terms of OS in patients with newly diagnosed GBM and to decide whether TMZ + IFNβ should be evaluated in a succeeding confirmatory phase III trial. Patients were randomized using a minimization method with biased-coin assignment to receive either the standard arm (TMZ + RT) or the experimental arm (TMZ + IFNβ + RT) at the JCOG Data Center, adjusting for factors including institution, age (≤ 49 vs. ≥ 50 years), ECOG performance status (0 vs. 1 or 2 [3 if this was due to brain tumor]), and residual tumor after resection (present vs. absent). The study protocol was approved by the JCOG Protocol Review Committee and the institutional review board of each participating institution, and carried out in accordance with the Declaration of Helsinki. This trial was registered at the UMIN Clinical Trials Registry as UMIN000003466 (http://www.umin.ac.jp/ctr/index.htm).
Statistical consideration
The primary endpoint was OS. OS was calculated from the date of randomization until death from any cause. The secondary endpoints were progression-free survival (PFS), complete response rate, overall response rate, and adverse events. PFS was calculated from the date of randomization until the date of documented progression or death. Responses were evaluated according to Response Evaluation Criteria in Solid Tumors version 1.0. Toxicities were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.
The planned sample size was 120 and the expected number of events was 70, with a one-sided alpha of 0.2 and power of 0.8 to detect a difference between arms. The 1-year survival was presumed to be 65% in the TMZ + RT arm, and was expected to be 75% in the TMZ + IFNβ + RT arm. The planned accrual and follow-up period were 1.5 and 2 years, respectively. Primary analysis was conducted 2 year after the accrual completion.
One interim analysis was scheduled after the half of the planned sample size was enrolled to assess the futility of this study. Multiplicity was not taken into consideration because terminating the trial due to superiority of TMZ + IFNβ + RT arm was not planned. Results of interim analysis were reviewed by the JCOG Data and Safety Monitoring Committee and investigators were masked to the results.
OS was analyzed by the stratified log-rank test with residual tumor after resection (present vs. absent) as a strata. Hazard ratio was estimated by stratified Cox proportional hazard model with residual tumor after resection (present vs. absent) as a strata. PFS was analyzed by the unstratified log-rank test and unstratified Cox proportional hazard model. OS and PFS curves were estimated by the Kaplan–Meier method. The efficacy analyses were by intention-to treat and safety analyses were by all patients who received protocol treatment. All analyses were performed by the JCOG Data Center using SAS 9.2 (SAS Institute, Cary, NC).
Results
CONSORT diagram and characteristics of the ITT population
From April 2010 to January 2012, 122 patients were accrued, of whom 63 and 59 patients were assigned to the TMZ + RT and TMZ + IFNβ + RT arms, respectively (Fig. 2). All the tumors were proven to be GBM by the central pathological review. In addition, IDH1/2 mutation in each tumor was not detected though anti-IDH1-R132H immunohistochemistry and Sanger sequencing (Table 1). The patients’ characteristics were as follows: median age (61 years [range 22–75 years] vs. 61 years [range 30–73 years]), male/female (38/25 vs. 35/24), ECOG performance status 0/1–3 (16/47 vs. 12/47), residual tumor resection absent/present (31/32 vs. 33/26) (Table 1). One patient in the TMZ + IFNβ + RT arm was off-protocol before the initiation of protocol treatment owing to liver dysfunction and thus was excluded from the safety analysis. Two patients in the TMZ + IFNβ + RT arm terminated protocol treatment during the concomitant period due to progression and adverse events (grade 3 anorexia and grade 2 erythema multiforme) and 12 patients terminated protocol treatment due to progression, adverse events and patient refusal with adverse events (7, 3, and 2 patients) in the interval between the concomitant and the maintenance treatments. In the TMZ + RT arm, 8 patients terminated protocol treatment during the interval between the concomitant and the maintenance treatments because of progression or adverse events (7 and 1 patients). In the maintenance period, 55 patients started TMZ, but 45 patients terminated the maintenance treatment owing to progression, adverse events, and patient refusal with adverse events (34, 3, and 6 patients) in the TMZ + RT arm. In the TMZ + IFNβ + RT, 44 patients started TMZ + IFNβ, but 36 patients terminated the maintenance treatment owing to progression, adverse events, patient refusal with adverse events, and another reason (30, 1, 3, and 1 patients) (Table 2). One treatment related death (TRD) was observed in TMZ + IFNβ + RT arm during the maintenance therapy (severe renal failure). The post-protocol treatments are listed in Table 3. Chemotherapy using either TMZ or TMZ + IFNβ was administered as post-protocol treatments in 11 and 14 patients, respectively. Other chemotherapies were applied in 1 and 6 patients. Bevacizumab was used in 3 patients in the TMZ + RT arm.
Fig. 2.
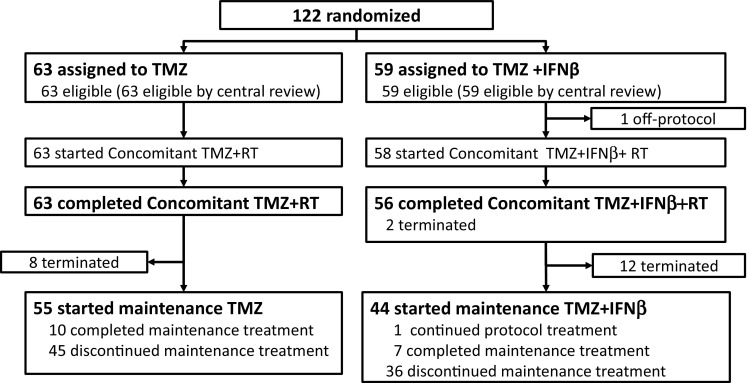
Consort diagram. From April 2010 to January 2012, 122 patients were accrued, of whom 63 and 59 patients were assigned to the TMZ + RT and TMZ + IFNβ + RT arms, respectively
Table 1.
Characteristics of the ITT population
| TMZ + RT (n = 63) | TMZ + IFNβ + RT (n = 59) | |
|---|---|---|
| Age, median (range) | 61 (22–75) | 61 (30–73) |
| Gender | ||
| Male | 38 | 35 |
| Female | 25 | 24 |
| ECOG PS | ||
| 0 | 16 | 12 |
| 1–3 | 47 | 47 |
| Residual tumor after resection | ||
| Absent | 31 | 33 |
| Present | 32 | 26 |
| IDH1/2 status | ||
| No mutation | 57 | 58 |
| Not examined | 6 | 1 |
Table 2.
Number of courses of the maintenance treatments
| Number of treatment course | TMZ + RT (n = 63) | TMZ + IFNβ + RT (n = 56) | n = 119 |
|---|---|---|---|
| 0 | 8(12.7%) | 12(21.4%) | 20 |
| 1–12 | 39(61.9%) | 29(51.8%) | 68 |
| 13–31 | 16(25.4%) | 15(26.8%) | 31 |
Table 3.
Post-protocol treatments
| RT/TMZ (n = 39) | RT/TMZ/IFNβ (n = 39) | |
|---|---|---|
| 1. Same as protocol treatment | 11 | 14 |
| TMZ | 9 | 5 |
| TMZ + IFNβ | 2 | 9 |
| 2. Other chemotherapy (ACNU, Irinotecan, ICE, other TMZ regimens) | 1 | 6 |
| 3.SRS, SRT | 4 | 7 |
| 4. Surgery | 18 | 11 |
| 5. Others | 5 | 1 |
| Bevacizumab | 3 | 0 |
| Vaccine | 2 | 1 |
Overall and progression-free survival
The median survival time was 20.3 months (95% CI 15.4–26.9 months) and 24.0 months (95% CI 18.8–27.4 months) in the TMZ + RT arm and the TMZ + IFNβ + RT arm, respectively (HR 1.00, 95% CI 0.65–1.55; one-sided log rank P = 0.51). OS did not statistically differ between the two arms (Fig. 3a).
Fig. 3.
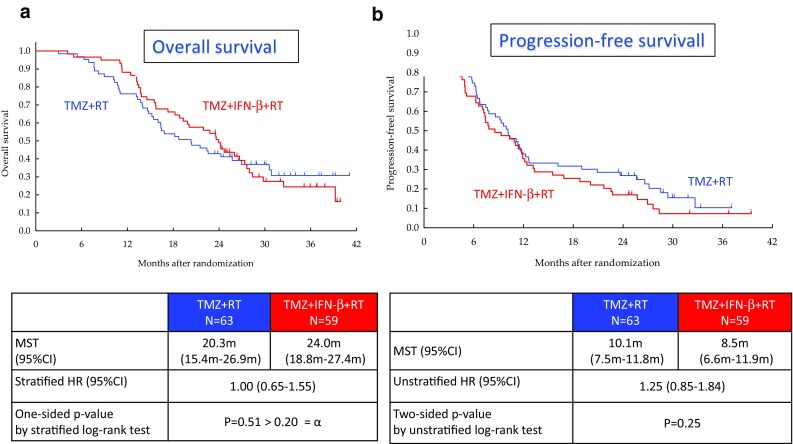
Clinical outcomes. The median survival time was 20.3 months (95% CI 15.4–26.9 months) and 24.0 months (95% CI 18.8–27.4 months) in the TMZ + RT arm and the TMZ + IFNβ + RT arm, respectively (HR 1.00, 95% CI 0.65–1.55; one-sided log rank P = 0.51). OS did not statistically differ between the two arms (a). The median PFS was 10.1 months (95% CI 7.5–11.8 months) and 8.5 months (95% CI 6.6–11.9 months) in the TMZ + RT arm and the TMZ + IFNβ + RT arm, respectively (HR 1.25, 95% CI 0.85–1.84; two-sided P = 0.25) (b)
The median PFS was 10.1 months (95% CI 7.5–11.8 months) and 8.5 months (95% CI 6.6–11.9 months) in the TMZ + RT arm and the TMZ + IFNβ + RT arm, respectively (HR 1.25, 95% CI 0.85–1.84; two-sided P = 0.25) (Fig. 3b).
Subgroup analyses were performed for OS by sex (male/female), age (≤ 49 years/≥ 50 years), residual tumor after resection (absent/present) and ECOG PS (0/1/2–3) (Fig. 4). Male, Younger patients (≤ 49 years) and ECOG PS 0 in the TMZ + IFNβ + RT arm showed good OS compared with RT/TMZ arm.
Fig. 4.
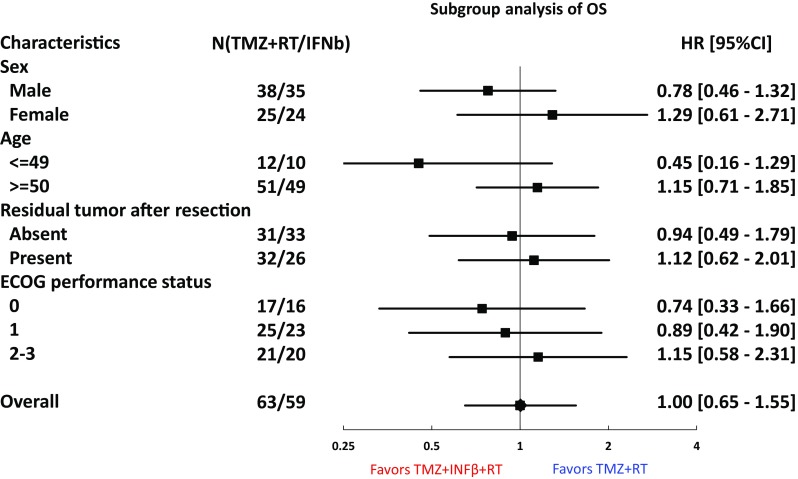
Subgroup analyses were performed for OS by sex (male/female), age (≤ 49 years/≥ 50 years), residual tumor after resection (absent/present) and ECOG PS (0/1/2–3). Male, Younger patients (≤ 49 years) and ECOG PS 0 in the TMZ + IFNβ + RT arm showed good OS compared with RT/TMZ arm
Adverse events
The incidence of grade 3 and 4 neutropenia was higher in the TMZ + IFNβ + RT arm (Table 4). The difference was more marked in the patients aged ≥ 50 years. Among the non-hematological adverse events, fever, nausea/vomiting, and appetite loss tended to be more frequent in the TMZ + IFNβ + RT arm.
Table 4.
(a) Adverse events (concomitant chemoradiotherapy), (b) adverse events (maintenance therapy)
| (a) | TMZ + RT (N = 63) | TMZ + IFNβ + RT (N = 58) | ||||
|---|---|---|---|---|---|---|
| Grade 1–2 (%) | Grade 3 (%) | Grade 4 (%) | Grade 1–2 (%) | Grade 3 (%) | Grade 4 (%) | |
| Hematological | ||||||
| Neutropenia | 25 (39.7) | 4 (6.3) | 4 (6.3) | 32 (55.2) | 10 (17.2) | 2 (3.5) |
| Lymphopenia | 24 (38.1) | 28 (44.4) | 6 (9.5) | 20 (34.5) | 30 (51.7) | 7 (12.1) |
| Non-hematological | ||||||
| Fever | 12 (19.0) | 0 | 0 | 18 (31.0) | 1 (1.7) | 0 |
| Nausea | 18 (28.6) | 0 | 0 | 18 (31.0) | 2 (3.4) | 0 |
| Vomiting | 7 (11.1) | 0 | 0 | 10 (17.2) | 1 (1.7) | 0 |
| Anorexia | 26 (41.3) | 0 | 0 | 26 (44.8) | 5 (8.6) | 0 |
| Febrile neutropenia | – | 1 (1.6) | 0 | – | 2 (3.4) | 0 |
| ALT elevation | 35 (55.6) | 6 (9.5) | 0 | 31 (53.4) | 5 (8.6) | 1 (1.7) |
| Hyponatremia | 13 (20.6) | 3 (4.8) | 0 | 15 (25.9) | 5 (8.6) | 0 |
| Skin rash | 13 (20.6) | 1 (1.6) | 0 | 4 (6.9) | 0 | 0 |
| (b) | TMZ + RT (N = 55) | TMZ + IFNβ + RT (N = 43)a | ||||
|---|---|---|---|---|---|---|
| Grade 1–2 (%) | Grade 3 (%) | Grade 4 (%) | Grade 1–2 (%) | Grade 3 (%) | Grade 4 (%) | |
| Hematological | ||||||
| Neutropenia | 31 (56.4) | 2 (3.6) | 0 | 21 (48.8) | 4 (9.3) | 0 |
| Lymphopenia | 29 (52.7) | 17 (30.9) | 2 (3.6) | 22 (51.2) | 17 (39.5) | 1 (2.3) |
| Non-hematological | ||||||
| Fever | 3 (5.5) | 0 | 0 | 5 (11.6) | 0 | 0 |
| Nausea | 14 (25.5) | 0 | 0 | 6 (14.0) | 0 | 0 |
| Vomiting | 4 (7.3) | 1 (1.8) | 0 | 2 (4.7) | 0 | 0 |
| Anorexia | 16 (29.1) | 2 (3.6) | 0 | 7 (16.3) | 0 | 0 |
| Febrile neutropenia | – | 0 | 0 | – | 0 | 0 |
| ALT elevation | 30 (54.5) | 1 (1.8) | 0 | 20 (46.5) | 0 | 0 |
| Hyponatremia | 11 (20.0) | 0 | 0 | 7 (16.3) | 2 (4.7) | 0 |
| Skin rash | 8 (14.5) | 0 | 0 | 8 (18.6) | 0 | 0 |
According to the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0
aData of one patient is missing
Discussion
IFNs exert pleiotropic antitumor effects by direct anticancer mechanisms though p53 induction and miR-21 downregulation or by regulating the immune system through the CD8 lymphocyte and macrophage activation [7]. This study was a randomized screening phase II trial to explore the superiority of TMZ + IFNβ therapy to TMZ alone for the patients with newly diagnosed GBM. In the present study, the superiority of TMZ + IFNβ + RT to TMZ + RT in OS was not demonstrated.
There are some possibilities that we failed to show the superiority of TMZ + IFNβ + RT to TMZ + RT. One potential reason is that TMZ + IFNβ + RT treatment was more toxic than expected. Before we started this trial, we assumed that additional IFNβ would not increase much toxicity because it had been suggested in some reports using nitrosourea anti-tumor agent with IFNβ. However, the proportion of severe (grade 3–4) hematological and non-hematological adverse events was higher in the TMZ + IFNβ + RT arm than in the TMZ + RT arm, which implied such unexpected severe toxicities could cause negative impact on the survival in the TMZ + IFNβ + RT arm. Due to the severe toxicities, treatment compliance was also deteriorated in the TMZ + IFNβ + RT arm. In fact, the number of patients who terminated protocol treatment before the start of the maintenance treatments was larger in the TMZ + IFNβ + RT arm, which was possibly caused by the toxicities as mentioned above. MGMT methylation status has not been investigated yet, but we are planning to evaluate the biomarkers including MGMT gene expression and methylation using tumor tissues and blood samples.
Subgroup analyses showed IFNβ could be possibly beneficial for younger, male, better PS, no residual tumor patients. It may suggest that the better tolerability against IFNβ toxicities might be predictive factors of IFNβ efficacy, but further studies would be needed to confirm this hypothesis.
As the future direction, we will seek for the promising combination therapy with TMZ + RT and other agents than IFNβ as a candidate of the following study for GBM. Now we just started a randomized phase III trial to confirm the superiority of dose-dense TMZ (ddTMZ) followed by bevacizumab at ddTMZ failure to bevacizumab alone for patients with first recurrence or progression of GBM (JCOG1308C).
In conclusion, although the combination therapy of TMZ + IFNβ + RT showed favorable survival, the superiority of TMZ/IFNβ + RT to TMZ + RT in overall survival was not demonstrated. Therefore TMZ + IFNβ + RT was not considered promising as the test treatment in the following phase III study for newly diagnosed GBM and TMZ + RT remained to be a most promising treatment.
Acknowledgements
We thank all the members of the JCOG Brain Tumor Study Group and the staff of the JCOG Data Center. This work was supported in part of the National Cancer Center Research and Development Fund (20S-4, 23-A-20, 26-A-4, 29-A-3), the Health and Labour Science Research Grant (H20-19), and the Grant-in Aid for Cancer Research (H23-013) from the ministry of Health, Labour and Welfare, Japan.
Abbreviations
- TMZ
Temozolomide
- IFNβ
Interferonβ
- RT
Radiotherapy
- OS
Overall survival
- MU
Million unit
- GBM
Glioblastoma
- MGMT
O6-methylguanine-DNA methyltransferase
- PTV
Planning target volume
- GTV
Gross tumor volume
- CTV
Clinical target volume
- PFS
Progression-free survival
- CTCAE
Common terminology criteria for adverse events
- ACNU
Nimustine hydrochloride
- HR
Hazard ratio
- JNS
Japan Neurosurgical Society
- IARC
International Agency for Research on Cancer
Compliance with ethical standards
Conflict of interest
T.W. has received research funding from Toray Co, Ltd, and MSD Co. Ltd. K.S. received honorarium fundings from MSD. M.N. received honorarium and research fundings from MSD, Chugai, and Eisai, and research funding from Daiichi-Sankyo. R.N. received honorarium and research fundings from MSD, Chugai, and Eisai. The other authors declare that they have no conflict of interest. All authors have registered online Self-reported COI Disclosure Statement Forms through the website for Japan Neurosurgical Society members.
References
- 1.Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017;19:v1–v88. doi: 10.1093/neuonc/nox158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups. National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M, Jeraj R, Brown PD, Jaeckle KA, Schiff D, Stieber VW, Brachman DG, Werner-Wasik M, Tremont-Lukats IW, Sulman EP, Aldape KD, Curran WJ, Jr, Mehta MP. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, Brandes AA, Hilton M, Abrey L, Cloughesy T. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 6.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 7.Galani V, Papadatos SS, Alexiou G, Galani A, Kyritsis AP. In vitro and in vivo preclinical effects of type I IFNs on gliomas. J Interferon Cytokine Res. 2017;37:139–146. doi: 10.1089/jir.2016.0094. [DOI] [PubMed] [Google Scholar]
- 8.GuhaSarkar D, Su Q, Gao G, Sena-Esteves M. Systemic AAV9-IFNbeta gene delivery treats highly invasive glioblastoma. Neuro Oncol. 2016;18:1508–1518. doi: 10.1093/neuonc/now097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chawla-Sarkar M, Lindner DJ, Liu YF, Williams BR, Sen GC, Silverman RH, Borden EC. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis. 2003;8:237–249. doi: 10.1023/A:1023668705040. [DOI] [PubMed] [Google Scholar]
- 10.Kawaji H, Tokuyama T, Yamasaki T, Amano S, Sakai N, Namba H. Interferon-beta and temozolomide combination therapy for temozolomide monotherapy-refractory malignant gliomas. Mol Clin Oncol. 2015;3:909–913. doi: 10.3892/mco.2015.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wakabayashi T, Hatano N, Kajita Y, Yoshida T, Mizuno M, Taniguchi K, Ohno T, Nagasaka T, Yoshida J. Initial and maintenance combination treatment with interferon-beta, MCNU (Ranimustine), and radiotherapy for patients with previously untreated malignant glioma. J Neurooncol. 2000;49:57–62. doi: 10.1023/A:1006405512579. [DOI] [PubMed] [Google Scholar]
- 12.Natsume A, Ishii D, Wakabayashi T, Tsuno T, Hatano H, Mizuno M, Yoshida J. IFN-beta down-regulates the expression of DNA repair gene MGMT and sensitizes resistant glioma cells to temozolomide. Cancer Res. 2005;65:7573–7579. doi: 10.1158/0008-5472.CAN-05-0036. [DOI] [PubMed] [Google Scholar]
- 13.Natsume A, Wakabayashi T, Ishii D, Maruta H, Fujii M, Shimato S, Ito M, Yoshida J. A combination of IFN-beta and temozolomide in human glioma xenograft models: implication of p53-mediated MGMT downregulation. Cancer Chemother Pharmacol. 2007;61:653–659. doi: 10.1007/s00280-007-0520-x. [DOI] [PubMed] [Google Scholar]
- 14.Wakabayashi T, Kayama T, Nishikawa R, Takahashi H, Hashimoto N, Takahashi J, Aoki T, Sugiyama K, Ogura M, Natsume A, Yoshida J. A multicenter phase I trial of combination therapy with interferon-beta and temozolomide for high-grade gliomas (INTEGRA study): the final report. J Neurooncol. 2011;104:573–577. doi: 10.1007/s11060-011-0529-1. [DOI] [PubMed] [Google Scholar]
- 15.Wakabayashi T, Kayama T, Nishikawa R, Takahashi H, Yoshimine T, Hashimoto N, Aoki T, Kurisu K, Natsume A, Ogura M, Yoshida J. A multicenter phase I trial of interferon-beta and temozolomide combination therapy for high-grade gliomas (INTEGRA study) Jpn J Clin Oncol. 2008;38:715–718. doi: 10.1093/jjco/hyn095. [DOI] [PubMed] [Google Scholar]
- 16.Motomura K, Natsume A, Kishida Y, Higashi H, Kondo Y, Nakasu Y, Abe T, Namba H, Wakai K, Wakabayashi T. Benefits of interferon-beta and temozolomide combination therapy for newly diagnosed primary glioblastoma with the unmethylated MGMT promoter: a multicenter study. Cancer. 2011;117:1721–1730. doi: 10.1002/cncr.25637. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida J, Kajita Y, Wakabayashi T, Sugita K. Long-term follow-up results of 175 patients with malignant glioma: importance of radical tumour resection and postoperative adjuvant therapy with interferon, ACNU and radiation. Acta Neurochir. 1994;127:55–59. doi: 10.1007/BF01808547. [DOI] [PubMed] [Google Scholar]
- 18.Hatano N, Wakabayashi T, Kajita Y, Mizuno M, Ohno T, Nakayashiki N, Takemura A, Yoshida J. Efficacy of post operative adjuvant therapy with human interferon beta, MCNU and radiation (IMR) for malignant glioma: comparison among three protocols. Acta Neurochir. 2000;142:633–638. doi: 10.1007/s007010070106. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe T, Katayama Y, Yoshino A, Fukaya C, Yamamoto T. Human interferon beta, nimustine hydrochloride, and radiation therapy in the treatment of newly diagnosed malignant astrocytomas. J Neurooncol. 2005;72:57–62. doi: 10.1007/s11060-004-2160-x. [DOI] [PubMed] [Google Scholar]
- 20.Colman H, Berkey BA, Maor MH, Groves MD, Schultz CJ, Vermeulen S, Nelson DF, Mehta MP, Yung WK, Radiation Therapy Oncology G. Phase II Radiation Therapy Oncology Group trial of conventional radiation therapy followed by treatment with recombinant interferon-beta for supratentorial glioblastoma: results of RTOG 9710. Int J Radiat Oncol Biol Phys. 2006;66:818–824. doi: 10.1016/j.ijrobp.2006.05.021. [DOI] [PubMed] [Google Scholar]


