Abstract
The NAC transcription factors involved plant development and response to various stress stimuli. However, little information is available concerning the NAC family in the woodland strawberry. Herein, 37 NAC genes were identified from the woodland strawberry genome and were classified into 13 groups based on phylogenetic analysis. And further analyses of gene structure and conserved motifs showed closer relationship of them in every subgroup. Quantitative real-time PCR evaluation different tissues revealed distinct spatial expression profiles of the FvNAC genes. The comprehensive expression of FvNAC genes revealed under abiotic stress (cold, heat, drought, salt), signal molecule treatments (H2O2, ABA, melatonin, rapamycin), biotic stress (Colletotrichum gloeosporioides and Ralstonia solanacearum). Expression profiles derived from quantitative real-time PCR suggested that 5 FvNAC genes responded dramatically to the various abiotic and biotic stresses, indicating their contribution to abiotic and biotic stresses resistance in woodland strawberry. Interestingly, FvNAC genes showed greater extent responded to the cold treatment than other abiotic stress, and H2O2 exhibited a greater response than ABA, melatonin, and rapamycin. For biotic stresses, 3 FvNAC genes were up-regulated during infection with C. gloeosporioides, while 6 FvNAC genes were down-regulated during infection with R. solanacearum. In conclusion, this study identified candidate FvNAC genes to be used for the genetic improvement of abiotic and biotic stress tolerance in woodland strawberry.
Introduction
Transcription factors containing the highly conserved NAC domain (NAM-ATAF1/2-CUC) in the N-terminal region compromise is one of the largest transcription factors families in plants [1–3]. The C-terminal region that contains the protein binding activity domain is greatly variable and plays an important role in transcriptional regulation [2, 4–7]. The NAC proteins play crucial roles in various aspects of plant growth and development (including the maintenance of the shoot apical meristem, cell division and expansion, nutrient remobilization, secondary cell wall biosynthesis, fiber development, lateral root development, leaf senescence, flower formation, seed development) [1, 3, 8–18], and adaption to the environment [19], such as abiotic stresses [20–29], and response to pathogen infections [6,22,29–33], including Magnaporthe oryzae [22,34], Phytophthora infestans [35], Sclerotinia sclerotiorum [36], and Colletotrichum graminicola [33], to name a few.
To date, genome-wide analyses have identified a large number of NAC family members in several species, including 117 NAC genes in the model plant Arabidopsis thaliana [7], 151 NAC genes in Oryza sativa [7], 152 NAC genes in Nicotiana tabacum [37], 163 NAC genes in Populus trichocarpa [38], 74 NAC genes in Vitis vinifera [39], 147 putative NAC genes in Setaria italic [40], 145 NAC genes in Gossypium raimondii [41], 167 NAC genes in Musa acuminate [42], 71 NAC genes in Cicer arietinum [43], 96 NAC genes in Manihot esculenta [44], 148 NAC genes in Zea mays [45], 79 NAC genes in Morus notabilis [46], 82 NAC genes in Cucumis melo [47], and 104 NAC genes in Solanum lycopersicum [48]. However, no information is available on the NAC family in the rosaceous fruit crop, woodland strawberry (Fragaria vesca L.).
Notably, accumulated evidence has confirmed that a large number of NAC genes induced by abiotic stresses play critical roles in the regulation of plant tolerance to abiotic stress. Three Arabidopsis NAC genes (ANAC019, ANAC055 and ANAC072) showed up-regulation after drought, high salinity, and abscisic acid (ABA) treatments, and positively regulate drought tolerance in the overexpression plants [20]. Similarly, overexpression of ATAF1 in Arabidopsis enhanced plant tolerance to drought, ABA, salt, and oxidative stress [19]. While, ATAF1 was negatively regulate the defense response to necrotrophic fungi (Alternaria brassicicola and Botrytis cinerea) and bacterial pathogens (Pseudomonas syringae pv. tomato) [29]. The same function of NAC genes, in increasing the tolerance of plants to abiotic stress, was also found in rice. SNAC1, an NAC gene from O. sativa, can improve drought and salt tolerance in rice, and transgenic plants were found to produce a 22–34% higher yield than the control in the field under severe drought stress conditions [21]. Accordingly, OsNAC10-overexpressing rice plants showed increased grain yield in comparison to the controls under both normal and drought conditions [23]. Overexpression of 3 rice drought-responsive NAC genes (OsNAC5, OsNAC6, and OsNAC10) increased plant tolerance to drought and salt stresses [23,49], and the overexpression of OsNAC6 led to increased resistance towards rice blast [22,34]. The genes ZmNAC41 and ZmNAC100 were found to be induced in Z. mays during infection with C. graminicola [33]. Similarly, BnNAC1-1, BnNAC5-1, and BnNAC5-7 were induced in Brassica campestris during infection with S. sclerotiorum. NAC family genes thus appear to be crucial regulators of plant tolerance to abiotic and biotic stress, as well as crop yield.
The cultivated strawberry (Fragaria × ananassa Duch.) is one of the most important fruit crops in the world. The woodland strawberry (F. vesca, 2n = 2x = 14) has a smaller genome (~ 240 Mb) that is highly congenic with the cultivated strawberry [50]. Robust in vitro regeneration and transformation systems have been established for studying the counterparts of many important genes in rosaceous fruit crops [50–53].
Based on the significance of NACs in the regulation of plant growth, development and adaption to the environment, the NAC family was selected for systematic analysis in woodland strawberry. In the present study, we identified 37 NAC genes from woodland strawberry and performed a detailed investigation of their phylogeny, conserved motifs, gene structure, expression profiles in various tissues and in response to cold, heat, drought, salt stress, signaling of H2O2, melatonin, ABA and rapamycin, and response to the pathogens Colletotrichum gloeosporioides and Ralstonia solanacearum. Our results should provide a basis for future research on the evolutionary mechanisms and biotic and abiotic stress responses mediated by NACs in the woodland strawberry.
Materials and methods
Plant materials and treatments
Woodland strawberry (F. vesca L., 2n = 2x = 14) seedlings were cultivated in a growth chamber at 24±2°C under a 14 h/10 h light/dark photoperiod with 80% relative humidity. The F. vesca seeds collected from Beimu Experimental Station, Yumin County, Xinjiang Province of China. Hundred-day-old woodland strawberry tissues (leaf, stem, root, flower, full reddening fruit) were collected from the greenhouse for the treatments. The cold and heat stress treatments was performed by transferring the plants to a low temperature (4°C) for 48 h following recovery, or to a high temperature (40°C) for 4 h following recovery. The leaves of the plants treated with cold and heat were then collected at 2 h, 6 h, 12 h, 2 d, 7 d, 14 d post-treatment (dpt). Drought or salt stresses were simulated by irrigating potted strawberry plants with 200 mM mannitol, 100 mM NaCl, respectively. The leaves of the plants treated with drought and salt were collected at 2 h, 6 h, 3 d, 14 d, 18 d, 24 d post-treatment. Signal molecule treatments were performed by spraying the woodland strawberry leaves with a solution containing 10 mM H2O2, 0.1 mM ABA, 0.5 mM melatonin, 0.01 mM rapamycin, and the leaves were collected at 2 h, 6 h, 12 h, 1 d, 2 d, 3 d post-treatment. The control woodland strawberry seedlings were similarly irrigating or spray with distilled water. 1×106 conidiospores/mL C. gloeosporioides inoculation leaves were collected at 12 h, 22 h, 40 h, 60 h, 96 h, 120 h post-inoculation (hpi), 1×108 CFU R. solanacearum irrigating woodland strawberry seedlings were collected at 2 h, 6 h, 12 h, 24 h,48 h, 72 h post-inoculation (hpi), and uninfected leaves served as a negative control. All materials harvested from each treatment were immediately frozen in liquid nitrogen and stored at -70°C before for RNA isolation.
Identification and phylogenetic analyses of the NAC gene family in the woodland strawberry
Whole protein sequences of woodland strawberry were obtained from the Plant Transcription Factor Database version 4.0 (PlantTFDB; http://planttfdb.cbi.pku.edu.cn/) [54], Phytozome version 12.0 (https://phytozome.jgi.doe.gov/pz/portal.html) and Pfam databases (http://pfam.xfam.org/) [55]. The Arabidopsis (Arabidopsis thaliana) NAC amino acid sequences were acquired from PlantTFDB version 4.0. The rice (Oryza sativa subsp. japonica) NAC amino acid sequences were acquired from PhantTFDB and Nuruzzaman et al. [7]. Additionally, all the Arabidopsis and rice NACs were entered as queries in BLAST and were used to identify the predicted NACs in the woodland strawberry database. The full-length amino acid sequences of the NAC proteins from Arabidopsis, rice and woodland strawberry were used to generate a phylogenetic tree based on ClustalX 2.0 alignment [56] and the unrooted neighbor-joining (NJ) method with 1,000 bootstrap replicates in MEGA 6.06 [57].
Protein properties and sequence analyses
The molecular weight and isoelectric points (pI) of the presumed FvNACs were predicted using the online ExPASy proteomics server database (http://web.expasy.org/compute_pi/)[58]. Information for each FvNAC was retrieved from the F. vesca version 1.1 database. The FvNAC genomic sequences and CDS sequences extracted from PlantTFDB were compared using the online GSDS 2.0 software (http://gsds.cbi.pku.edu.cn/) [59] to infer the exon/intron organization of the FvNAC genes. The conserved motifs of the FvNAC genes were obtained from the online Multiple Expectation Maximization for Motif Elicitation (MEME) (Suite version 4.11.2; http://meme-suite.org/tools/meme).
Quantitative RT-PCR evaluation of retrotransposon expression
Total RNA was isolated using an RNAprep Pure Plant Kit (TIANGEN, China, Cat. DP441) and the concentration and purity were evaluated by NanoDrop 2000c (Thermo Scientific, USA). Reverse transcription was implemented using 1 μg total RNA of each sample with a FastQuant RT Kit (With gDNase; TIANGEN, China, Cat. KR106-01). Expression of FvNAC genes by quantitative real-time PCR (qRT-PCR) analysis, performed in an Applied Biosystems QuantStudioTM 6 Flex Real-Time PCR System using the UltraSYBR mixture from Beijing CoWin Biotech (Cat. CW2601M). The reaction mixture was as follows: 1 μL cDNA template, 10μL 2 × PCR Mix, 0.5μL forward and reverse primers each (10μM), and 8μL ddH2O. PCR reactions were carried out with a denaturing step (95°C 10 min) and 40 cycles of denaturing at 95°C for 10 s, followed by annealing at 56°C for 30 s, and elongation at 72°C for 32 s. Primers with high specificity and efficient amplification on the basis of a dissociation curve analysis and agarose gel electrophoresis were used to conduct the quantification analysis. The relative expressions of the target genes were determined using the 2–ΔΔCt method [60]. Actin-7 gene (LOC101313051) was used as an internal reference gene for all the quantitative real-time PCR analyses in this study [61]. The primers used for qRT-PCR are listed in S1 Table. All experiments were conducted three times with three biological replicates for qRT-PCR analyses. Relative expression levels greater than 2-fold (2-fold higher than control) were considered up-regulated, whereas relative expression levels that were expressed less than 0.5 fold (2-fold lower than control) were considered down-regulated.
Results
Genome-wide identification of NACs in the woodland strawberry
A total of 37 FvNAC genes were identified in the woodland strawberry genome as putative members of the NAC family from PlantTFDB v4.0 and Phytozome v12, and were designated as FvNAC01-FvNAC37. The characteristic parameters of all predicted FvNAC proteins are listed in S2 Table, including chromosome location, protein length, molecular weight, theoretical pI and intron numbers. The 37 FvNAC proteins ranged from 64 (FvNAC23) to 1, 317 (FvNAC02) amino acid residues with an average of 426.3 aa, and the pIs ranged from 4.53 (FvNAC16) to 9.40 (FvNAC32) with 25 members showing pI <7, 67.57% and others, pI >7, 32.43% (S2 Table).
Phylogenetic analysis of woodland strawberry NACs
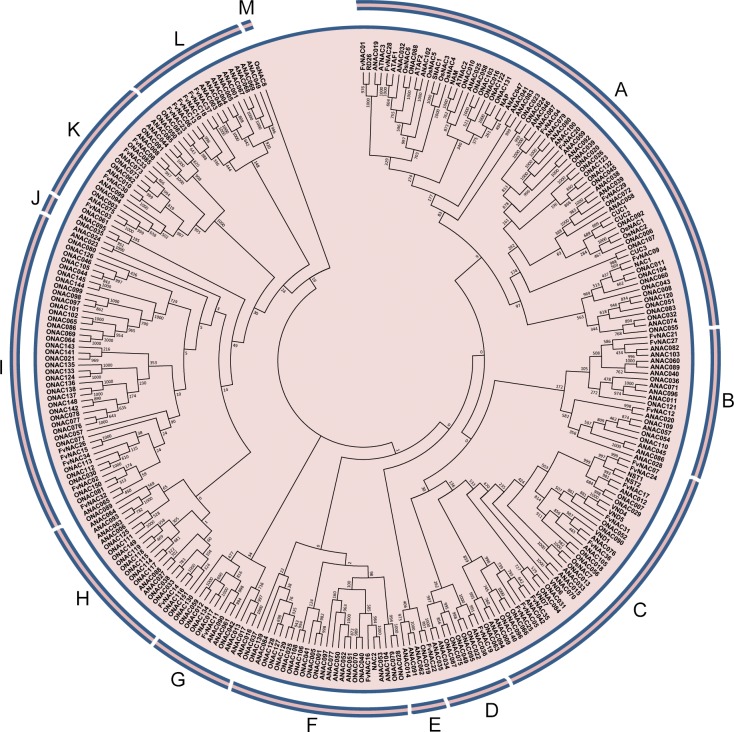
To study the evolutionary relationships between woodland strawberry NAC proteins and known NACs from Arabidopsis and rice, an unrooted neighbor-joining phylogenetic tree was constructed with the amino acid sequences of the NAC family proteins from the woodland strawberry, Arabidopsis, and rice. The results indicated that 37 FvNACs could be divided into 13 groups (A to M) together with their orthologs of Arabidopsis and rice (Fig 1, S3 Table). Phylogenetic analysis also showed that there were some closely related orthologous NACs between the woodland strawberry and Arabidopsis (FvNAC01 and RD26, FvNAC28 and ATAF1, FvNAC09 and NAC1, FvNAC35 and ANAC042, FvNAC19 and ANAC035, FvNAC32 and ANAC065, FvNAC03 and ANAC076, FvNAC30 and ANAC010, FvNAC33 and ANAC073, FvNAC08 and ANAC008) and between the woodland strawberry and rice (FvNAC22 and ONAC019, FvNAC14 and ONAC116, FvNAC23 and ONAC082), suggesting that FvNACs are closely associated with the proteins from Arabidopsis.
Fig 1. Phylogenetic analysis of NAC proteins from woodland strawberry, rice, and Arabidopsis.
The full-length amino acid sequences of NAC genes from woodland strawberry (FvNACs), Arabidopsis (ANACs), and rice (ONACs) were aligned using ClustalX 2.0, and the phylogenetic tree was constructed using the neighbor-joining method with 1000 bootstrap replicates with MEGA 6.06.
Gene structure and conserved motifs of woodland strawberry NACs
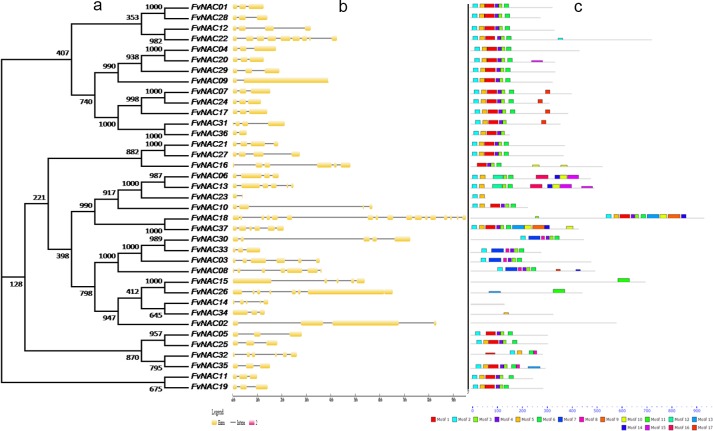
The diversification of gene structure during the evolution of multigene families facilitated the evolutionary co-option of genes for new functions in order to adapt to changes in the environment. To further examine the structural features of FvNAC genes, intron/exon distribution and conserved motifs were analyzed according to their phylogenetic relationships (Fig 2A). Gene structure analysis (Fig 2B) indicated that the number of introns of FvNACs varied from 1 (FvNAC23, FvNAC36) to 16 (FvNAC18), and it was found that 16 of the 37 FvNAC genes had 2 introns, while 7 of the 37 FvNAC genes had 3 introns. Most of the FvNAC members in the same group exhibited similar exon-intron structure. The conserved intron numbers in each subfamily supports their close evolutionary relationship and group classification.
Fig 2. Phylogenetic relationships, exon-intron structure, and motif compositions of FvNAC genes.
(a) The unrooted phylogenetic tree was constructed using full-length protein sequences of 37 FvNAC genes by the neighbor-joining method using 1000 bootstrap replicates. (b) Exon-intron structure analyses of FvNAC genes were performed by using the online tool GSDS 2.0. Lengths of exons and introns of each FvNAC genes were exhibited proportionally. (c) The motif composition related to each FvNAC protein. The motifs numbered 1–17 are displayed in different colored boxes. The sequence information for each motif is provided in S1 Fig.
To further examine the structural diversity of FvNACs, 17 conserved motifs were predicted using Multiple Em for Motif Elicitation (MEME) and subsequent annotation with InterPro (Fig 2C, S1 Fig). The FvNACs identified in this study possessed conserved features of the NAC family. Interestingly, it was found that most of the conserved motifs were located in the N-terminal of the NAC proteins that are highly conserved for DNA-binding, indicating that these motifs may be essential for the functioning of NAC proteins. However, the FvNAC18 motifs were located in the C-terminal. FvNAC02 and FvNAC14, lacking motif, and FvNAC34 and FvNAC15, with only a domain, were excluded from further analysis as they cannot be used to construct acceptable phylogenies. In general, the NAC proteins clustered into the same groups and shared similar motif compositions, indicating functional similarities among members of the same group.
FvNACs expression profiles in different organs
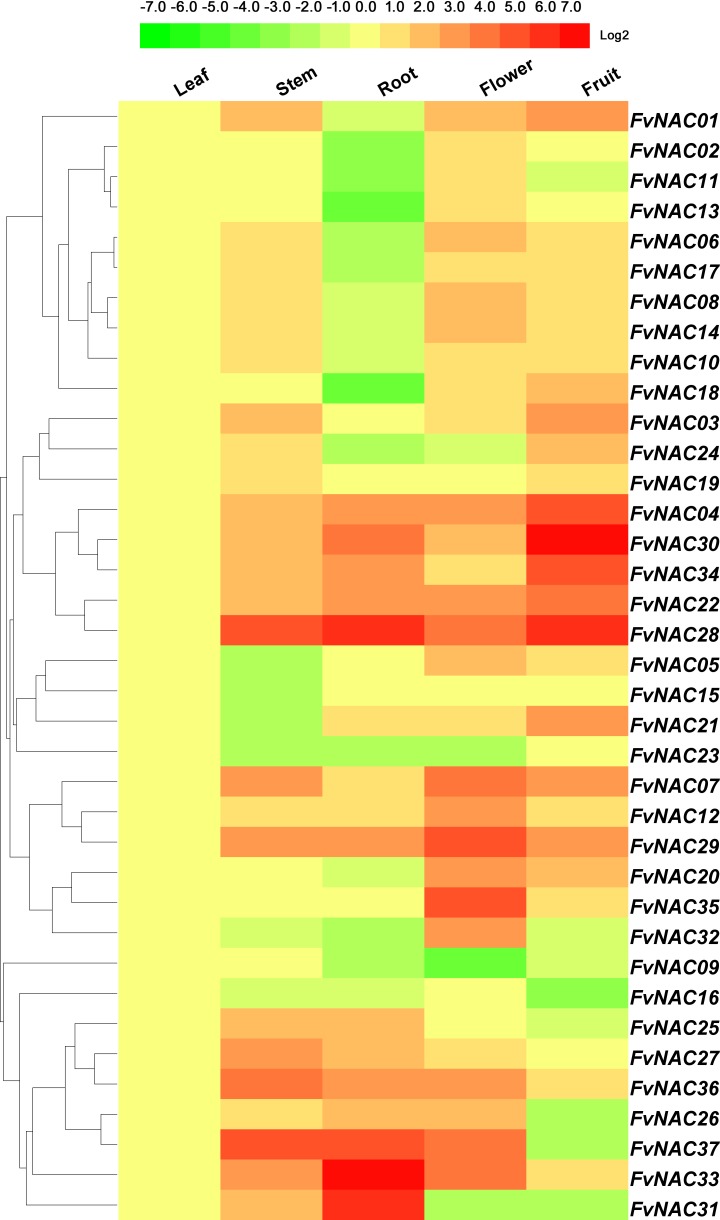
To determine the biological roles of NACs in the woodland strawberry, the distribution of the 37 FvNACs transcripts were surveyed in 5 major organs (leaf, stem, root, flower, full reddening fruit) under non-stress conditions. As shown in Fig 3, 10 FvNAC genes (FvNAC-04, -07, -12, -22, -28, -29, -30, -33, -34, and -35) showed lower transcript abundance in leaves than in the stems, roots, flowers, and full reddening fruit. All 37 FvNAC genes showed transcripts in different tissues, in which 24 (64.9%), 16 (43.2%), 29 (78.4%), and 23 (62.1%) genes exhibited high transcriptional abundance (value >2-fold) in the stems, roots, flowers, and full reddening fruit tissues, respectively. FvNAC13 and FvNAC18 did not show any transcripts in the roots (S4 Table).
Fig 3. Expression profiles of FvNAC genes in different organs of the woodland strawberry.
The expression profiles were generated by qRT-PCR and visualized as heat map. The heat map was constructed using HemI 1.0 software. The color scale represents log2 expression values, with green indicates low expression and red indicates high expression.
FvNACs expression profiles upon exposure to cold, heat, drought and salt
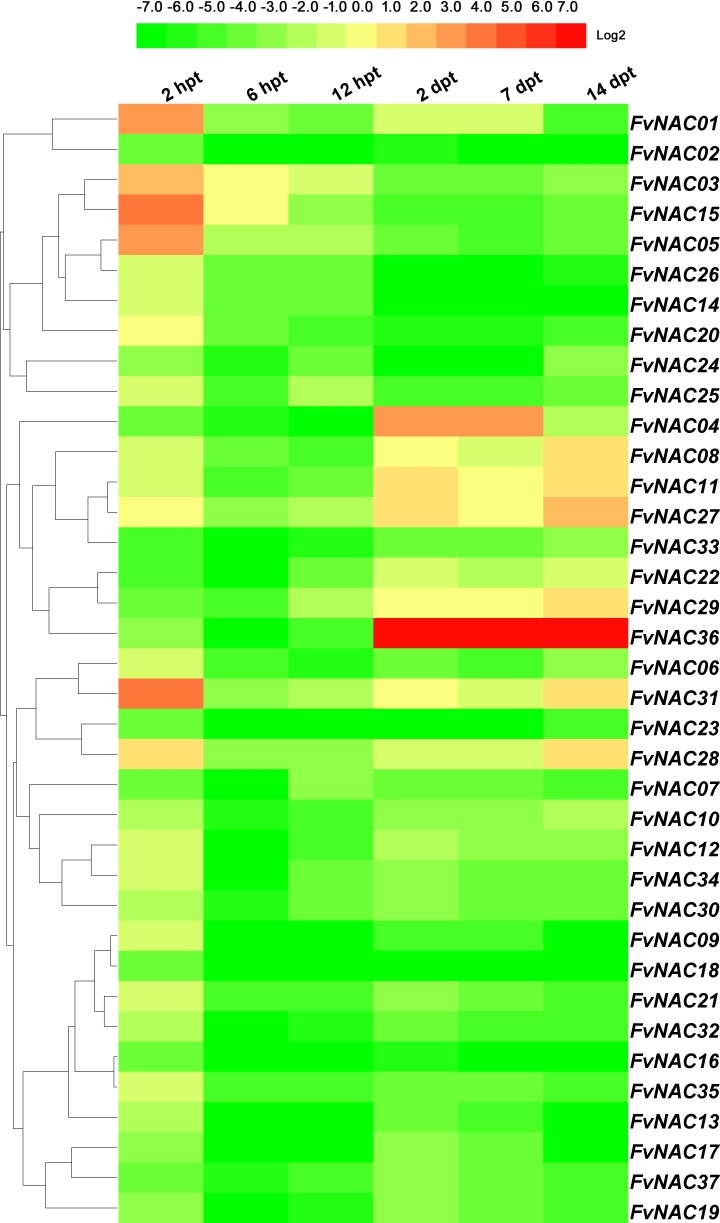
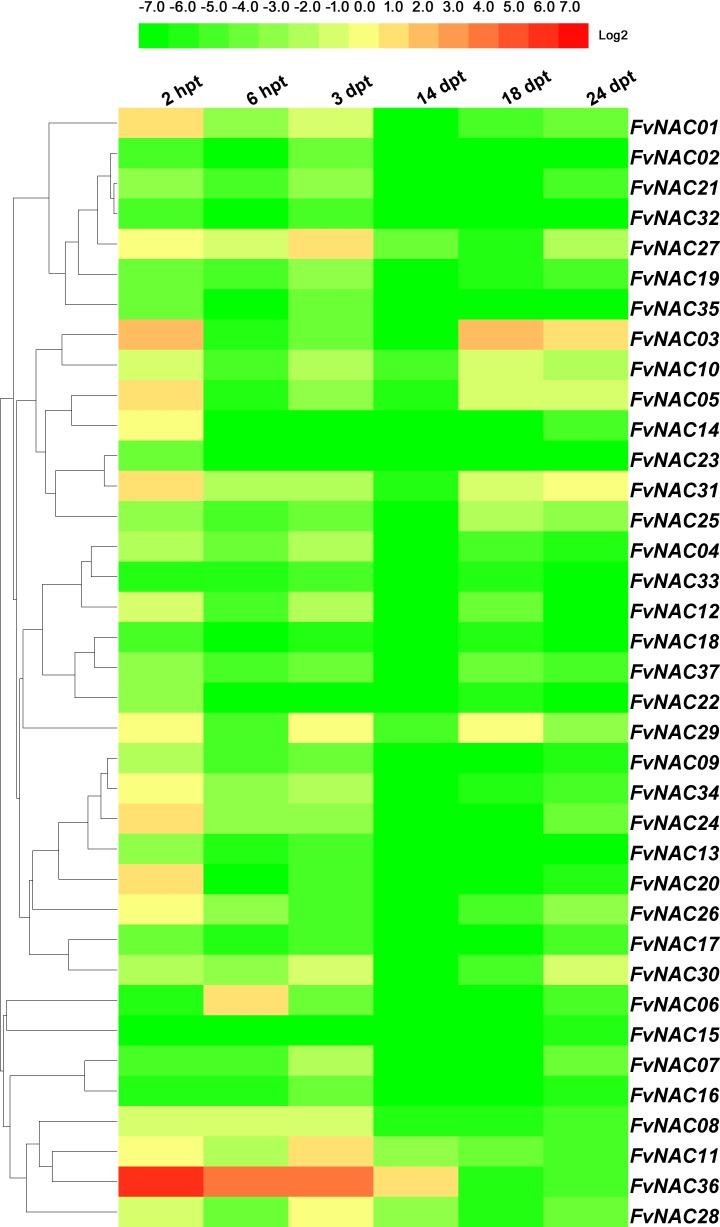
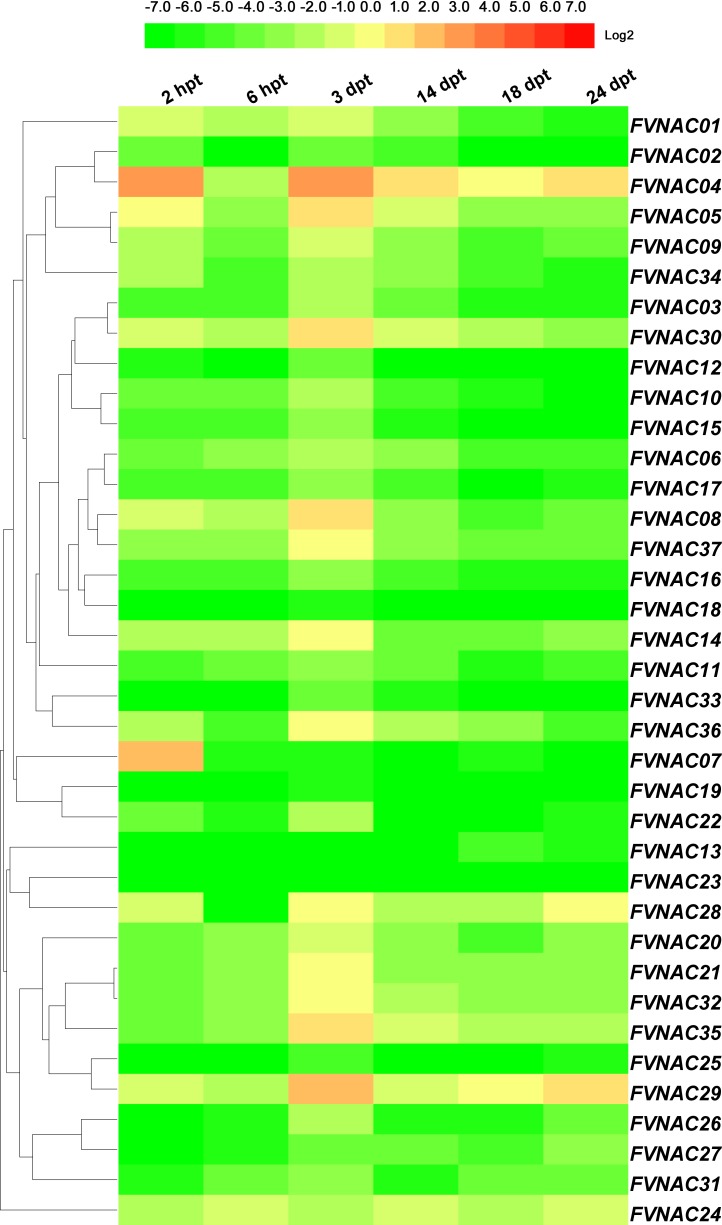
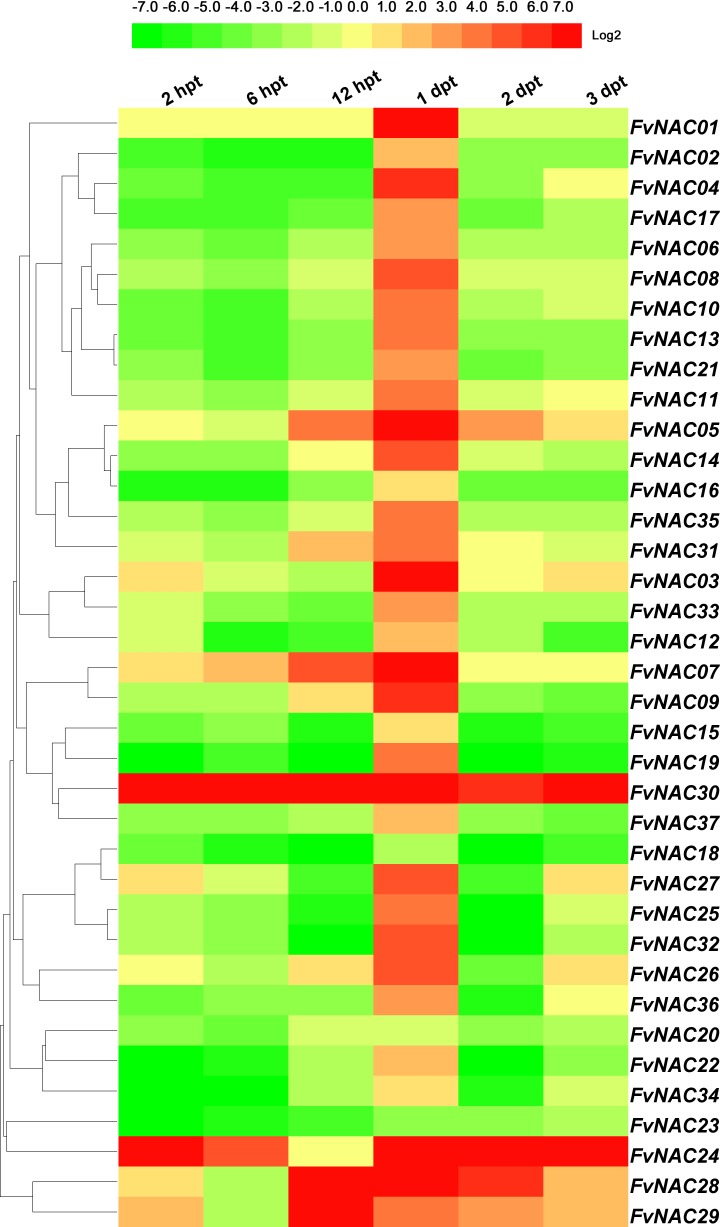
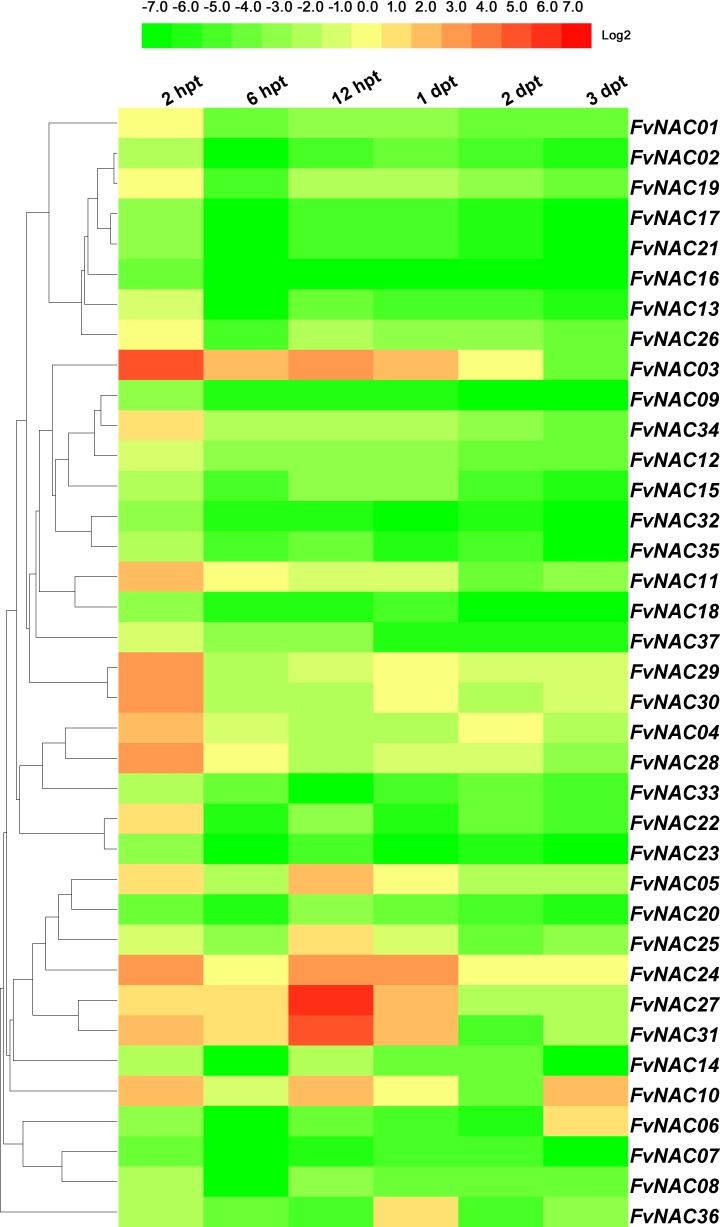
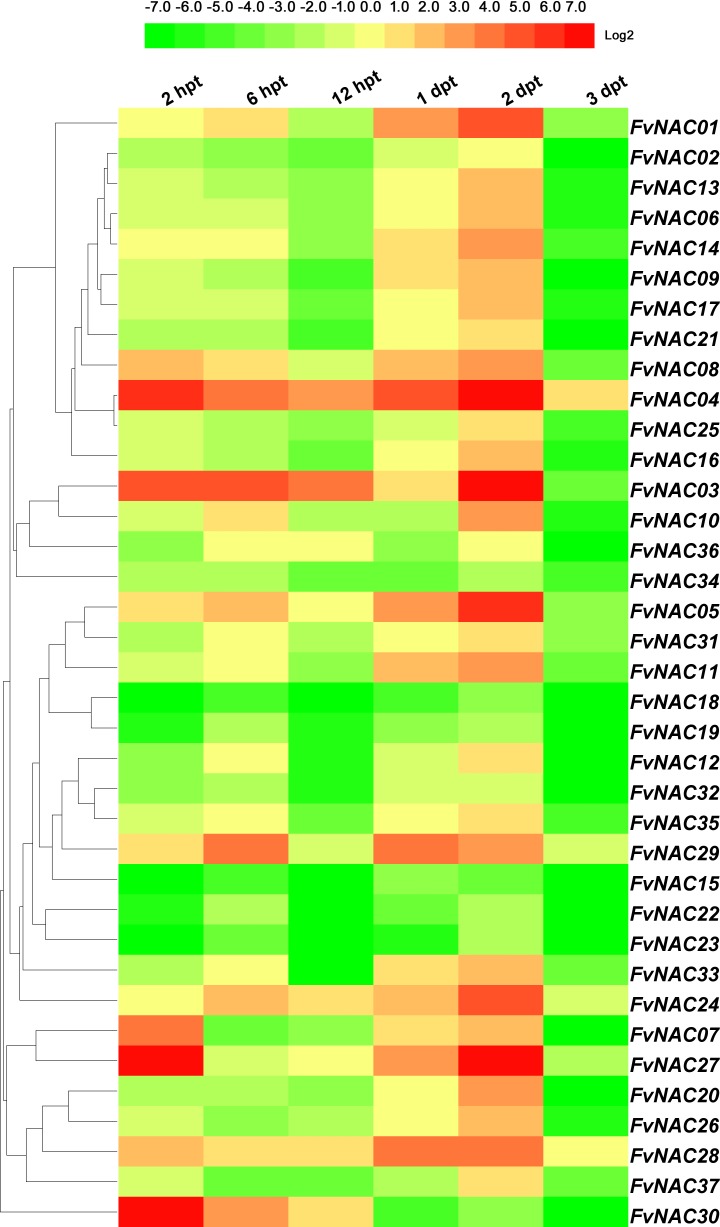
To examine the response of FvNAC genes to cold (4°C), heat (40°C), drought, and salt stresses at the transcriptional level, the transcripts of FvNAC genes under these treatments were determined using quantitative real-time PCR. As shown in Fig 4 (S5 Table), the steady expression of 6 FvNAC genes under cold stress at all treated time points was observed, with FvNAC03 and FvNAC31 being up-regulated, and FvNAC02, FvNAC06, FvNAC09, and FvNAC32 being down-regulated. As shown in Fig 5 (S6 Table), 23 FvNAC genes (FvNAC-02, -06, -07, -09, -10, -12, -13, -14, -16, -17, -18, -19, -21, -22, -23, -24, -25, -30, -32, -33, -34, -35, and -37) were down-regulated under heat stress, while no FvNAC genes were up-regulated. As shown in Fig 6 (S7 Table), 21 FvNAC genes (FvNAC-02, -04, -07, -08, -09, -10, -12, -13, -15, -16, -17, -18, -19, -21, -22, -23, -32, -33, -35, and -37) were down-regulated under conditions of drought stress. Additionally, as shown in Fig 7 (S8 Table), FvNAC04 and FvNAC29 were mostly up-regulated under salt stress, while 20 FvNAC genes (FvNAC-02, -03, -06, -10, -11, -12, -13, -15, -16, -17, -18, -19, -22, -23, -25, -26, -27, -31, -33, and -34) were mostly down-regulated.
Fig 4. Expression profiles of FvNAC genes in leaves under cold stress.
The cold stress treatment was performed by transferring the plants to a low temperature (4°C) for 48 h following recovery. Log2 based values from cold stress of qRT-PCR data were used to create the heat map.
Fig 5. Expression profiles of FvNAC genes in leaves under heat stress.
The heat stress treatment was performed by transferring the plants to a high temperature (40°C) for 4 h following recovery. Log2 based values from cold stress of qRT-PCR data were used to create the heat map.
Fig 6. Expression profiles of FvNAC genes in leaves under drought stress.
The drought stress treatment was simulated by irrigating potted woodland strawberry plants with 200 mM mannitol. Log2 based values from cold stress of qRT-PCR data were used to create the heat map.
Fig 7. Expression profiles of FvNAC genes in leaves under salt stress.
The salt stress treatment was simulated by irrigating potted woodland strawberry plants with 100 mM NaCl. Log2 based values from cold stress of qRT-PCR data were used to create the heat map.
FvNACs expression profiles upon exposure to H2O2, ABA, melatonin and rapamycin
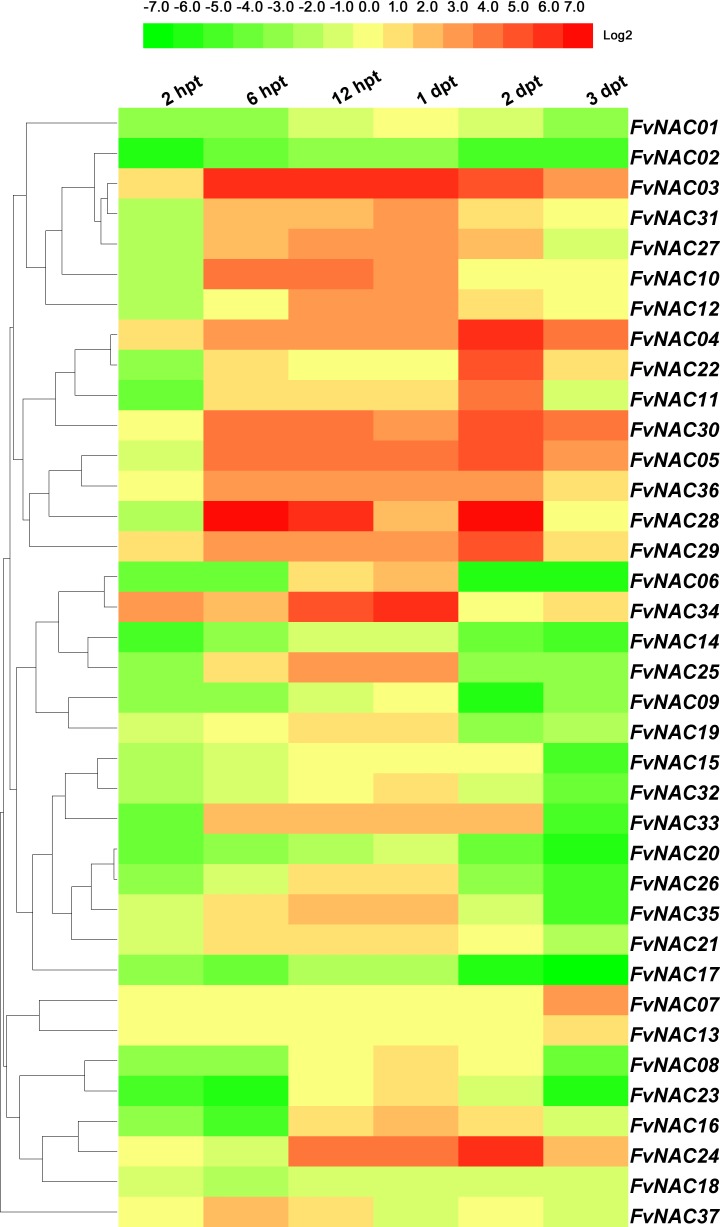
To examine the response of FvNAC genes to H2O2, ABA, melatonin, and rapamycin stress at the transcriptional level, the transcripts of the FvNAC genes under these treatments were determined by quantitative real-time PCR. As shown in Fig 8 (S9 Table), the steady expression of 7 FvNAC genes was observed under H2O2 stress, with FvNAC03, FvNAC04 and FvNAC29 being up-regulated, and FvNAC02, FvNAC14, FvNAC17 and FvNAC20 being down-regulated. As shown in Fig 9 (S10 Table), the steady expression of 4 FvNAC genes was documented under ABA stress, with FvNAC30 being up-regulated, and FvNAC18, FvNAC20 and FvNAC23 being down-regulated. As shown in Fig 10 (S11 Table), 18 FvNAC genes (FvNAC-02, -07, -08, -09, -12, -13, -14, -15, -16, -17, -18, -20, -21, -23, -32, -33, -35 and -37) were down-regulated under melatonin stress, while no FvNAC genes were up-regulated. Moreover, as shown in Fig 11 (S12 Table), the steady expression of 8 FvNAC genes was observed under rapamycin stress, with FvNAC04 being up-regulated, and FvNAC15, FvNAC18, FvNAC19, FvNAC22, FvNAC23, FvNAC32 and FvNAC34 being down-regulated. Notably, FvNAC18 tended to be down-regulated under all 4 stress treatments.
Fig 8. Expression profiles of FvNAC genes in leaves under H2O2 stress.
The H2O2 stress treatment was performed by spraying the woodland strawberry leaves with a solution containing 10 mM H2O2. Log2 based values from cold stress of qRT-PCR data were used to create the heat map.
Fig 9. Expression profiles of FvNAC genes in leaves under ABA stress.
The ABA stress treatment was performed by spraying the woodland strawberry leaves with a solution containing 0.1 mM ABA. Log2 based values from cold stress of qRT-PCR data were used to create the heat map.
Fig 10. Expression profiles of FvNAC genes in leaves under melatonin stress.
The melatonin stress treatment was performed by spraying the woodland strawberry leaves with a solution containing 0.5 mM melatonin. Log2 based values from cold stress of qRT-PCR data were used to create the heat map.
Fig 11. Expression profiles of FvNAC genes in leaves under rapamycin stress.
The rapamycin stress treatment was performed by spraying the woodland strawberry leaves with a solution containing 0.01 mM rapamycin. Log2 based values from cold stress of qRT-PCR data were used to create the heat map.
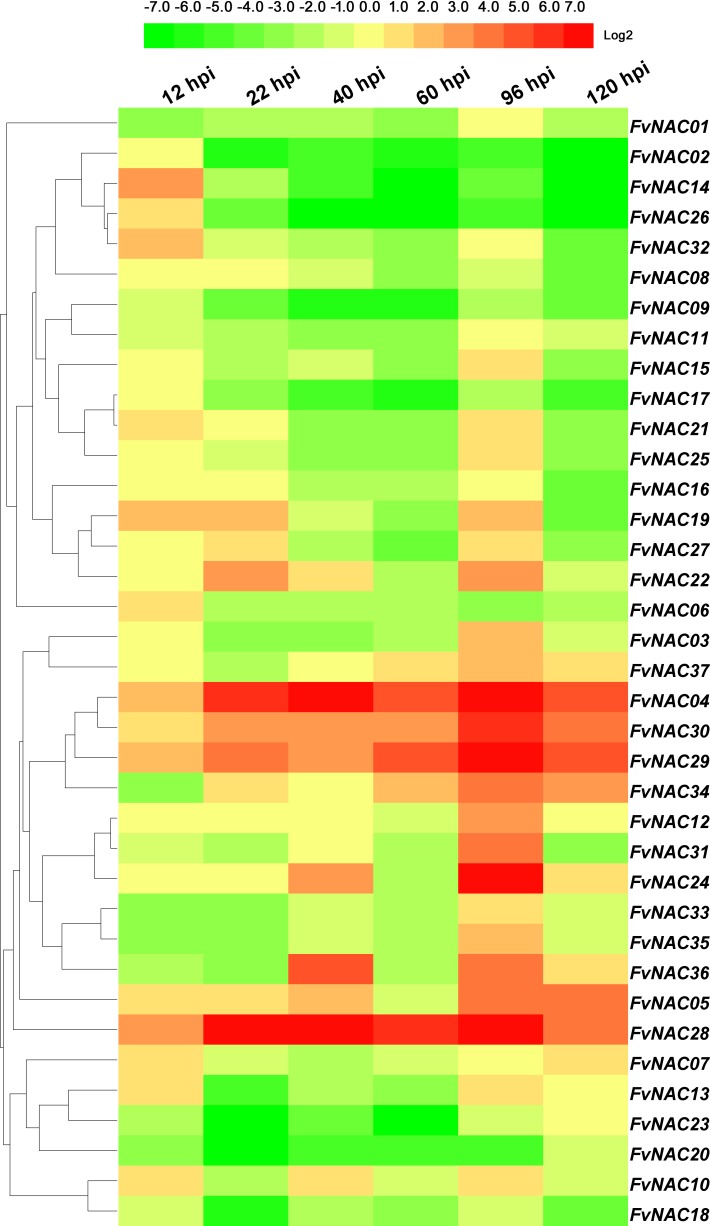
FvNACs expression profiles in response to C. gloeosporioides and R. solanacearum infection
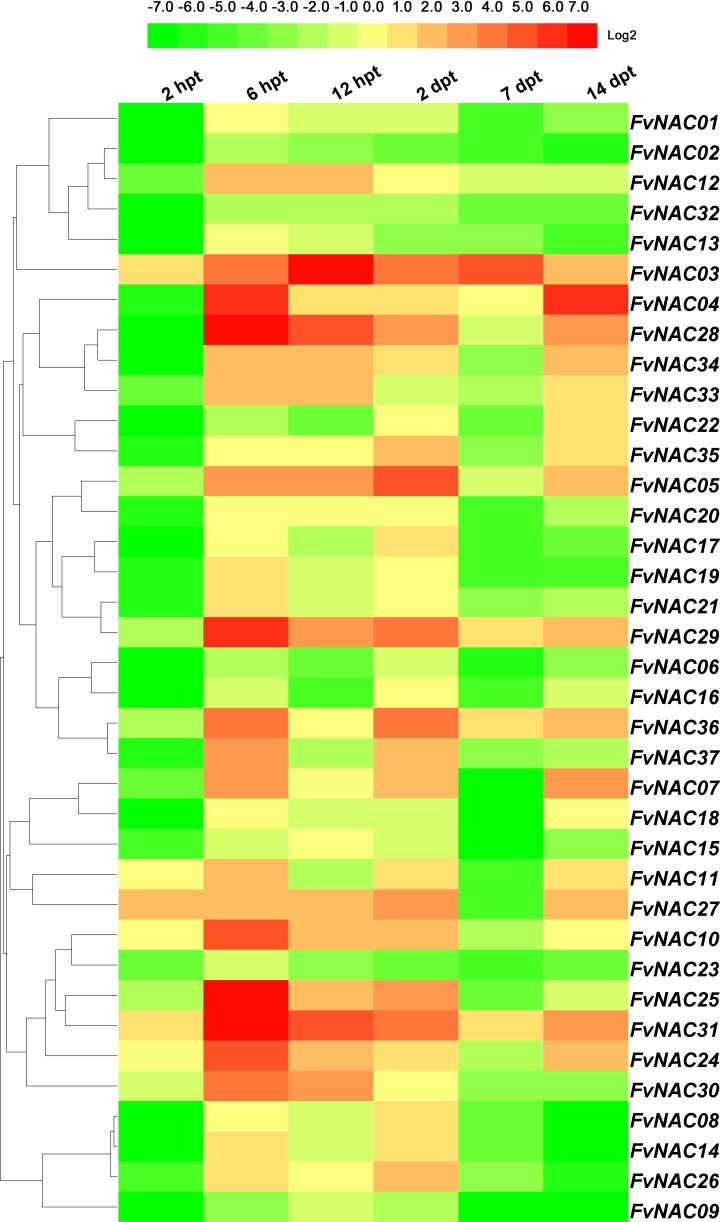
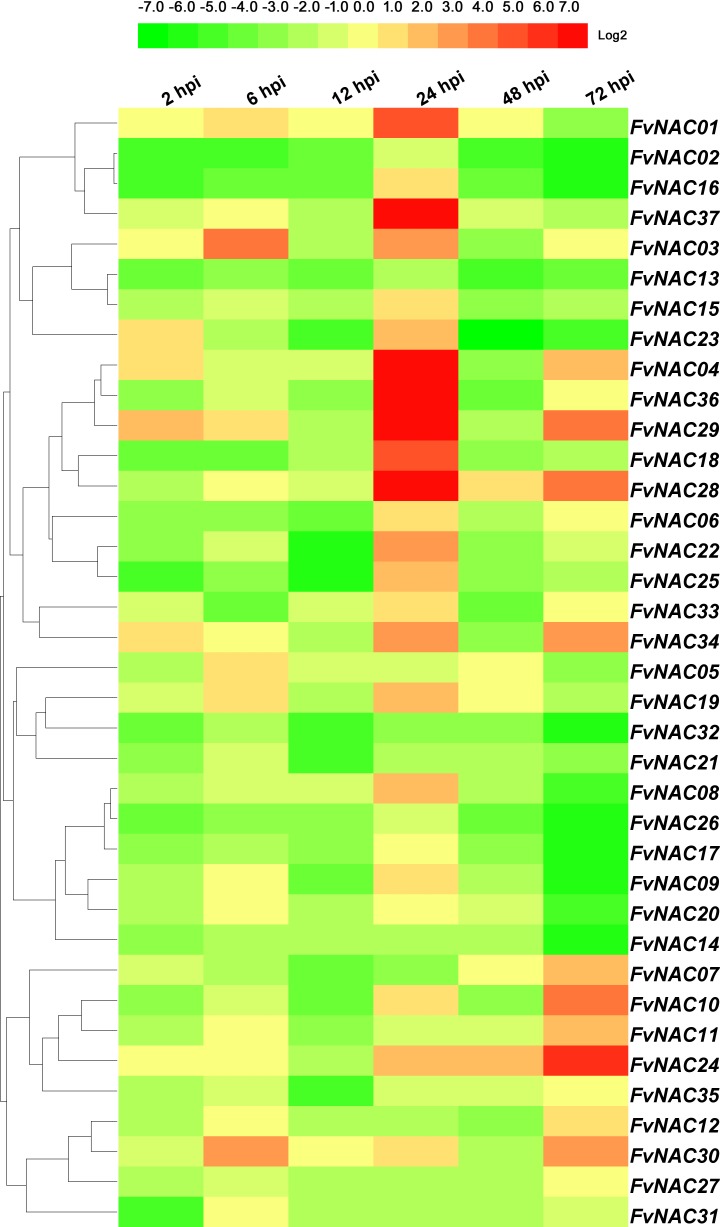
To investigate the possible role of FvNAC genes in plant-pathogen interactions, quantitative real-time PCR was used to analyze the response of woodland strawberry leaf infected with C. gloeosporioides and R. solanacearum in comparison to a control. As shown in Fig 12 (S13 Table), the steady expression of 6 FvNAC genes occurred under C. gloeosporioides infection, with FvNAC04, FvNAC28 and FvNAC29 being up-regulated, and FvNAC09, FvNAC18 and FvNAC20 being down-regulated. As shown in Fig 13 (S14 Table), FvNAC genes (FvNAC-02, -13, -14, -21, -26, and -32) were steadily expressed under R. solanacearum infection and were found to be down-regulated, while no FvNAC genes were steadily up-regulated. However, FvNAC01, -03, -04, -18, -28, -29, -34, 36 and -37 tended to be up-regulated (value>10-fold) at 1 d post-infection.
Fig 12. Expression profiles of FvNAC genes in leaves under C. gloeosporioides infection.
The C. gloeosporioides infection stress was performed by spraying the conidiospores (1×106 conidiospores/mL) to woodland strawberry leaves surface. Log2 based values from cold stress of qRT-PCR data were used to create the heat map.
Fig 13. Expression profiles of FvNAC genes in leaves under R. solanacearum infection.
The R. solanacearum infection stress was irrigating pathogenic bacteria suspension (1×108 CFU) woodland strawberry seedlings.
Discussion
Woodland strawberries face severe destruction from various abiotic and biotic stresses (particularly C. gloeosporioides and R. solanacearum) during the growth and developmental stages. In order to address this, farmers and researchers have been increasing the cultivation area and improving cultivated techniques. However, the effects of this appear to be very limited [62,63]. Considering the common involvement of NACs in the plant stress response, FvNACs were chosen as candidate genes for further investigation for their potential use in genetic breeding in woodland strawberries. Overall, 37 FvNACs were identified from across the genome and the phylogenetic evolution of these genes was also revealed. Using quantitative real-time PCR analysis, the comprehensive expression profiles of the 37 FvNACs were assessed. To our knowledge, this is the first study to extend our understanding of the FvNAC gene family. Generally, the expression profiles could be divided into 2 sections. The first involves the expression of 37 FvNACs at different developmental stages, or in different tissues, and comprises the basic information of this gene family. We discovered that the transcripts of some FvNACs have strong expression levels for specific tissue and developmental stage, indicating their possible roles in specific growth or developmental stages, such as in the flowers or fruit. The other section involves gene expression in response to various abiotic and biotic stresses, which intends to identify several candidate genes commonly regulated by various stresses for stress-related genetic breeding.
In this study, multiple abiotic and biotic stress-responsive FvNACs were identified, and were in accordance with previous studies of the NAC gene family in other plant species, including Arabidopsis [7], O. sativa [7], Nicotiana tabacum [37], P. trichocarpa [38], V. vinifera [39], S. italic [40], G. raimondii [41], M. acuminate [42], C. arietinum [43], M. esculenta [44], Z. mays [45], M. notabilis [46], C. melo [47], and S. lycopersicum [48].
ANAC042, which showed a high degree of similarity with FvNAC35, was previously reported to be involved in the regulation of camalexin biosynthesis, and oxidative and heat stress resistance [24,32]. FvNAC28 shared high similarity with ATAF1/ANAC002, which was previously shown to be involved in abiotic (drought, salt, and ABA) and biotic (necrotrophic pathogen B. cinerea) stress responses [8]. These results provide firm evidence of the protective roles of plant NACs in abiotic and biotic stress responses.
Cold stress is a major environmental factor affecting crop productivity and plant growth, development [64]. In Arabidopsis, ANAC053 and ANAC017 were found to be induced by cold stress [65]. In rice, 16 NAC genes (ONAC007, -010, -015, -027, -028, -039, -045, -059, -067, -068, -073, -074, -085, -103, -122 and -132) showed up-regulation under cold treatment [66]. In our study, under cold treatment following recovery, FvNAC03 and FvNAC31 were up-regulated at all treated time points, and constituted the most highly induced genes (over 15-fold at 4 time points, respectively; Fig 4, S5 Table).
In tea (Camellia sinensis) plants, 7 CsNAC genes (CsNAC02, CsNAC17, CsNAC26, CsNAC29, CsNAC30, and CsNAC32) showed up-regulation under heat (40°C) stress at 24 h [67]. Similarly, FvNAC15 and FvNAC31 were up-regulated at 2 h, and FvNAC36 between 2 d and 14 d, constituting the most highly induced genes (over 15-fold; Fig 5, S6 Table).
Under drought treatment, FvNAC36 showed significant induction between 2 h and 3 d (Fig 6, S7 Table). In Arabidopsis, rice, maize, chickpea, and apple, 6 ANAC genes [19,20,68,69], 4 OsNAC genes [22,23,49,21,70], 8 ZmNAC genes [71], 14 CaNAC genes [43], and 12 MdNAC genes [72], were up-regulated under drought treatment, respectively.
Furthermore, under salt treatment, FvNAC04 and FvNAC29 showed significant up-regulation at all treated time points (Fig 7, S8 Table). In Arabidopsis, 10 ANAC genes [8,15,19,73–78], 21 OsNAC genes [21,66,79], 4 CsNAC genes [67], and 8 MdNAC genes [72] were up-regulated under drought treatment. These studies indicate that NAC family genes may be positively involved in the salt stress response.
Under H2O2 treatment, FvNAC03, FvNAC04, and FvNAC29 showed significant up-regulation at all treated time points (Fig 8, S9 Table). In Arabidopsis, some evidence suggests that NAC genes, including ANAC013, ANAC042, and ANAC059/ORS1, play a positive role in the response to oxidative stress.
Under ABA treatment, FvNAC30 tended to be up-regulated at all treated time points. In addition to FvNAC18, FvNAC20, and FvNAC23, all FvNAC genes tended to be up-regulated at the 1 d time point (over 2-fold; Fig 9, S10 Table). In Arabidopsis, several ANAC genes have been shown to regulate ABA mediated processes [14,69,73,77,80–83]. Among them, FvNAC30 and ANAC010, FvNAC28 and ATAF1/ANAC002, FvNAC17 and ANAC012, and FvNAC28 and ATAF1/ANAC002 were found to be closely related and orthologous. Furthermore, the rice gene OsNAC19 has been shown to regulate ABA mediated processes [34]. However, FvNAC22 and OsNAC19 were found to be closely related orthologs. The response of woodland strawberry NAC genes to ABA treatment suggested a possible role for FvNAC genes in ABA signaling.
Melatonin is a widely investigated, endogenously produced molecule in all plant species [84]. Previous reports have demonstrated the protective effects of melatonin to alleviate biotic and abiotic stresses [84]. FvNAC03, FvNAC24, FvNAC29, FvNAC30 at 2 h, FvNAC03, FvNAC27, FvNAC31 at 12 h, and FvNAC24 at 1 d constituted the most highly induced genes (over 10-fold). On the contrary, 18 FvNAC genes under melatonin stress were found to be down-regulated (Fig 10, S11 Table). Thus, FvNAC family genes may be positively or negatively involved in the melatonin stress response.
Rapamycin, as an antifungal agent against pathogenic fungi, plays a crucial role in plant growth and metabolism [85–89]. Under rapamycin treatment, FvNAC04 was up-regulated at all treated time points, and FvNAC03 and FvNAC28 tended to be up-regulated at 1 h to 2 d. However, 7 FvNAC genes were found to be down-regulated at all treated time points (Fig 11, S12 Table).
Plant NACs are involved in plant-pathogen interactions [25,29,31,90–92]. The Arabidopsis ATAF1 is a regulator of the defense response against B. cinerea, Blumeria graminis f.sp. hordei, and P. syringae pv. Tomato [26]. Additionally, HvSNAC1 has been found to be important for the resistance of barley inoculated with Ramularia leaf spot [93]. Under C. gloeosporioides infection, FvNAC04, FvNAC28, and FvNAC29 tended to be up-regulated (Fig 12, S13 Table); meanwhile, under R. solanacearum infection, FvNAC01, -03, -04, -18, -28, -29, -34, -36, and -37 tended to be up-regulated (over 10-fold) at 24 h (Fig 13, S14 Table). Moreover, ATAF1 and FvNAC28 were found to be closely related orthologs. This suggests that FvNAC28 exhibits a similar function to ATAF1. Therefore, the NACs in the woodland strawberry are regulators of the defense response against C. gloeosporioides and R. solanacearum.
In conclusion, this study is the first to examine the FvNAC gene family along with their specific expression profiles, which may be used as potential candidates for further studies to dissect the function of FvNAC in response to stress stimuli.
The gene expression profiles obtained during 4°C, 40°C, drought, salt, H2O2, ABA, melatonin, rapamycin, C. gloeosporioides and R. solanacearum infection, and phytohormone treatments suggest that several woodland strawberry NAC genes could play important roles in environmental stress adaptation.
Supporting information
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
Conserved motifs and the sequence logos were generated using the MEME search tool. Numbers on the horizontal axis represent the sequence positions in the motifs and the vertical axis represent the information content measured in bits.
(TIF)
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No. 31460455).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by the National Natural Science Foundation of China (No. 31460455). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Souer E. The no apical meristem gene of petunia is required for pattern formation in embryos and flowers and is expressed at meristem and primordia boundaries. Cell.1996; 85:159 [DOI] [PubMed] [Google Scholar]
- 2.Aida M, Ishida T, Fukaki H, Fujisawa H, Tasaka M. Genes involved in organ separation in Arabidopsis: an analysis of the cup-shaped cotyledon mutant. Plant Cell. 1997; 9:841–857. doi: 10.1105/tpc.9.6.841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao D, Wei Q, Xu W, Syrenne RD, Yuan JS, Su Z. Comparative genomic analysis of NAC transcriptional factors to dissect the regulatory mechanisms for cell wall biosynthesis. BMC Bioinformatics. 2012; 13:S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kikuchi K, Uequchi-Tanaka M, Yoshida KT, Nagato Y, Matausoka M, Hirano HY. Molecular analysis of the NAC gene family in rice. Mol Genet Genomics. 2000; 262:1047–1051. [DOI] [PubMed] [Google Scholar]
- 5.Ooka H, Satoh K, Doi K, Nagata T, Otomo Y, Murakami K, et al. Comprehensive analysis of NAC family genes in Oryza sativa and Arabidopsis thaliana. DNA Res. 2013; 10:239–247. [DOI] [PubMed] [Google Scholar]
- 6.Olsen AN, Ernst HA, Leggio LL, Skriver K. NAC transcription factors: structurally distinct, functionally diverse. Trends Plant Sci. 2005; 10, 79–87. doi: 10.1016/j.tplants.2004.12.010 [DOI] [PubMed] [Google Scholar]
- 7.Nuruzzaman M, Manimekalai R, Sharoni AM, Satoh K, Kondoh H, Ooka H, et al. Genome-wide analysis of NAC transcription factor family in rice. Gene. 2010; 465:30–44. doi: 10.1016/j.gene.2010.06.008 [DOI] [PubMed] [Google Scholar]
- 8.Kim SG, Kim SY, Park CM. A membrane-associated NAC transcription factor regulates salt-responsive flowering via FLOWERING LOCUST in Arabidopsis. Planta. 2007; 226:647–654. doi: 10.1007/s00425-007-0513-3 [DOI] [PubMed] [Google Scholar]
- 9.Kim YS, Kim SG, Park JE, Park HY, Lim MH, Chua NH, et al. A membrane-bound NAC transcription factor regulates cell division in Arabidopsis. Plant Cell. 2006; 18:3132–3144. doi: 10.1105/tpc.106.043018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uaury C, Distelfeld A, Fahima T, Blechl A, Dubcovsky J. A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science. 2006; 314:1298–1301. doi: 10.1126/science.1133649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong R, Richardson EA, Ye ZH. Two NAC domain transcription factors, SND1 and NST1, function redundantly in regulation of secondary wall synthesis in fibers of Arabidopsis. Planta. 2007; 225:1603–1611. doi: 10.1007/s00425-007-0498-y [DOI] [PubMed] [Google Scholar]
- 12.Ko JH, Yang SH, Park AH, Lerouxel O, Han KH. ANAC012, a member of the plant-specific NAC transcription factor family, negatively regulates xylary fiber development in Arabidopsis thaliana. Plant J. 2007; 50:1035–1048. doi: 10.1111/j.1365-313X.2007.03109.x [DOI] [PubMed] [Google Scholar]
- 13.Xie Q, Frugis G, Colgan D, Chua NH. Arabidopsis NAC1 transduces auxin signal downstream of TIR1 to promote lateral root development. Gene Dev. 2000; 14:3024–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He XJ, Mu RL, Cao WH, Zhang ZG, Zhang JS, Chen SY. AtNAC2, a transcription factor downstream of ethylene and auxin signaling pathways, is involved in salt stress response and lateral root development. Plant J. 2005; 44:903–916. doi: 10.1111/j.1365-313X.2005.02575.x [DOI] [PubMed] [Google Scholar]
- 15.Guo Y, Gan S. AtNAP, a NAC family transcription factor, has an important role in leaf senescence. Plant J. 2006; 46, 601–612. doi: 10.1111/j.1365-313X.2006.02723.x [DOI] [PubMed] [Google Scholar]
- 16.Kim HJ, Hong SH, Kim YW, Lee IH, Jun JH, Phee BK, et al. Gene regulatory cascade of senescence-associated NAC transcription factors activated by ETHYLENE-INSENSITIVE2-mediated leaf senescence signalling in Arabidopsis. J Exp Bot. 2014; 65:4023–4036. doi: 10.1093/jxb/eru112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sablowski RW, Meyerowitz EM. A homolog of no apical meristem is an immediate target of the floral homeotic genes APETALA3/PISTILLATA. Cell. 1998; 92:93–103. [DOI] [PubMed] [Google Scholar]
- 18.Sperotto RA, Ricachenevsky FK, Duarte GL, Boff T, Lopes KL, Sperb ER, et al. Identification of up-regulated genes in flag leaves during rice grain filling and characterization of OsNAC5, a new ABA-dependent transcription factor. Planta. 2009; 230:985–1002. doi: 10.1007/s00425-009-1000-9 [DOI] [PubMed] [Google Scholar]
- 19.Wu Y, Deng Z, Lai J, Zhang Y, Yang C, Yin B, et al. Dual function of Arabidopsis ATAF1 in abiotic and biotic stress responses. Cell Res. 2009; 19:1279–1290. doi: 10.1038/cr.2009.108 [DOI] [PubMed] [Google Scholar]
- 20.Tran LS, Nakashima K, Sakuma Y, Simpson SD, Fujita Y, Maruyama K, et al. Isolation and functional analysis of Arabidopsis stress inducible NAC transcription factors that bind to a drought responsive cis-element in the early responsive to dehydration stress 1 promoter. Plant Cell. 2004; 16:2481–2498. doi: 10.1105/tpc.104.022699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu H, Dai M, Yao J, Xiao B, Li X, Zhang Q, et al. Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. P Natl Acad Sci USA. 2006; 103:12987–12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakashima K, Tran LS, Van Nguyen D, Fujita M, Maruyama K, Todaka D, et al. Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice. Plant J. 2007; 51:617–630. doi: 10.1111/j.1365-313X.2007.03168.x [DOI] [PubMed] [Google Scholar]
- 23.Jeong JS, Kim YS, Baek KH, Jung H, Ha SH, Do Choi Y, et al. Root-specific expression of OsNAC10 improves drought tolerance and grain yield in rice under field drought conditions. Plant Physiol. 2010; 153:185–197. doi: 10.1104/pp.110.154773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shahnejat-Bushehri S, Mueller-Roeber B, Balazadeh S. Arabidopsis NAC transcription factor JUNGBRUNNEN1 affects thermo memory-associated genes and enhances heat stress tolerance in primed and unprimed conditions. Plant Signal Behav. 2012; 7:1518–1521. doi: 10.4161/psb.22092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delessert C, Kazan K, Wilson IW, Van Der Straeten D, Manners J, Dennis ES, et al. The transcription factor ATAF2 represses the expression of pathogenesis-related genes in Arabidopsis. Plant J, 2005; 43:745–757. doi: 10.1111/j.1365-313X.2005.02488.x [DOI] [PubMed] [Google Scholar]
- 26.Lu PL, Chen NZ, An R, Su Z, Qi BS, Ren F, et al. A novel drought-inducible gene, ATAF1, encodes a NAC family protein that negatively regulates the expression of stress-responsive genes in Arabidopsis. Plant Mol Biol. 2007; 63:289–305. doi: 10.1007/s11103-006-9089-8 [DOI] [PubMed] [Google Scholar]
- 27.Jensen MK. The HNAC6 transcription factor: A positive regulator of penetration resistance in barley and Arabidopsis. Plant Mol Biol. 2007; 65:137–150. doi: 10.1007/s11103-007-9204-5 [DOI] [PubMed] [Google Scholar]
- 28.Jensen MK, Hagedorn PH, de Torres-Zabala M, Grant MR, Rung JH, Collinge DB, et al. Transcriptional regulation by a NAC (NAM-ATAF1, 2-CUC2) transcription factor attenuates ABA signalling for efficient basal defence towards Blumeria graminis f. sp. hordei in Arabidopsis. Plant J. 2008; 56:867–880. doi: 10.1111/j.1365-313X.2008.03646.x [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Basnayake BM, Zhang H, Li G, Li W, Virk N, et al. The Arabidopsis ATAF1, a NAC transcription factor, is a negative regulator of defense responses against necrotrophic fungal and bacterial pathogens. Mol Plant Microbe In. 2009; 22:1227–1238. [DOI] [PubMed] [Google Scholar]
- 30.Xie Q, Sanz-Burgos AP, Guo H, Garcia JA, Gutierrez C. GRAB proteins, novel members of the NAC domain family, isolated by their interaction with a geminivirus protein. Plant Mol Biol. 1999; 39:647–656. [DOI] [PubMed] [Google Scholar]
- 31.Ren T, Qu F, Morris TJ. HRT gene function requires interaction between a NAC protein and viral capsid protein to confer resistance to Turnip Crinkle Virus. Plant Cell. 2000; 12:1917–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saga H, Ogawa T, Kai K, Suzuki H, Ogata Y, Sakurai N, et al. Identification and characterization of ANAC042, transcription factor family gene involved in the regulation of camalexin biosynthesis in Arabidopsis. Mol Plant Microbe In. 2012; 25:684–696. [DOI] [PubMed] [Google Scholar]
- 33.Voitsik AM, Muench S, Deising HB, Voll LM. Two recently duplicated maize NAC transcription factor paralogs are induced in response to Colletotrichum graminicola infection. BMC Plant Biol.2013; 13:1–16. doi: 10.1186/1471-2229-13-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin R, Zhao W, Meng X, Wang M, Peng Y. Rice gene OsNAC19 encodes a novel NAC-domain transcription factor and responds to infection by Magnaporthe grisea. Plant Sci. 2007; 172:120–130. [Google Scholar]
- 35.Collinge M, Boller T. Differential induction of two potato genes, Stprx2 and StNAC, in response to infection by Phytophthora infestans and to wounding. Plant Mol Biol. 2001; 46:521–529. [DOI] [PubMed] [Google Scholar]
- 36.Hegedus D, Yu M, Baldwin D, Gruber M, Sharpe A, Parkin I, et al. Molecular characterization of Brassica napus NAC domain transcriptional activators induced in response to biotic and abiotic stress. Plant Mol Biol. 2003; 53:383–397. [DOI] [PubMed] [Google Scholar]
- 37.Rushton PJ, Bokowiec MT, Han S, Zhang H, Brannock JF, Chen X, et al. Tobacco transcription factors: novel insights into transcriptional regulation in the Solanaceae. Plant Physiol. 2008; 147:280–295. doi: 10.1104/pp.107.114041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu R, Qi G, Kong Y, Kong D, Gao Q, Zhou G. Comprehensive analysis of NAC domain transcription factor gene family in Populus trichocarpa. BMC Plant Biol. 2010; 10:145 doi: 10.1186/1471-2229-10-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang N, Zheng Y, Xin H, Fang L, Li S. Comprehensive analysis of NAC domain transcription factor gene family in Vitis vinifera. Plant Cell Rep. 2013; 32:61–75. doi: 10.1007/s00299-012-1340-y [DOI] [PubMed] [Google Scholar]
- 40.Puranik S, Sahu PP, Mandal SN, B VS, Parida SK, Prasad M. Comprehensive genome-wide survey, genomic constitution and expression profiling of the NAC transcription factor family in oxtail millet (Setaria italica L.). PLoS One. 2013; 8:e64594 doi: 10.1371/journal.pone.0064594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shang H, Li W, Zou C, Yuan Y. Analyses of the NAC transcription factor gene family in Gossypium raimondii Ulbr.: chromosomal location, structure, phylogeny, and expression patterns. J Integr Plant Biol. 2013; 55:663–676. doi: 10.1111/jipb.12085 [DOI] [PubMed] [Google Scholar]
- 42.Cenci A, Guignon V, Roux N, Rouard M. Genomic analysis of NAC transcription factors in banana (Musa acuminata) and definition of NAC orthologous groups for monocots and dicots. Plant Mol Biol. 2014; 85:63–80. doi: 10.1007/s11103-013-0169-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ha CV, Esfahani MN, Watanabe Y, Tran UT, Sulieman S, Mochida K, et al. Genome-wide identification and expression analysis of the CaNAC family members in chickpea during development, dehydration and ABA treatments. PLoS One. 2014; 9:e114107 doi: 10.1371/journal.pone.0114107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu W, Wei Y, Xia Z, Yan Y, Hou X, Zou M, et al. Genome-wide identification and expression analysis of the NAC transcription factor family in cassava. PLoS One. 2015; 10:e0136993 doi: 10.1371/journal.pone.0136993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng X, Zhao Y, Li X, Wu M, Chai W, Sheng L, et al. Genome-wide identification, classification and analysis of NAC type gene family in maize. J Genet. 2015; 94:377–390. [DOI] [PubMed] [Google Scholar]
- 46.Baranwal VK, Khurana P. Genome-wide analysis, expression dynamics and varietal comparison of NAC gene family at various developmental stages in Morus notabilis. Mol Genet Genomics. 2016; 291:1305–1317. doi: 10.1007/s00438-016-1186-z [DOI] [PubMed] [Google Scholar]
- 47.Wei S, Gao L, Zhang Y, Zhang F, Yang X, Huang D. Genome-wide investigation of the NAC transcription factor family in melon (Cucumis melo L.) and their expression analysis under salt stress. Plant Cell Rep. 2016; 35:1–13. doi: 10.1007/s00299-015-1872-z [DOI] [PubMed] [Google Scholar]
- 48.Su H, Zhang S, Yuan X, Chen C, Wang XF, Hao YJ. Genome-wide analysis of NAM-ATAF1, 2-CUC2 transcription factor family in Solanum lycopersicum. J Plant Biochem Biot. 2015; 24:176–183. [DOI] [PubMed] [Google Scholar]
- 49.Song SY, Chen Y, Chen J, Dai XY, Zhang WH. Physiological mechanisms underlying OsNAC5-dependent tolerance of rice plants to abiotic stress. Planta. 2011; 234:331–345. doi: 10.1007/s00425-011-1403-2 [DOI] [PubMed] [Google Scholar]
- 50.Shulaev V, Sargent DJ, Crowhurst RN, Mockler TC, Folkerts O, Delcher AL, et al. The genome of woodland strawberry (Fragaria vesca). Nat Genet. 2011; 43:109–116. doi: 10.1038/ng.740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruiz-Rojas JJ, Sargent DJ, Shulaev V, Dickerman AW, Pattison J, Holt SH. et al. SNP discovery and genetic mapping of T-DNA insertional mutants in Fragaria vesca L. Theor Appl Genet. 2010; 121:449–463. doi: 10.1007/s00122-010-1322-9 [DOI] [PubMed] [Google Scholar]
- 52.Hu Y, Han YT, Wei W, Li YJ, Zhang K, Gao YR, et al. Identification, isolation, and expression analysis of heat shock transcription factors in the diploid woodland strawberry Fragaria vesca. IEEE T Geosci Remote. 2009; 47:3313–3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu H, Xie WF, Zhang L, Valpuesta V, Ye ZW, Gao QH, et al. Auxin biosynthesis by the YUCCA6 flavin monooxygenase gene in woodland strawberry. J Integr Plant Biol. 2014; 56:350–363. doi: 10.1111/jipb.12150 [DOI] [PubMed] [Google Scholar]
- 54.Jin JP, Tian F, Yang DC, Meng YQ, Kong L, Luo JC, et al. PlantTFDB 4.0: toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2017; 45:1040–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Finn RD, Coggill P, Eberhardt RY, Eddy SR, Mistry J, Mitchell AL, et al. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res. 2016; 44:D279 doi: 10.1093/nar/gkv1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007; 23:2947–2948. doi: 10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
- 57.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011; 28:2731–2739. doi: 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilkins MR, Gasteiger E, Bairoch A, Sanchez JC, Williams KL, Appel RD, et al. Protein identification and analysis tools in the ExPASy server The Proteomics Protocols Handbook. Humana Press, 2007; 571–607. [DOI] [PubMed] [Google Scholar]
- 59.Hu B, Jin J, Guo AY, Zhang H, Luo J, Gao G. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics. 2014; 31:1296 doi: 10.1093/bioinformatics/btu817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 61.Zhang Q, Jia M, Xing Y, Qin L, Li B, Jia W. Genome-wide identification and expression analysis of MRLK family genes associated with strawberry (Fragaria vesca) fruit ripening and abiotic stress responses. PLoS One. 2016; 11:e0163647 doi: 10.1371/journal.pone.0163647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang QY, Zhang LQ, Song LL, Duan K, Li N, Wang YX, et al. The different interactions of Colletotrichum gloeosporioides with two strawberry varieties and the involvement of salicylic acid. Hortic Res, 2016; 3:16007 doi: 10.1038/hortres.2016.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goto M, Shiramatsu T, Nozaki K, Kawaguchi K. Studies on bacterial wilt of strawberry caused by Pseudomonas solanacearum (Smith) Smith[J]. Ann Phytopath Soc Japan, 2009; 44:270–276. [Google Scholar]
- 64.Sanghera GS, Wani SH, Hussain W, Singh NB. Engineering cold stress tolerance in crop plants. Curr Genomics. 2011; 12:30–43. doi: 10.2174/138920211794520178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim SY, Kim SG, Kim YS, Seo PJ, Bae M, Yoon HK, et al. Exploring membrane-associated NAC transcription factors in Arabidopsis: implications for membrane biology in genome regulation. Nucleic Acids Res.2007; 35:203–213. doi: 10.1093/nar/gkl1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fang Y, You J, Xie K, Xie W, Xiong L. Systematic sequence analysis and identification of tissue-specific or stress-responsive genes of NAC transcription factor family in rice. Mol Genet Genomics. 2008; 280:547–563. doi: 10.1007/s00438-008-0386-6 [DOI] [PubMed] [Google Scholar]
- 67.Wang YX, Liu ZW, Wu ZJ, Li H, Zhuang J. Transcriptome-wide identification and expression analysis of the NAC gene family in tea plant [Camellia sinensis (L.) O. Kuntze]. PLoS One. 2016; 11:e0166727 doi: 10.1371/journal.pone.0166727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ng S, Ivanova A, Duncan O, Law SR, Van Aken O, De Clercq I, et al. A membrane-bound NAC transcription factor, ANAC017, mediates mitochondrial retrograde signaling in Arabidopsis. Plant Cell. 2013; 25:3450–3471. doi: 10.1105/tpc.113.113985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu ZY, Kim SY, Hyeon do Y, Kim DH, Dong T, Park Y, et al. The Arabidopsis NAC transcription factor ANAC096 cooperates with bZIP-type transcription factors in dehydration and osmotic stress responses. Plant Cell. 2013; 25:4708–4724. doi: 10.1105/tpc.113.119099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hu H, You J, Fang Y, Zhu X, Qi Z, Xiong L. Characterization of transcription factor gene SNAC2 conferring cold and salt tolerance in rice. Plant Mol Biol. 2008; 67:169–181. doi: 10.1007/s11103-008-9309-5 [DOI] [PubMed] [Google Scholar]
- 71.Shiriga K, Sharma R, Kumar K, Yadav SK, Hossain F, Thirunavukkarasu N. Genome-wide identification and expression pattern of drought-responsive members of the NAC family in maize. Meta Gene. 2014; 2:407–417. doi: 10.1016/j.mgene.2014.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Su H, Zhang S, Yuan X, Chen C, Wang XF, Hao YJ. Genome-wide analysis and identification of stress-responsive genes of the NAM-ATAF1, 2-CUC2 transcription factor family in apple. Plant Physiol Bioch. 2013; 71:11–21. [DOI] [PubMed] [Google Scholar]
- 73.Yang SD, Seo PJ, Yoon HK, Park CM. The Arabidopsis NAC transcription factor VNI2 integrates abscisic acid signals into leaf senescence via the COR/RD genes. Plant Cell. 2011; 23:2155–2168. doi: 10.1105/tpc.111.084913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim YS, Sakuraba Y, Han SH, Yoo SC, Paek NC. Mutation of the Arabidopsis NAC016 transcription factor delays leaf senescence. Plant Cell Physiol. 2013; 54:1660–1672. doi: 10.1093/pcp/pct113 [DOI] [PubMed] [Google Scholar]
- 75.Jiang H, Li H, Bu Q, Li C. The RHA2a-interacting proteins ANAC019 and ANAC055 may play a dual role in regulating ABA response and jasmonate response. Plant Signal Behav. 2009; 4:464–466. doi: 10.1104/pp.109.135269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fujita M, Fujita Y, Maruyama K, Seki M, Hiratsu K, Ohme-Takagi, et al. A dehydration-induced NAC protein, RD26, is involved in a novel ABA-dependent stress-signaling pathway. Plant J. 2004; 39:863–876. doi: 10.1111/j.1365-313X.2004.02171.x [DOI] [PubMed] [Google Scholar]
- 77.Kim SG, Lee AK, Yoon HK, Park CM. A membrane bound NAC transcription factor NTL8 regulates gibberellic acid-mediated salt signaling in Arabidopsis seed germination. Plant J. 2008; 55:77–88. doi: 10.1111/j.1365-313X.2008.03493.x [DOI] [PubMed] [Google Scholar]
- 78.Park J, Kim YS, Kim SG, Jung JH, Woo JC, Park CM. Integration of auxin and salt signals by the NAC transcription factor NTM2 during seed germination in Arabidopsis. Plant Physiol. 2011; 156:537–549. doi: 10.1104/pp.111.177071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takasaki H, Maruyama K, Kidokoro S, Ito Y, Fujita Y, Shinozaki K, et al. The abiotic stress-responsive NAC-type transcription factor OsNAC5 regulates stress-inducible genes and stress tolerance in rice. Mol Genet Genomics. 2010; 284:173–183. doi: 10.1007/s00438-010-0557-0 [DOI] [PubMed] [Google Scholar]
- 80.Kou X, Watkins CB, Gan SS. Arabidopsis AtNAP regulates fruit senescence. J Exp Bot. 2012; 63:6139–6147. doi: 10.1093/jxb/ers266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seo PJ, Park CM. A membrane-bound NAC transcription factor as an integrator of biotic and abiotic stress signals. Plant Signal Behav. 2010; 5:481–483. doi: 10.4161/psb.11083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sakuraba Y, Kim YS, Han SH, Lee BD, Paek NC. The Arabidopsis transcription factor NAC016 promotes drought stress responses by repressing AREB1 transcription through a trifurcate feed-forward regulatory loop involving NAP. Plant Cell. 2015; 15:00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee S, Seo PJ, Lee HJ, Park CM. A NAC transcription factor NTL4 promotes reactive oxygen species production during drought-induced leaf senescence in Arabidopsis. Plant J. 2012; 70:831–844. doi: 10.1111/j.1365-313X.2012.04932.x [DOI] [PubMed] [Google Scholar]
- 84.Reiter RJ, Tan DX, Zhou Z, Cruz MH, Fuentes-Broto L, Galano A. Phytomelatonin: assisting plants to survive and thrive. Molecules. 2015; 20:7396–7437. doi: 10.3390/molecules20047396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiong Y, Sheen J. The role of target of rapamycin signaling networks in plant growth and metabolism. Plant Physiol. 2014; 164:499–512. doi: 10.1104/pp.113.229948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xiong F, Zhang R, Meng Z, Deng K, Que Y, Zhuo F, et al. Brassinosteriod Insensitive 2 (BIN2) acts as a downstream effector of the target of rapamycin (TOR) signaling pathway to regulate photoautotrophic growth in Arabidopsis. New Phytol. 2017; 213:233 doi: 10.1111/nph.14118 [DOI] [PubMed] [Google Scholar]
- 87.Yu F, Gu Q, Yun Y, Yin Y, Xu JR, Shim WB, et al. The TOR signaling pathway regulates vegetative development and virulence in Fusarium graminearum. New Phytol. 2014; 203:219–232. doi: 10.1111/nph.12776 [DOI] [PubMed] [Google Scholar]
- 88.Sehgal SN, Baker H, Vezina C. Rapamycin (AY-22, 989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J Antibiotics (Tokyo). 1975; 28:727–732. [DOI] [PubMed] [Google Scholar]
- 89.Marroquinguzman M, Wilson RA. GATA-dependent glutaminolysis drives appressorium formation in Magnaporthe oryzae by suppressing TOR inhibition of cAMP/PKA signaling. PLoS Pathog. 2015; 11:e1004851 doi: 10.1371/journal.ppat.1004851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bu Q, Jiang H, Li CB, Zhai Q, Zhang J, Wu X, et al. Role of the Arabidopsis thaliana NAC transcription factors ANAC019 and ANAC055 in regulating jasmonic acid-signaled defense responses. Cell Res. 2008; 18:756–767. doi: 10.1038/cr.2008.53 [DOI] [PubMed] [Google Scholar]
- 91.Yoshii M, Shimizu T, Yamazaki M, Higashi T, Miyao A, Hirochika H, et al. Disruption of a novel gene for a NAC-domain protein in rice confers resistance to Rice Dwarf Virus. Plant J. 2008; 57:615–625. doi: 10.1111/j.1365-313X.2008.03712.x [DOI] [PubMed] [Google Scholar]
- 92.Kim HS, Park HC, Kim KE, Jung MS, Han HJ, Kim SH, et al. A NAC transcription factor and SNI1 cooperatively suppress basal pathogen resistance in Arabidopsis thaliana. Nucleic Acids Res. 2012; 40:9182 doi: 10.1093/nar/gks683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McGrann GR, Steed A, Burt C, Goddard R, Lachaux C, Bansal A, et al. Contribution of the drought tolerance-related Stress-responsive NAC1 transcription factor to resistance of barley to Ramularia leaf spot. Mol Plant Pathol. 2015; 16:201 doi: 10.1111/mpp.12173 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
Conserved motifs and the sequence logos were generated using the MEME search tool. Numbers on the horizontal axis represent the sequence positions in the motifs and the vertical axis represent the information content measured in bits.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.