Abstract
Superparamagnetic nanoparticles (SPMNPs) have attracted interest for various biomedical applications due to their unique magnetic behavior, excellent biocompatibility, easy surface modification, and low cost. Their unique magnetic properties, superparamagnetism, and magnetophoretic mobility have led to their inclusion in immunoassays to enhance biosensor sensitivity and allow for rapid detection of various analytes. In this review, we describe SPMNP characteristics valuable for incorporation into biosensors, including the use of SPMNPs to increase detection capabilities of surface plasmon resonance and giant magneto-resistive biosensors. The current status of SPMNP-based immunoassays to improve the sensitivity of rapid diagnostic tests is reviewed, and suggested strategies for the successful adoption of SPMNPs for immunoassays are presented.
Keywords: superparamagnetic nanoparticles, immunoassay, biosensor, surface plasmon resonance, giant magnetoresistive sensors, rapid diagnostic test
Introduction
Nanotechnology is providing exciting new capabilities for research in materials science, biomedical engineering, and environmental engineering. In particular, bionanotechnology has emerged as a promising area with various commercial biomedical products already using nanoparticles.1 The global market for nanoparticles in biotechnology, drug development, and drug delivery has been estimated to reach $79.8 billion in 2019, with a compound annual growth rate of 22%.2 These are used for applications as varied as nanoparticle silver with antibiotic capabilities, quantum dots for noninvasive imaging, and superparamagnetic nanoparticles for enhanced oil recovery.3,4
Superparamagnetic nanoparticles (SPMNPs) exhibit superparamagnetic behavior (i.e., hysteresis-free reversible magnetization) and quick magnetophoretic response, which are attractive properties for biomedical applications. Indeed, a wide spectrum of applications, including drug delivery,5−7 magnetic resonance imaging,5,6,8,9 and hyper-thermic cancer treatments,10−13 have been successfully developed with these particles. Localized heating using SPMNPs has also been used in nonmedical applications such as precision polymer gelation14 (for details, see Laurent et al.29 and Pollert and Zaveta15). While many different kinds of nanoparticles have been synthesized and evaluated, iron-oxide SPMNPs (synthetic γ-Fe2O3 or Fe3O4 particles with a size of ∼10 nm) are of particular interest for their excellent magnetic properties, low cost, and low toxicity.
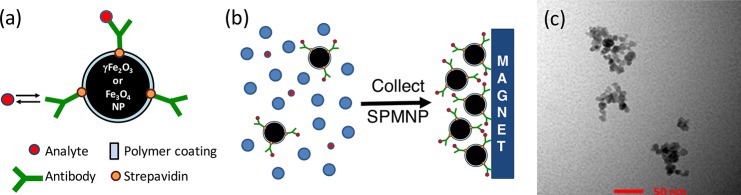
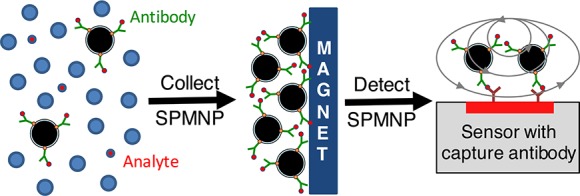
When SPMNPs are in the presence of a controlled external magnetic field, they act as “nano-magnets” and, accordingly, acquire several useful properties: (i) they can move in a desired direction; (ii) their presence can be remotely detected; (iii) they can generate highly localized, intense heat; and (iv) they lose their magnetism instantly and completely once the external magnetic field is removed. Furthermore, the SPMNP surface can be decorated with reactive functional groups without affecting these magnetic properties. When decorated with antibodies, this allows for SPMNP-based detection of a target biological or other chemical entity. The particles can then be manipulated for sensing, filtration, fractionation, collection, and other purposes. The tremendously large surface area per mass of particle offers the potential to detect a very dilute concentration of analyte, as the complex of particles and target molecules can be magnetically concentrated 10–1000-fold for improved detection sensitivity (Figure 1).
Figure 1.
Modification of SPMNPs for analyte detection. (a) For incorporation into immunoassays, SPMNPs can be covalently decorated with antibodies specific for a given analyte and concentrated using an external magnet. (b) This allows for capture of the analyte in a complex mixture, even when the analyte is present at dilute concentrations. (c) Direct visualization of Fe3O4 SPMNPs using transmission electron microscopy. SPMNPs have spherical shape and an average diameter of ∼10 nm and often form clusters or small aggregates with a size of ∼50 nm. The scale bar indicates 50 nm. (c) Reproduced with permission from Ko et al. (2017).16
Because of these capabilities, considerable effort has been invested in exploring the use of SPMNPs for medical, biological, and other applications.17 Many of these require high-end sophisticated equipment and will likely be more appropriate for research laboratories and large hospitals. However, there are also opportunities to incorporate SPMNPs into rapid diagnostic test (RDT) kits. This is an attractive option, since SPMNPs have the potential to increase device sensitivity by detecting molecules present at very dilute concentrations in biological samples such as in saliva or urine, for touch-free and rapid magnetic sensing, and for particle reuse.16,18 Among the various SPMNPs developed, magnetite (Fe3O4) nanoparticles are of particular interest, because they can be produced easily and cheaply, as already demonstrated with successful applications in removing arsenic from drinking water19 and treatment of oilfield water.16,20 As any RDT kit, for example, for malaria detection, must be cheap, portable, and easy to use, the low-cost and large-quantity availability of magnetite nanoparticles are key advantages of SPMNP for immunoassay applications.
While still in the early stages, several recent studies have reported SPMNP-based immunoassays for disease detection in developing countries. For example, Castilho et al.21 reported an electrochemical magneto immunoassay to detect the malarial Plasmodium falciparum histidine-rich protein 2 (PfHRP2) with higher sensitivity as compared to conventional methods. Nash et al.22 used a mixture of polymer-coated SPMNPs and gold nanoparticles (AuNPs) to concentrate captured antigens 50-fold before use in commercial lateral flow assays to detect two malarial antigens, namely, pan-aldose and PfHRP2.
This review focuses first on the characteristics of SPMNPs that are relevant for biosensor applications and describes biosensors currently in development for SPMNP-based immunoassays. The detection sensitivity of reported SPMNP-based immunoassays is compared with conventional methods including enzyme-linked immune-sorbent assay (ELISA) and commercial RDT kits for various analytes, with a special emphasis on malaria antigens. Finally, we describe strategies for improving the sensitivity and practical usability of SPMNP-based immunoassays.
Attractive Characteristics of Magnetic Nanoparticles for Immunoassay Applications
There is an extensive literature covering SPMNP fundamentals, their behavior, and applications.23,24 Therefore, in this review, we describe only those unique SPMNP properties relevant for immunoassay development.
Magnetic Behavior
The most useful SPMNP feature in this respect is their magnetic ability, which can be described quantitatively and predictably with physical analyses. When SPMNPs are subjected to an external magnetic field (H), they magnetize and generate an induced field (M) (see, e.g., Guimaraes23):
where
is the magnetic susceptibility of the SPMNP dispersion, ϕ is the volume fraction of the particles; μo is magnetic permittivity in free space (or in vacuum); Md is the bulk magnetization of the material; d is the diameter of the particle; k is the Boltzmann constant, and T is the absolute temperature.
The implication of this magnetic susceptibility is twofold. First, nanoparticles dispersed in a biological medium can collect upon application of a magnetic field, allowing quantification and analyses of the nanoparticles. Second, as the magnetization is proportional to the SPMNP volume fraction, shown in the equation above, the nanoparticle concentration (and accordingly the concentration of attached analytes) can be measured. The SPMNPs can be ushered in a desired direction or to a desired location by application of a magnetic field gradient, because they experience the following force:25
where VP is the SPMNP volume; MP is their magnetization; and ∇ H is the gradient of the external magnetic field. Therefore, by collecting the SPMNPs to an analysis location, the concentration of SPMNPs and associated analytes can be increased many-fold.
Synthesis
Various types of magnetic nanoparticles (MNPs) have been synthesized, including those based on iron oxides (e.g., γ-Fe2O3 and Fe3O4), pure metals such as Fe, Co, and Ni, spinel-type ferromagnets (e.g., MgFe2O4 and CoFe2O4), alloys (e.g., CoPt3 and FePt), and multifunctional composites (e.g., Fe3O4–Ag, Fe3O4–Au, and FePt-Ag).26,27 These can be synthesized by wet chemical methods28 (e.g., coprecipitation, high-temperature reactions, reactions in steric environments, sol–gel reactions, decomposition of organometallic precursors, polyol methods, etc.), physical methods (e.g., gas-phase deposition and electron-beam lithography), and microbial methods.26 The most popular synthesis method is coprecipitation, because this technique can achieve a narrow size distribution (10–50 nm) of nanoparticles.28,29 Iron oxide nanoparticles (e.g., magnetite (Fe3O4) and maghemite (γ-Fe2O3) with superparamagnetism are most widely used in biomedical applications due to their low toxicity, excellent biocompatibility, facile surface modification, and low cost.
Superparamagnetism
Because of their small size and high surface area per volume, SPMNPs have different properties compared with those of bulk materials, including magnetic behavior and lower melting and boiling points.30 Namely, in the absence of an external magnetic field, the spins of SPMNPs can orient randomly resulting in zero magnetic moment. However, in the presence of a magnetic field, a high magnetic response is induced. Superparamagnetism appears in ferromagnetic nanoparticles with sizes ranging from a few nanometers to several tenths of nanometers.31 This characteristic is highly dependent on the size, material, temperature, and surface modification of the nanoparticle.32 This hysteresis-free, reversible magnetization property allows for a rapid “on–off” switching capability that can be controlled by the presence of an external magnet.30 In the presence of a magnetic field, the SPMNPs will aggregate, but when the field is removed, the magnetization rapidly drops to zero, allowing the particles to redisperse. In the case of an immunoassay, this allows for reuse of the sensor and potentially even the same SPMNPs for subsequent detection events.
Surface Modification for Stability in Aqueous Solution
A small particle size (5–25 nm) with a narrow size distribution is necessary to yield not only superparamagnetic character but also uniform physical and chemical properties.33 However, because of the high surface area-to-volume ratios and the hydrophobic nature of bare magnetic nanoparticles, these readily aggregate, causing several problems. Large nanoparticle clusters experience strong magnetic dipole–dipole attractions between clusters, leading to ferromagnetic behavior, in which clusters retain magnetic properties even after the magnetic field is removed.34 Plasma proteins also readily coat the individual or clustered SPMNPs, which can interfere with analyte detection.
Surface modifications can help to passivate the surface and maintain SPMNP dispersion stability. Coating the nanoparticles with a shell of polymeric materials (e.g., poly(vinylpyrrolidone) [PVP], poly(lactic-glycolic acid) [PLGA], poly(ethylene glycol) [PEG], and poly(vinyl acetate) [PVA]), ionic and nonionic amphiphiles (e.g., oleic acid and stearic acid), inorganic materials (e.g., silica and gold), and carbon-based materials (e.g., carbon precursor such as glucose)35 are all strategies used to stabilize SPMNPs. The coating provides repulsion (of either electrostatic or steric/entropic origin) between the nanoparticles to overcome the attractive van der Waals forces, thereby preventing aggregation.36 Indeed, Torrisi et al.37 demonstrated that PEG-coated SPMNPs are stable in cell culture media for over one month. The uses of polymer-coated SPMNPs in biomedical applications and their morphology, advantages, and magnetization were recently reviewed in Wu et al.36
Surface Functionalization for Analyte Detection
Surface functionalization of SPMNPs for effective immobilization of protein is critical for developing immunoassays. There have been various attempts to functionalize nanoparticles using covalent bonding, noncovalent bonding (e.g., physical adsorption), and affinity bonding (e.g., streptavidin–biotin interaction).68,69 For most biological assays, the SPMNPs require a specific binding functionality to recognize the desired analyte, in addition to the reduced aggregation and nonspecific protein adsorption conferred by a polymeric coating. A convenient and modular approach is to first functionalize the particles with streptavidin, which can then capture biotinylated antibodies. Streptavidin is a homotetrameric protein from the actinobacterium Streptomycetes that can bind up to four molecules of biotin with extremely high affinity (equilibrium dissociation constant Kd of ∼1 × 10–14 M). Because of biotin’s small size (244 Da), it can be conjugated to larger proteins such as antibodies with minimal effect on their properties. The streptavidin–biotin system has been successfully used for immunoassays as well as solid-phase assays using magnetic and polystyrene nanoparticles.61,62,64,70
For nanoparticle decoration, a simple approach is to use a polymer coating with functional groups that can covalently bond to streptavidin. For example, the carboxylic acid groups present on polymers such as carboxyl-PEG can react with the terminal amino groups of surface-exposed lysine residues in strepavidin. The subsequent capture of biotinylated ligands is quite efficient, due to the high affinity of the interaction. Eberbeck et al.71 reported that ∼85% of SPMNPs decorated with streptavidin bound to biotinylated beads, while only 20% of SPMNPS decorated with antibiotin-antibody bound to biotinylated beads. Other approaches to immobilize streptavidin include the addition of a six-residue peptide linker, including a single unpaired cysteine, to facilitate thiol-based conjugation methods and act as a tether to separate streptavidin from the solid surface.72 Ylikotila et al.73 introduced active thiol groups through primary amines in the streptavidin, providing higher biotin binding efficiency of streptavidin conjugated flat solid surface.
Magnetophoresis
Magnetophoresis refers to the controlled motion of SPMNPs in a viscous medium induced by the application of an external magnetic field.38 This useful characteristic can be used to isolate, wash, and concentrate SPMNPs and any attached material. Purification and enrichment of dilute SPMNP samples in a biological medium (e.g., blood, saliva, etc.) can be performed by three simple steps. First, an external magnetic field is applied to collect SPMNP and associated material, the supernatant is removed, and the SPMNPs are washed if necessary before resuspension in a smaller volume of buffer. The concentrated sample can be obtained by small-scale magnetophoresis, negating the need for electricity or specialized machinery such as centrifugation. This is the key advantage of SPMNPs for biomedical applications, especially RDT and other diagnostics, because the SPMNPs conjugated with the analyte can be purified and concentrated before detection, which improves the signal-to-noise ratio and detection sensitivity.22,39 Indeed, SPMNPs have been successfully used to separate and purify specific cells,40 antigens,22 and DNA strands.41,42 Previous studies43−45 have demonstrated that the average magnetophoretic velocities of microbeads conjugated with analyte were proportional to the analyte concentration.
Biosensors Based on Magnetic Nanoparticles
Because of their high biocompatibility and potential for sensitive detection of analytes with a high signal-to-noise ratio and a short analysis time,26 SPMNPs are finding applications in biosensors using various detection methods.46 The SPMNPs can be used to facilitate sample concentration, amplify the signal, or directly contribute to the detected signal.
Reported devices include voltammetric, electro-chemiluminescent, superconducting quantum interference device (SQUID) sensors, nuclear magnetic resonance (NMR), and surface plasmon resonance (SPR). In addition, there are spintronic sensors such as giant magneto-resistance (GMR) and tunneling magnetoresistance (TMR), which measure magnetoresistance changes caused by binding target analytes to the SPMNPs. Voltammetric sensors measure electrochemical signal changes (e.g., voltage or current), while electro-chemiluminescent sensors detect photons released from chemical reactions. For example, Li et al. (2013)47 developed an electro-chemiluminescent sensor to detect Bacillus thuringiensis Cry1Ac using a gold-coated iron oxide nanoparticle. In this study, a primary antibody was complexed with the nanoparticles and immobilized on a glass carbon electrode using an external magnet. Next, antigen was captured and detected with a second antibody followed by a glucose oxidase conjugated antibody and signal produced by the reaction of luminol and hydrogen peroxide in the presence of the enzyme.
The SQUID immunoassays rely on an immobilized antibody that captures antigen that is in turn detected by a second biotinylated antibody, and finally, avidin-coated SPMNPs. The signal results from the slowly decaying magnetic flux of immobilized SPMNPs when the magnetic field is turned off. Free SPMNPs cannot be detected due to the random orientation of the dipole moments resulting from Brownian motion.48,49 Antibody-bound SPMNPs cannot rotate, and thus relaxation occurs slowly after magnetization by this Neel mechanism, producing a decaying magnetic signal. NMR measures the 1H proton signal. Because more surrounding water protons can be affected by MNPs than other materials,50 MNP-based nuclear magnetic resonance has higher detection sensitivity than regular nuclear magnetic resonance. This modification has the potential to improve magnetic resonance imaging.51
The SPR and GMR sensors, which work by measuring optical and magnetic field changes, respectively, are being combined with SPMNP-based immunoassays. Therefore, we briefly describe the SPR and GMR phenomena and discuss the use of SPMNPs to improve SPR and GMR sensor sensitivity in recent studies (summarized in Table 1).
Table 1. SPR and GMR Sensors Based on Magnetic Nanoparticles.
| sensor | SPMNP composition | NP size | analyte | detection limit | detection range | ref |
|---|---|---|---|---|---|---|
| SPR | streptavidin conjugated Fe2O3 magnetic nanoparticles | 50 nm | brain natriuretic peptide | ND | 0.025–1 ng/mL | (82) |
| streptavidin conjugated superparamagnetic nanoparticles | 50 nm | Staphylococcal enterotoxin B | ND | 0.10–10 ng/mL | (83) | |
| tosyl-activated superparamagnetic nanoparticles | 1 μm | prostate specific antigen | 10 fg/mL | 1 fg/mL–100 ng/mL | (52) | |
| magnetic nanoparticles with iron oxide core | 220 ± 63 nm | beta human chorionic gonadotropin | 0.45 pM | ND | (84) | |
| carboxyl group modified Fe3O4 magnetic nanoparticles | 10.5 nm | thrombin | 0.017 nM | 0.27–27 nM | (85) | |
| core/shell Fe3O4/SiO2 nanoparticles | 16 nm | rabbit IgG | ND | 1.25–20 μg/mL | (86) | |
| core/shell Fe3O4/Ag/SiO2 nanoparticles | 19 nm | rabbit IgG | ND | 0.30–20 μg/mL | (86) | |
| core/shell Fe3O4/Au nanoparticles modified with 3-mercaptopropionic acid (MPA) | 8–30 nm | human IgM | ND | 0.30–20 μg/mL | (87) | |
| Iron oxide carboxyl-modified magnetic nanoparticles | 200 nm | Ochratoxin A | 0.94 ng/mL | 1–50 ng/mL | (88) | |
| core/shell Fe3O4/Au nanoparticles | 25–30 nm | A-fetoprotein | 0.65 ng/mL | 1.0–200.0 ng/mL | (54) | |
| Fe3O4–Au nanorod (50 × 15 with 0.05 mmol/L AgNO3, 65 × 30 with 0.1 mmol/L AgNO3) | goat IgM | ND | 0.15–40.00 μg/mL | (55) | ||
| Fe3O4/Ag/Au nanocomposite | 35 nm | Dog IgG | 0.15 μg/mL | 0.15–40.00 μg/mL | (56) | |
| carboxyl group modified Fe3O4 magnetic nanoparticles | 6.53 ± 0.22 nm | Salmonella enteritidis | 14 cfu/mL | 14–1.4 × 109 cfu/mL | (89) | |
| GMR | streptavidin conjugated superparamagnetic nanoparticles | 300 nm | parathyroid hormone | 10 pM | ND | (90) |
| cubic FeCo nanoparticles | 12.8 ± 1.58 nm | Interleukin-6 | ND | 125 fM–41.5 pM | (91) | |
| streptavidin conjugated Fe2O3 nanoparticles | 50 nm | Aflatoxin B1 | 50 pg/mL | 0.050–50 ng/mL | (62) | |
| streptavidin conjugated microbeads | ND | allergen Ara h 1 | 7. 0 ng/mL | 7.0–>2000 ng/mL | (61) | |
| streptavidin conjugated microbeads | ND | allergen Ara h 2 | 0.2 ng/mL | 0.2–>250 ng/mL | (61) | |
| streptavidin conjugated microbeads | ND | allergen gliadin | 1.5 ng/mL | 1.5–4000 ng/mL | (61) | |
| streptavidin conjugated cubic FeCo nanoparticles | 12.8 ± 1.58 nm | endoglin | 83 fM | ND | (70) | |
| streptavidin conjugated superparamagnetic nanoparticles | 50 nm | Staphylococcal enterotoxin A | 0.1 ng/mL | ND | (64) | |
| streptavidin conjugated superparamagnetic nanoparticles | 50 nm | toxic shock syndrome toxin | 0.3 ng/mL | ND | (64) | |
| streptavidin conjugated magnetic nanoparticles | ND | Flt3 ligand | ND | 0.020–3 μg/mL | (59) | |
| streptavidin conjugated magnetic nanoparticles | ND | serum amyloid A1 | ND | 3–50 ng/mL | (59) | |
| streptavidin conjugated magnetic nanoparticles | ND | influenza A virus | 1.5 × 102 TCIDa 50/mL | 1.5 × 102–1.0 × 105 TCID 50/mL | (66) |
TCID, tissue culture infective dose.
Surface Plasmon Resonance (SPR) Sensors
SPR is an optical method that measures refractive index changes caused by an increase of mass on the metallic sensor surface and corresponding change in refractive index. In this case, the mass increases when analytes are captured by immobilized antibodies. SPR response units are related to the amount of bound analyte per test area (one response unit ≈ 1 pg/mm2).52 SPR has the advantages of rapid and label-free detection, real-time analysis, and small sample volume requirements. Thus, SPR biosensors have been widely used for qualitative and quantitative measurements of biomolecular interactions.53 However, since the SPR signal is related to the mass of protein bound to the sensor surface, it has poor sensitivity for small molecular weight molecules or low concentrations due to the small refractive index changes created by binding of these analytes on the SPR surfaces.54
SPMNPs have been used to amplify the signal in SPR biosensors simply due to their large mass, concentrate antigens, and higher refractive index, and, when the SPMNP includes silver or gold, they produce an SPR signal by their ability to propagate surface plasmons. For instance, antibody immobilized on the sensor surface can be used to capture an analyte, which is then bound by a second antibody decorating an SPMNP, which greatly increases the refractive index relative to the analyte alone (Figure 2).55−58 Wang et al.56 was able to detect canine immunoglobulins at a concentration range of 1.25–20.00 μg/mL using a standard SPR detection strategy, but the detection range improved to 0.15–40.00 μg/mL when SPMNPs/Ag composite was included. Additionally, biological analytes associated with SPMNPs can be concentrated and washed using an external magnet before application to the SPR (Figure 2). This purification strategy efficiently removes nontarget analytes present in biological samples, thereby reducing background signal. Finally, using magnetic pillars, SPMNPs can be attached and immobilized on the SPR surface to enhance the biosensor sensitivity.55,56
Figure 2.
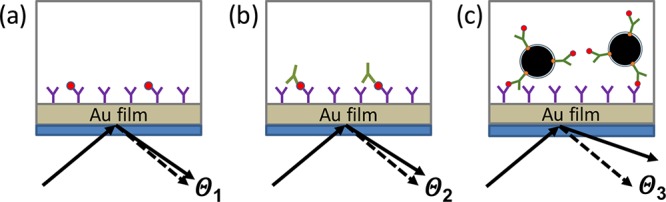
SPMNPs to enhance SPR sensitivity. (a) SPR devices typically function by immobilizing a capture antibody on a supported gold film. Polarized light is refracted by the gold film at an angle Θ1 greater than would be expected due to the mass of bound antigen affecting the surface plasmon resonance. If the antigen has a small mass, the angle is small and can be difficult to detect. (b) Detection sensitivity can be increased by detecting bound antigen with a second detection antibody, thereby increasing the amount of mass bound and the reflection angle Θ2 per bound antigen. (c) Sensitivity can be further increased by using a detection antibody bound to an SPMNP with a large total mass, resulting in a much larger angle Θ3 per antigen molecule bound, and thereby lowering the concentration of antigen that can be reliably detected.
Giant Magnetoresistive (GMR) Sensors
Magnetoresistance is the dependence of a material’s electrical resistance to an externally applied magnetic field. GMR is a quantum mechanical effect that causes a significant change in electrical resistance of a thin-film layered structure induced by changing the external magnetic field.59
The GMR sensor developed by Osterfeid et al.60 utilized antibody-conjugated magnetic nanoparticles to measure antigen concentrations (Figure 3). They used spin-valve GMR sensors consisting of the top “free” ferromagnetic layer and the bottom “fixed” ferromagnetic layer.61 A capture antibody was immobilized on the sensor surface and used to capture antigen from a complex sample. This was in turn recognized by a second biotinylated detection antibody, which binds a separate site on the antigen. Finally, streptavidin-coated SPMNPs bind the biotinylated antibody to generate a change in the magnetization of the top free layer. The electrical resistance of the sensor changes in real-time, resulting in the detected signal.
Figure 3.
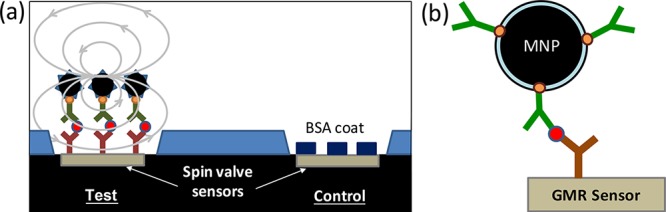
Spin-valve GMR sensor to detect analyte. (a) A test sensor is first functionalized with a capture antibody specific for the target analyte. This is used to capture analyte and create an immune sandwich culminating in SPMNPs. The magnetic signal from the immobilized SPMNPs enables quantification of the analyte concentration using a standard curve. A separate control sensor is coupled with bovine serum albumin (BSA) to detect background signal resulting from nonspecific binding. (b) The molecular assembly used for detection includes the capture antibody covalently coupled to the sensor surface that binds the target analyte. This is then detected by a second antibody binding a different epitope on the analyte. Since the detection antibody is biotinylated, it can in turn be bound by a streptavidin-conjugated SPMNP. This sensor was reported in Osterfeld et al.60
Recent studies have used GMR sensors to detect various analytes such as food allergens, potential cancer markers,60 and mycotoxins62 with fast detection and high sensitivity. For example, Osterfeld et al.60 detected multiple potential cancer markers (e.g., interleukin-1-alpha, interleukin-10, etc.) at sub-picomolar concentrations with a more than four-log range in sensitivity. In addition, Mak et al.62 detected multiple mycotoxins (e.g., aflatoxin B1, zearalenone, and HT-2) in real-time using a streptavidin-linked SPMNP for detection and achieved a detection limit of 50 pg/mL. The MNPs used in GMR sensors are superparamagnetic or ferromagnetic with a high magnetic moment and large susceptibility, which is essential for magnetization in a low magnetic field. In addition, the SPMNPs must have a homogeneous size distribution and high stability in physiological solutions to ensure a stable relationship between number of analyte-complexed SPMNPs and the resulting magnetic signal.26,63
Current Development of SPMNP-Based Immunoassays
Immunoassays that incorporate SPMNPs are under development for monitoring of various infectious diseases, including malaria and staphylococcal enterotoxins. Here, we describe these assays and compare their detection sensitivity to conventional assays including commercial RDT kits and ELISA.
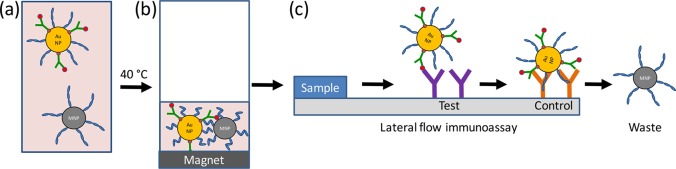
Nash et al.22 developed a system with polymer-coated SPMNPs and gold nanoparticles to concentrate malaria antigens before application to commercial lateral flow assays. The SPMNPs and AuNPs were decorated with the thermally responsive polymer poly(N-isopropylacrylamide) (pNIPAm). The coated AuNPs were next conjugated to streptavidin and coated with a biotinylated antibody recognizing a malaria antigen (either aldolase, which is common to all malarial parasites, or PfHRP2). The particles were then incubated with human plasma samples spiked with malaria antigens and combined with the SPMNPs. Heating for 15 min at 40 °C resulted in polymer phase transition and coaggregation of the AuNPs and SPMNPs, which were then concentrated using an external magnet for an additional 15 min at 40 °C, resulting in a 50-fold reduction in volume (Figure 4). The concentrated AuNPs-antigen-SPMNP mixture was directly applied to existing lateral flow immunoassays with control and test lines. This enrichment method improved detection sensitivity 4.4-fold over a conventional commercial assay, as measured by visual inspection and integrated pixel intensity.
Figure 4.
Sample concentration using mixed a AuNP/SPMNP system for lateral flow immunoassays. (a) Polymer and antibody-coated AuNPs were combined with polymer-coated SPMNPs and human plasma spiked with malaria antigens. (b) Heating to 40 °C induced a polymer phase transition and coaggregation of the AuNPs and SPMNPs, which were collected using an external magnet. The supernatant was removed, and the particles were redispersed in a smaller volume of buffer. (c) The concentrated particles were directly applied to a standard lateral flow immunoassay and migrated down the paper strip by capillary action. The gold nanoparticles accumulated at the test and control lines, allowing for visual inspection of the results, while the SPMNPs were transported into the waste. Figure inspired by Nash et al.22
Orlov et al.64,65 developed separate magnetic immunoassays to detect staphylococcal enterotoxins in contaminated foods and water and the cancer marker prostate specific antigen. In the first case, they created an immune sandwich with a capture antibody on three-dimensional fiber filters, followed by toxin and detection antibody coupled to a magnetic nanoparticle.64 This was able to detect concentrations as low as 4 pg/mL from a 30 mL sample. In the second case, they combined a traditional lateral immunoassay with capture antibodies linked to 200 nm magnetic nanoparticles and thicker nitrocellulose (260 μm) to allow access to a larger sample volume and magnetic particle quantification.65 This resulted in a detection limit of 25 pg/mL in serum.
Finally, Ng et al.61 demonstrated detection of several food allergens using GMR sensor arrays. With a molecular assembly comprised of food allergens, then biotinylated antibodies followed by streptavidin-conjugated SPMNPs, the resistance of GMR sensors correlated with allergen concentrations. The authors showed that limit of detection (LOD) of GMR sensor assays were approximately an order of magnitude higher than for a conventional ELISA, ranging from 0.2 to 7.0 ng/mL (Table 2). Furthermore, multiple food allergens (peanut Arah1, peanut Arah2, and wheat gliadin) were detected with little to no cross-reactivity.
Table 2. Comparison of SPMNPs Immunoassays and Conventional ELISA Limit of Detection.
| analyte | ELISA LOD | sensor type | SPMNPs immunoassay LOD | ref |
|---|---|---|---|---|
| peanut Ara h 1 | 31.5 ng/mL | GMR | 7.0 ng/mL | (61) |
| peanut Ara h 2 | 2 ng/mL | GMR | 0.2 ng/mL | (61) |
| wheat gliadin | 40 ng/mL | GMR | 1.5 ng/mL | (61) |
| Staphylococcal enterotoxins (SEs) | 0.1–0.5 ng/mL | magnetic particle quantification (MPQ) method | 0.3 ng/mL (express MIA) | (64) |
| 10 pg/mL (HV MIA) | ||||
| prostate specific antigen (PSA) | ∼100 pg/mL | magnetic particle quantification (MPQ) method | 25 pg/mL | (65) |
Nash et al.22 and Orlov et al.65 developed modified lateral flow immunoassays, which used color changes and a magnetic particle quantification reader for quantitative detection of the results, respectively. The diagnostic methods developed in these two studies would be appropriate for qualitative biomarker detection in the field. The GMR sensors reported by Nash et al.22 can incorporate multiple sensor arrays in one GMR chip, which allows for simultaneous detection of several biomarkers from one sample. It was reported that GMR bioassays can be portable, sensitive immunoassays for on-site application and thus have potential applications for developing world diagnostics to support sero-epidemiology studies.66,67
Strategies to Improve SPMNP-Based Immunoassays
Increase Magnetic Nanoparticle Stability
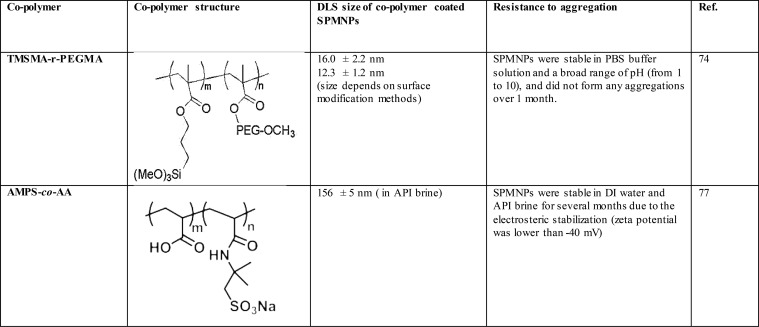
Even with the polymer coatings described above, SPMNPs remain subject to aggregation in complex cell culture media and sera, which have a higher ionic strength (∼150 mM) than buffers often used for nonbiological applications.74 To further stabilize the SPMNPs, copolymers have employed to coat the particles. These copolymers are typically random combinations of two monomers: one provides an “anchor” functionality, while the other confers chemical functionality. The anchor monomer adsorbs to or forms covalent bonds with the SPMNP surface, allowing the “functional” monomers to extend from the SPMNP surface, preventing SPMNPs aggregation via electrostatic stabilization or steric repulsion. Additionally, the functional monomer often provides chemical reactivity to connect to streptavidin or antibodies.
In one example, Jon et al.75 synthesized a random copolymer composed of (trimethoxysilyl) propyl methacrylate (the anchor) and PEG methacrylate (the functional), denoted as poly(TMSMA-r-PEGMA). The trimethoxysilyl group is surface-reactive, forming covalent bonds with the solid oxide (SiO2) surface, effectively anchoring the copolymer to the surface. The hydrophilic PEG components extend away from the surface, into the aqueous solvent, acting as an aggregation and protein-resistant shell via steric exclusion.76 The authors reported that the TMSMA-r-PEGMA copolymer significantly reduced nonspecific protein adsorption by insulin, lysozyme, and fibrinogen on Si/SiO2 wafers. Lee et al.74 also successfully synthesized poly(TMSMA-r-PEGMA)-coated magnetic nanoparticles and reported that these particles resisted aggregation during one month of storage in phosphate-buffered saline.
These promising results have prompted exploration with other copolymers to stabilize and functionalize SPMNPs (Table 3). Ureña-Benavides et al.77 used poly(2-acrylamido-2-methyl-1-propanesulfonic acid-co-acrylic acid), denoted as poly(AMPS-co-AA), to stabilize magnetic iron oxide nanoparticles under harsh conditions such as high salinity and high temperatures observed in enhanced oil recovery operations. In this copolymer, poly(AMPS-co-AA), the AA groups have anchor function, which form strong complexes with SPMNPs, and the AMPS groups support electrosteric stabilization. The authors reported that poly(AMPS-co-AA)-coated SPMNPs were stable against aggregation at 90 °C in standard American Petroleum Institute (API) brine (8 wt % NaCl and 2 wt % CaCl2) for 24 h. The hydrodynamic diameter of poly(AMPS-co-AA)-coated SPMNPs was measured by dynamic light scattering after 24 h at 90 °C in standard API brine and found to be 183 ± 58, which is slightly higher than 136 ± 25 measured in API brine at room temperature. Thus, surface modification of SPMNPs with copolymeric materials is likely necessary immunoassay applications, which require a similar high stability in biological media.
Table 3. Copolymer-Coated Magnetic Nanoparticles.
In addition to increased stability, polymeric coatings can provide other functions to improve immunoassay performance. Thermally responsive polymers, such as pNIPAm, have been conjugated to SPMNPs to purify and to concentrate AuNP-immune complexes, as described above for detection of malaria antigens.22 Similarly, gold-magnetite composite nanoparticles coated with poly(acrylic acid) were conjugated with Treponema pallidum (Tp) antigens and used to detect the presence of anti-Tp antibodies by lateral flow immunoassay. This resulted in a detection limitation as low as 1 national clinical unit/mL.78 Finally, SPMNPs and the anticancer drug doxorubicin were coencapsulated with a biocompatible amphiphilic block copolymer and conjugated with an antibody recognizing the breast cancer antigen HER2. These were then able to detect breast cancer cells by magnetic resonance imaging and demonstrated high sensitivity in mice,79 suggesting the approach may be useful for immunoassays as well.
Reusable Immunoassays
Immunosensors whose surfaces can be regenerated and reused without loss of sensitivity are of great interest for reducing assay cost and waste and may be especially attractive for developing world applications. Thus, far, immunoassays have been regenerated by detaching bound antigen–antibody complexes with treatment such as low pH, which disrupts the complex but also often leads to loss or inactivation the immobilized antibody.80 In one example, Kandimalla et al.81 compared different antigen-dissociating agents observing that Gly-HCl (pH 2.3) buffer with 1% dimethyl sulfoxide (DMSO) was most effective, removing 97% of the bound material.
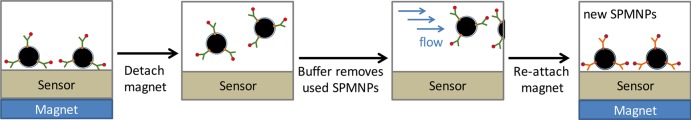
In contrast, SPMNP immunoassays can be easily regenerated by replacing the used nanoparticles with fresh ones. In essence, SPMNPs are first immobilized on the sensor surface using an external magnet. Thus, contaminated SPMNPs are released by detaching the external magnet and removed by flowing buffer solution. The introduction of new SPMNPs and their immobilization with the magnet results in a fresh surface for a new assay. Previous studies successfully regenerated SPMNPs immunoassays by these simple steps54,55,78,80 (Figure 5).
Figure 5.
Reusable SPMNP immunoassays. After analyte capture by both the antibody-coated SPMNPs, antigen-bound SPMNPs are concentrated at the sensor surface by a magnet for signal detection. These are then released by detaching the external magnet and removed by flowing buffer solution. New SPMNPs are easily attached on the sensor surface by attaching the external magnet and ready for a new assay.
Conclusion and Outlook
Current RDT assays are inexpensive, simple to use with high sensitivity, and provide rapid results. However, the desire to achieve ever-lower detection limits and analyze samples with very low analyte concentrations (namely, urine and saliva) demands analytical techniques with far greater sensitivity. The inclusion of superparamagnetic SPMNPs in immunoassays is a promising strategy to increase assay sensitivity by facilitating sample concentration, amplify or directly produce signal, depending on the specific sensor used. In this review, we introduced strategies by which SPMNPs can be generated, incorporated into immunoassays, and discuss recent efforts to develop SPMNP-based immunoassays. On the basis of these initial reports, assays using SPMNPs are likely to become increasingly common tool for diagnostics.
Acknowledgments
This research was supported by Grand Challenges Exploration (Grant OPP 1150977) from the Bill & Melinda Gates foundation.
Glossary
Abbreviations
- AuNP
gold nanoparticle
- ELISA
enzyme-linked immune-sorbent assay
- PfHRP2
Plasmodium falciparum histidine-rich protein 2
- GMR
giant magneto-resistive
- LOD
limit of detection
- MNP
magnetic nanoparticle
- RDT
rapid diagnostic test
- SPMNP
superparamagnetic nanoparticle
- SPR
surface plasma resonance
- SQUID
superconducting quantum interference device
The authors declare no competing financial interest.
References
- Arruebo M.; Valladares M.; Gonzalez-Fernandez A. Antibody-Conjugated Nanoparticles for Biomedical Applications. J. Nanomater. 2009, 2009, 1–24. 10.1155/2009/439389. [DOI] [Google Scholar]
- BCC Research. Nanoparticles in Biotechnology, Drug Development and Drug Delivery. 2014, access on https://www.bccresearch.com.
- Panthani M. G.; Khan T. A.; Reid D. K.; Hellebusch D. J.; Rasch M. R.; Maynard J. A.; Korgel B. A. In Vivo Whole Animal Fluorescence Imaging of a Microparticle-Based Oral Vaccine Containing (Cuinse X S2–X)/Zns Core/Shell Quantum Dots. Nano Lett. 2013, 13, 4294–4298. 10.1021/nl402054w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko S.; Prigiobbe V.; Huh C.; Bryant S. L.; Bennetzen M. V.; Mogensen K.. Oil Droplet Removal from Produced Water Using Nanoparticles and Their Magnetic Separation , SPE Annual Technical Conference and Exhibition, Sept 26–28, 2014, Dubai, UAE; Society of Petroleum Engineers, 2016; SPE-181893-MS, DOI: 10.2118/181893-MS. [DOI] [Google Scholar]
- Chertok B.; Moffat B. A.; David A. E.; Yu F.; Bergemann C.; Ross B. D.; Yang V. C. Iron Oxide Nanoparticles as a Drug Delivery Vehicle for Mri Monitored Magnetic Targeting of Brain Tumors. Biomaterials 2008, 29, 487–496. 10.1016/j.biomaterials.2007.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C.; Lee J. S.; Zhang M. Magnetic Nanoparticles in Mr Imaging and Drug Delivery. Adv. Drug Delivery Rev. 2008, 60, 1252–1265. 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger T. S. B.; Hofmann H.; Hofmann M.; von Rechenberg B.; Schopf B. Superparamagnetic Nanoparticles for Biomedical Applications: Possibilities and Limitations of a New Drug Delivery System. J. Magn. Magn. Mater. 2005, 293, 483–496. 10.1016/j.jmmm.2005.01.064. [DOI] [Google Scholar]
- Smith B. R.; Heverhagen J.; Knopp M.; Schmalbrock P.; Shapiro J.; Shiomi M.; Moldovan N. I.; Ferrari M.; Lee S. C. Localization to Atherosclerotic Plaque and Biodistribution of Biochemically Derivatized Superparamagnetic Iron Oxide Nanoparticles (Spions) Contrast Particles for Magnetic Resonance Imaging (MRI). Biomed. Microdevices 2007, 9, 719–727. 10.1007/s10544-007-9081-3. [DOI] [PubMed] [Google Scholar]
- Hola K.; Markova Z.; Zoppellaro G.; Tucek J.; Zboril R. Tailored Functionalization of Iron Oxide Nanoparticles for Mri, Drug Delivery, Magnetic Separation and Immobilization of Biosubstances. Biotechnol. Adv. 2015, 33, 1162–1176. 10.1016/j.biotechadv.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Espinosa A.; Di Corato R.; Kolosnjaj-Tabi J.; Flaud P.; Pellegrino T.; Wilhelm C. Duality of Iron Oxide Nanoparticles in Cancer Therapy: Amplification of Heating Efficiency by Magnetic Hyperthermia and Photothermal Bimodal Treatment. ACS Nano 2016, 10, 2436–2446. 10.1021/acsnano.5b07249. [DOI] [PubMed] [Google Scholar]
- Hayashi K.; Nakamura M.; Miki H.; Ozaki S.; Abe M.; Matsumoto T.; Sakamoto W.; Yogo T.; Ishimura K. Magnetically Responsive Smart Nanoparticles for Cancer Treatment with a Combination of Magnetic Hyperthermia and Remote-Control Drug Release. Theranostics 2014, 4, 834–844. 10.7150/thno.9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossatz S.; Grandke J.; Couleaud P.; Latorre A.; Aires A.; Crosbie-Staunton K.; Ludwig R.; Dahring H.; Ettelt V.; Lazaro-Carrillo A.; Calero M.; Sader M.; Courty J.; Volkov Y.; Prina-Mello A.; Villanueva A.; Somoza A.; Cortajarena A. L.; Miranda R.; Hilger I. Efficient Treatment of Breast Cancer Xenografts with Multifunctionalized Iron Oxide Nanoparticles Combining Magnetic Hyperthermia and Anti-Cancer Drug Delivery. Breast Cancer Res. 2015, 17, 66. 10.1186/s13058-015-0576-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J.; Ouyang W.; Wang X.; Tang J.; et al. Anticancer Effect and Feasibility Study of Hyperthermia Treatment of Pancreatic Cancer Using Magnetic Nanoparticles. Oncol. Rep. 2011, 27, 719–726. 10.3892/or.2011.1567. [DOI] [PubMed] [Google Scholar]
- Panthi K.; Mohanty K. K.; Huh C.. Precision Control of Gel Formation Superparamagnetic Nanoparticle-Based Heating , Proceedings of SPE ATCE, Houston, TX, Sept 28–30, 2015; Society of Petroleum Engineers, 2015; SPE-175006-MS, DOI: 10.2118/175006-MS. [DOI] [Google Scholar]
- Pollert E. A.; Korel Z.. Nanocrystalline Oxides in Magnetic Fluid Hyperthermia. In Magnetic Nanoparticles. From Fabrication to Clinical Applications ;Thanh N. T. K., Ed.; CRC Press: Boca Raton, FL, 2012; pp 449–479. [Google Scholar]
- Ko S.; Kim E. S.; Park S.; Daigle H.; Milner T. E.; Huh C.; Bennetzen M. V.; Geremia G. A. Amine Functionalized Magnetic Nanoparticles for Removal of Oil Droplets from Produced Water and Accelerated Magnetic Separation. J. Nanopart. Res. 2017, 19, 132. 10.1007/s11051-017-3826-6. [DOI] [Google Scholar]
- Nikitin P. I.; Vetoshko P. M.; Ksenevich T. I. Magnetic Immunoassays. Sens. Lett. 2007, 5, 296–299. 10.1166/sl.2007.007. [DOI] [Google Scholar]
- Ko S.; Lee H.; Huh C.. Efficient Removal of Eor Polymer from Produced Water Using Magnetic Nanoparticles and Regeneration/Re-Use of Spent Particles , SPE Improved Oil Recovery Conference, Tulsa, OK, April 11–13, 2016,; Society of Petroleum Engineers, 2016; DOI: 10.2118/179576-MS [DOI] [Google Scholar]
- Yavuz C. T.; Mayo J. T.; Suchecki C.; Wang J.; Ellsworth A. Z.; D’Couto H.; Quevedo E.; Prakash A.; Gonzalez L.; Nguyen C.; Kelty C.; Colvin V. L. Pollution Magnet: Nano-Magnetite for Arsenic Removal from Drinking Water. Environ. Geochem. Health 2010, 32, 327–334. 10.1007/s10653-010-9293-y. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Prigiobbe V.; Huh C.; Bryant S. L. Alkaline Earth Element Adsorption onto Paa-Coated Magnetic Nanoparticles. Energies 2017, 10, 223. 10.3390/en10020223. [DOI] [Google Scholar]
- de Souza Castilho M.; Laube T.; Yamanaka H.; Alegret S.; Pividori M. I. Magneto Immunoassays for Plasmodium Falciparum Histidine-Rich Protein 2 Related to Malaria Based on Magnetic Nanoparticles. Anal. Chem. 2011, 83, 5570–5577. 10.1021/ac200573s. [DOI] [PubMed] [Google Scholar]
- Nash M. A.; Waitumbi J. N.; Hoffman A. S.; Yager P.; Stayton P. S. Multiplexed Enrichment and Detection of Malarial Biomarkers Using a Stimuli-Responsive Iron Oxide and Gold Nanoparticle Reagent System. ACS Nano 2012, 6, 6776–6785. 10.1021/nn3015008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães A. P.Principles of Nanomagnetism ;Springer: Heidelberg Germany, 2009. [Google Scholar]
- Thinh T. T.; Maenosono S.; Thanh N. T.K.. Next Generation Magnetic Nanoparticles for Biomedical Applications. In Magnetic Nanoparticles: From Fabrication to Clinical Applications ;Thanh N. T. K., Ed.; CRC Press: Boca Raton, FL, 2012; pp 99–129. [Google Scholar]
- Ryoo S.; Rahmani A. R.; Yoon K. Y.; Prodanović M.; Kotsmar C.; Milner T. E.; Johnston K. P.; Bryant S. L.; Huh C. Theoretical and Experimental Investigation of the Motion of Multiphase Fluids Containing Paramagnetic Nanoparticles in Porous Media. J. Pet. Sci. Eng. 2012, 81, 129–144. 10.1016/j.petrol.2011.11.008. [DOI] [Google Scholar]
- Rocha-Santos T. A. P. Sensors and Biosensors Based on Magnetic Nanoparticles. TrAC, Trends Anal. Chem. 2014, 62, 28–36. 10.1016/j.trac.2014.06.016. [DOI] [Google Scholar]
- Lu A. H.; Salabas E. L.; Schuth F. Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application. Angew. Chem., Int. Ed. 2007, 46, 1222–1244. 10.1002/anie.200602866. [DOI] [PubMed] [Google Scholar]
- Laurent S.; Forge D.; Port M.; Roch A.; Robic C.; Vander Elst L.; Muller R. N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- Colombo M.; Carregal-Romero S.; Casula M. F.; Gutierrez L.; Morales M. P.; Bohm I. B.; Heverhagen J. T.; Prosperi D.; Parak W. J. Biological Applications of Magnetic Nanoparticles. Chem. Soc. Rev. 2012, 41, 4306–4334. 10.1039/c2cs15337h. [DOI] [PubMed] [Google Scholar]
- Zhu Y. K. K.; NDong C.; Huang Y.-Y.; Shubitidze F.; Griswold K. E.; Baker I.; Zhang J. X. J.; Kekalo K. Magnetic Nanoparticle Based Immunoaasays on Chip: Materials Synthesis, Surface Functionalization, and Cancer Cell Screening. Adv. Funct. Mater. 2016, 26, 3953–3972. 10.1002/adfm.201504176. [DOI] [Google Scholar]
- Benz M.Superparamagnetism: Theory and Applications. Unpublished Manuscript, 2012. Cited Jan 30, 2018.
- Mikhaylova M.; Kim D. K.; Bobrysheva N.; Osmolowsky M.; Semenov V.; Tsakalakos T.; Muhammed M. Superparamagnetism of Magnetite Nanoparticles: Dependence on Surface Modification. Langmuir 2004, 20, 2472–2477. 10.1021/la035648e. [DOI] [PubMed] [Google Scholar]
- Gupta A. K.; Wells S. Surface-Modified Superparamagnetic Nanoparticles for Drug Delivery: Preparation, Characterization, and Cytotoxicity Studies. IEEE Trans. Nanobioscience 2004, 3, 66–73. 10.1109/TNB.2003.820277. [DOI] [PubMed] [Google Scholar]
- Gupta A. K.; Gupta M. Synthesis and Surface Engineering of Iron Oxide Nanoparticles for Biomedical Applications. Biomaterials 2005, 26, 3995–4021. 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- He J.; Huang M.; Wang D.; Zhang Z.; Li G. Magnetic Separation Techniques in Sample Preparation for Biological Analysis: A Review. J. Pharm. Biomed. Anal. 2014, 101, 84–101. 10.1016/j.jpba.2014.04.017. [DOI] [PubMed] [Google Scholar]
- Wu W.; Jiang C. Z.; Roy V. A. Designed Synthesis and Surface Engineering Strategies of Magnetic Iron Oxide Nanoparticles for Biomedical Applications. Nanoscale 2016, 8, 19421–19474. 10.1039/C6NR07542H. [DOI] [PubMed] [Google Scholar]
- Torrisi V.; Graillot A.; Vitorazi L.; Crouzet Q.; Marletta G.; Loubat C.; Berret J. F. Preventing Corona Effects: Multiphosphonic Acid Poly(Ethylene Glycol) Copolymers for Stable Stealth Iron Oxide Nanoparticles. Biomacromolecules 2014, 15, 3171–3179. 10.1021/bm500832q. [DOI] [PubMed] [Google Scholar]
- Li D.Encyclopedia of Microfluidics and Nanofluidics ;Springer: New York, 2008. [Google Scholar]
- Holzinger M.; Le Goff A.; Cosnier S. Nanomaterials for Biosensing Applications: A Review. Front. Chem. 2014, 2, 1–10. 10.3389/fchem.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossendorp F. A.; Bruning P. F.; Vaan den Brink J. A.; De Boer M. Efficient Selection of High-Affinity B Cell Hybridomas Using Antigen-Coated Magnetic Beads. J. Immunol. Methods 1989, 120, 191–200. 10.1016/0022-1759(89)90242-1. [DOI] [PubMed] [Google Scholar]
- He X.; Huo H.; Wang K.; Tan W.; Gong P.; Ge J. Plasmid DNA Isolation Using Amino-Silica Coated Magnetic Nanoparticles (ASMNPs). Talanta 2007, 73, 764–769. 10.1016/j.talanta.2007.04.056. [DOI] [PubMed] [Google Scholar]
- Min J. H.; Woo M. K.; Yoon H. Y.; Jang J. W.; Wu J. H.; Lim C. S.; Kim Y. K. Isolation of DNA Using Magnetic Nanoparticles Coated with Dimercaptosuccinic Acid. Anal. Biochem. 2014, 447, 114–118. 10.1016/j.ab.2013.11.018. [DOI] [PubMed] [Google Scholar]
- Hahn Y. K.; Jin Z.; Kang J. H.; Oh E.; Han M. K.; Kim H. S.; Jang J. T.; Lee J. H.; Cheon J.; Kim S. H.; Park H. S.; Park J. K. Magnetophoretic Immunoassay of Allergen-Specific Ige in an Enhanced Magnetic Field Gradient. Anal. Chem. 2007, 79, 2214–2220. 10.1021/ac061522l. [DOI] [PubMed] [Google Scholar]
- Hahn Y. K.; Chang J. B.; Jin Z.; Kim H. S.; Park J. K. Magnetophoretic Position Detection for Multiplexed Immunoassay Using Colored Microspheres in a Microchannel. Biosens. Bioelectron. 2009, 24, 1870–1876. 10.1016/j.bios.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Zborowski M.; Fuh C. B.; Green R.; Sun L.; Chalmers J. J. Analytical Magnetapheresis of Ferritin-Labeled Lymphocytes. Anal. Chem. 1995, 67, 3702–3712. 10.1021/ac00116a014. [DOI] [PubMed] [Google Scholar]
- Aguilar-Arteaga K.; Rodriguez J.; Barrado E. Magnetic Solids in Analytical Chemistry: A Review. Anal. Chim. Acta 2010, 674, 157–165. 10.1016/j.aca.2010.06.043. [DOI] [PubMed] [Google Scholar]
- Li J.; Xu Q.; Wei X.; Hao Z. Electrogenerated Chemiluminescence Immunosensor for Bacillus Thuringiensis Cry1ac Based on Fe3o4@ Au Nanoparticles. J. Agric. Food Chem. 2013, 61, 1435–1440. 10.1021/jf303774x. [DOI] [PubMed] [Google Scholar]
- Enpuku K.; Soejima K.; Nishimoto T.; Matsuda T.; Tokumitsu H.; Tanaka T.; Yoshinaga K.; Kuma H.; Hamasaki N. Biological Immunoassays without Bound/Free Separation Utilizing Magnetic Marker and Hts Squid. IEEE Trans. Appl. Supercond. 2007, 17, 816–819. 10.1109/TASC.2007.897699. [DOI] [Google Scholar]
- Chemla Y. R.; Grossman H. L.; Poon Y.; McDermott R.; Stevens R.; Alper M. D.; Clarke J. Ultrasensitive Magnetic Biosensor for Homogeneous Immunoassay. Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 14268–14272. 10.1073/pnas.97.26.14268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.; Shin T.-H.; Cheon J.; Weissleder R. Recent Developments in Magnetic Diagnostic Systems. Chem. Rev. 2015, 115, 10690–10724. 10.1021/cr500698d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.-T.; Kolhatkar A. G.; Zenasni O.; Xu S.; Lee T. R. Biosensing Using Magnetic Particle Detection Techniques. Sensors 2017, 17, 2300. 10.3390/s17102300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan S.; Mani V.; Wasalathanthri D.; Kumar C. V.; Rusling J. F. Attomolar Detection of a Cancer Biomarker Protein in Serum by Surface Plasmon Resonance Using Superparamagnetic Particle Labels. Angew. Chem., Int. Ed. 2011, 50, 1175–1178. 10.1002/anie.201005607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed I. H.; Huang X.; El-Sayed M. A. Surface Plasmon Resonance Scattering and Absorption of Anti-Egfr Antibody Conjugated Gold Nanoparticles in Cancer Diagnostics: Applications in Oral Cancer. Nano Lett. 2005, 5, 829–834. 10.1021/nl050074e. [DOI] [PubMed] [Google Scholar]
- Liang R. P.; Yao G. H.; Fan L. X.; Qiu J. D. Magnetic Fe3o4@Au Composite-Enhanced Surface Plasmon Resonance for Ultrasensitive Detection of Magnetic Nanoparticle-Enriched Alpha-Fetoprotein. Anal. Chim. Acta 2012, 737, 22–28. 10.1016/j.aca.2012.05.043. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Sun Y.; Wang J.; Zhang J.; Zhang H.; Zhou H.; Song D. Preparation and Application of Novel Nanocomposites of Magnetic-Au Nanorod in SPR Biosensor. Biosens. Bioelectron. 2012, 34, 137–143. 10.1016/j.bios.2012.01.032. [DOI] [PubMed] [Google Scholar]
- Wang J.; Song D.; Zhang H.; Zhang J.; Jin Y.; Zhang H.; Zhou H.; Sun Y. Studies of Fe3o4/Ag/Au Composites for Immunoassay Based on Surface Plasmon Resonance Biosensor. Colloids Surf., B 2013, 102, 165–170. 10.1016/j.colsurfb.2012.08.040. [DOI] [PubMed] [Google Scholar]
- Wu J.; Peng Y.; Liu X.; Li W.; Tang S. Evaluation of Wondfo Rapid Diagnostic Kit (Pf-Hrp2/Pan-Pldh) for Diagnosis of Malaria by Using Nano-Gold Immunochromatographic Assay. Acta Parasitol. 2014, 59, 267–271. 10.2478/s11686-014-0238-y. [DOI] [PubMed] [Google Scholar]
- Lee K. S.; Lee M.; Byun K. M.; Lee I. S. Surface Plasmon Resonance Biosensing Based on Target-Responsive Mobility Switch of Magnetic Nanoparticles under Magnetic Fields. J. Mater. Chem. 2011, 21, 5156–5162. 10.1039/c0jm03770b. [DOI] [Google Scholar]
- Kim D.; Marchetti F.; Chen Z.; Zaric S.; Wilson R. J.; Hall D. A.; Gaster R. S.; Lee J. R.; Wang J.; Osterfeld S. J.; Yu H.; White R. M.; Blakely W. F.; Peterson L. E.; Bhatnagar S.; Mannion B.; Tseng S.; Roth K.; Coleman M.; Snijders A. M.; Wyrobek A. J.; Wang S. X. Nanosensor Dosimetry of Mouse Blood Proteins after Exposure to Ionizing Radiation. Sci. Rep. 2013, 3, 2234. 10.1038/srep02234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterfeld S. J.; Yu H.; Gaster R. S.; Caramuta S.; Xu L.; Han S. J.; Hall D. A.; Wilson R. J.; Sun S.; White R. L.; Davis R. W.; Pourmand N.; Wang S. X. Multiplex Protein Assays Based on Real-Time Magnetic Nanotag Sensing. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 20637–20640. 10.1073/pnas.0810822105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng E.; Nadeau K. C.; Wang S. X. Giant Magnetoresistive Sensor Array for Sensitive and Specific Multiplexed Food Allergen Detection. Biosens. Bioelectron. 2016, 80, 359–365. 10.1016/j.bios.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Mak A. C.; Osterfeld S. J.; Yu H.; Wang S. X.; Davis R. W.; Jejelowo O. A.; Pourmand N. Sensitive Giant Magnetoresistive-Based Immunoassay for Multiplex Mycotoxin Detection. Biosens. Bioelectron. 2010, 25, 1635–1639. 10.1016/j.bios.2009.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X.; Ho D.; Lacroix L. M.; Xiao J. Q.; Sun S. Magnetic Nanoparticles for Magnetoresistance-Based Biodetection. IEEE Trans. Nanobioscience 2012, 11, 46–53. 10.1109/TNB.2011.2176509. [DOI] [PubMed] [Google Scholar]
- Orlov A. V.; Khodakova J. A.; Nikitin M. P.; Shepelyakovskaya A. O.; Brovko F. A.; Laman A. G.; Grishin E. V.; Nikitin P. I. Magnetic Immunoassay for Detection of Staphylococcal Toxins in Complex Media. Anal. Chem. 2013, 85, 1154–1163. 10.1021/ac303075b. [DOI] [PubMed] [Google Scholar]
- Orlov A. V.; Bragina V. A.; Nikitin M. P.; Nikitin P. I. Rapid Dry-Reagent Immunomagnetic Biosensing Platform Based on Volumetric Detection of Nanoparticles on 3D Structures. Biosens. Bioelectron. 2016, 79, 423–429. 10.1016/j.bios.2015.12.049. [DOI] [PubMed] [Google Scholar]
- Krishna V. D.; Wu K.; Perez A. M.; Wang J. P. Giant Magnetoresistance-Based Biosensor for Detection of Influenza A Virus. Front. Microbiol. 2016, 7, 400. 10.3389/fmicb.2016.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K.; Klein T.; Krishna V. D.; Su D.; Perez A. M.; Wang J.-P. Portable GMR Handheld Platform for the Detection of Influenza A Virus. ACS Sens. 2017, 2 (11), 1594–1601. 10.1021/acssensors.7b00432. [DOI] [PubMed] [Google Scholar]
- Jonkheijm P.; Weinrich D.; Schröder H.; Niemeyer C. M.; Waldmann H. Chemical Strategies for Generating Protein Biochips. Angew. Chem., Int. Ed. 2008, 47, 9618–9647. 10.1002/anie.200801711. [DOI] [PubMed] [Google Scholar]
- Jia F.; Narasimhan B.; Mallapragada S. Materials-Based Strategies for Multi-Enzyme Immobilization and Co-Localization: A Review. Biotechnol. Bioeng. 2014, 111, 209–222. 10.1002/bit.25136. [DOI] [PubMed] [Google Scholar]
- Srinivasan B.; Li Y.; Jing Y.; Xing C.; Slaton J.; Wang J. P. A Three-Layer Competition-Based Giant Magnetoresistive Assay for Direct Quantification of Endoglin from Human Urine. Anal. Chem. 2011, 83, 2996–3002. 10.1021/ac2005229. [DOI] [PubMed] [Google Scholar]
- Eberbeck D.; Bergemann C.; Wiekhorst F.; Steinhoff U.; Trahms L. Quantification of Specific Binding of Biomolecules by Magnetorelaxometry. J. Nanobiotechnol. 2008, 6, 4. 10.1186/1477-3155-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznik G. O.; Vajda S.; Cantor C. R.; Sano T. A Streptavidin Mutant Useful for Directed Immobilization on Solid Surfaces. Bioconjugate Chem. 2001, 12, 1000–1004. 10.1021/bc015507t. [DOI] [PubMed] [Google Scholar]
- Ylikotila J.; Valimaa L.; Takalo H.; Pettersson K. Improved Surface Stability and Biotin Binding Properties of Streptavidin Coating on Polystyrene. Colloids Surf., B 2009, 70, 271–277. 10.1016/j.colsurfb.2008.12.042. [DOI] [PubMed] [Google Scholar]
- Lee H.; Lee E.; Kim D. K.; Jang N. K.; Jeong Y. Y.; Jon S. Antibiofouling Polymer-Coated Superparamagnetic Iron Oxide Nanoparticles as Potential Magnetic Resonance Contrast Agents for in Vivo Cancer Imaging. J. Am. Chem. Soc. 2006, 128, 7383–7389. 10.1021/ja061529k. [DOI] [PubMed] [Google Scholar]
- Jon S.; Seong J.; Khademhosseini A.; Tran T-N T.; Laibinis P. E.; Langer R. Construction of Nonbiofoluing Surfaces by Polymeric Self-Assembled Monolayers. Langmuir 2003, 19, 9989–9993. 10.1021/la034839e. [DOI] [Google Scholar]
- Zhang Y.; Kohler N.; Zhang M. Surface Modification of Superparamagnetic Magnetite Nanoparticles and Their Intracellular Uptake. Biomaterials 2002, 23, 1553–1561. 10.1016/S0142-9612(01)00267-8. [DOI] [PubMed] [Google Scholar]
- Urena-Benavides E. E.; Lin E. L.; Foster E. L.; Xue Z.; Ortiz M. R.; Fei Y.; Larsen E. S.; Kmetz A. A. II; Lyon B. A.; Moaseri E.; Bielawski C. W.; Pennell K. D.; Ellison C. I.; Johnston K. P. Low Adsorption of Magnetite Nanoparticles with Uniform Polyelectolyte Coatings in Concentrated Brine on Model Silica and Sandstone. Ind. Eng. Chem. Res. 2016, 55, 1522–1532. 10.1021/acs.iecr.5b03279. [DOI] [Google Scholar]
- Yang M.; Guan Y.; Yang Y.; Xia T.; Xiong W.; Wang N.; Guo C. Peroxidase-Like Activity of Amino-Functionalized Magnetic Nanoparticles and Their Applications in Immunoassay. J. Colloid Interface Sci. 2013, 405, 291–295. 10.1016/j.jcis.2013.04.045. [DOI] [PubMed] [Google Scholar]
- Yang J.; Lee C. H.; Ko H. J.; Suh J. S.; Yoon H. G.; Lee K.; Huh Y. M.; Haam S. Multifunctional Magneto-Polymeric Nanohybrids for Targeted Detection and Synergistic Therapeutic Effects on Breast Cancer. Angew. Chem. 2007, 119, 8992–8995. 10.1002/ange.200703554. [DOI] [PubMed] [Google Scholar]
- Pan J.; Yang Q. Antibody-Functionalized Magnetic Nanoparticles for the Detection of Carcinoembryonic Antigen Using a Flow-Injection Electrochemical Device. Anal. Bioanal. Chem. 2007, 388, 279–286. 10.1007/s00216-007-1224-0. [DOI] [PubMed] [Google Scholar]
- Kandimalla V. B.; Neeta N. S.; Karanth N. G.; Thakur M. S.; Roshini K. R.; Rani B. E. A.; Pasha A.; Karanth N. G. K. Regeneration of Ethyl Parathion Antibodies for Repeated Use in Immunosensor: A Study on Dissociation of Antigens from Antibodies. Biosens. Bioelectron. 2004, 20 (4), 903–906. 10.1016/j.bios.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Teramura Y.; Arima Y.; Iwata H. Surface Plasmon Resonance-Based Highly Sensitive Immunosensing for Brain Natriuretic Peptide Using Nanobeads for Signal Amplification. Anal. Biochem. 2006, 357, 208–215. 10.1016/j.ab.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Soelberg S. D.; Stevens R. C.; Limaye A. P.; Furlong C. E. Surface Plasmon Resonance Detection Using Antibody-Linked Magnetic Nanoparticles for Analyte Capture, Purification, Concentration, and Signal Amplification. Anal. Chem. 2009, 81, 2357–2363. 10.1021/ac900007c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Dostalek J.; Knoll W. Magnetic Nanoparticle-Enhanced Biosensor Based on Grating-Coupled Surface Plasmon Resonance. Anal. Chem. 2011, 83, 6202–6207. 10.1021/ac200751s. [DOI] [PubMed] [Google Scholar]
- Wang J.; Zhu Z.; Munir A.; Zhou H. S. Fe3o4 Nanoparticles-Enhanced Spr Sensing for Ultrasensitive Sandwich Bio-Assay. Talanta 2011, 84, 783–788. 10.1016/j.talanta.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Wang L.; Sun Y.; Wang J.; Wang J.; Yu A.; Zhang H.; Song D. Preparation of Surface Plasmon Resonance Biosensor Based on Magnetic Core/Shell Fe3O4/SiO2 and Fe3O4/Ag/SiO2 Nanoparticles. Colloids Surf., B 2011, 84, 484–490. 10.1016/j.colsurfb.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Wang J.; Sun Y.; Wang L.; Zhu X.; Zhang H.; Song D. Surface Plasmon Resonance Biosensor Based on Fe3o4/Au Nanocomposites. Colloids Surf., B 2010, 81, 600–606. 10.1016/j.colsurfb.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Zamfir L.-G.; Geana I.; Bourigua S.; Rotariu L.; Bala C.; Errachid Al; Jaffrezic-Renault N. Highly Sensitive Label-Free Immunosensor for Ochratoxin a Based on Funcionalizaed Magnetic Nanoparticles and Eis/Spr Detection. Sens. Actuators, B 2011, 159, 178–184. 10.1016/j.snb.2011.06.069. [DOI] [Google Scholar]
- Liu X.; Hu Y.; Zheng S.; Liu Y.; He Z.; Luo F. Surface Plasmon Resonance Immunosensor for Fast, Highly Sensitive, and in Situ Detection of the Magnetic Nanoparticles-Enriched Salmonella Enteritidis. Sens. Actuators, B 2016, 230, 191–198. 10.1016/j.snb.2016.02.043. [DOI] [Google Scholar]
- Dittmer W. U.; de Kievit P.; Prins M. W.; Vissers J. L.; Mersch M. E.; Martens M. F. Sensitive and Rapid Immunoassay for Parathyroid Hormone Using Magnetic Particle Labels and Magnetic Actuation. J. Immunol. Methods 2008, 338, 40–46. 10.1016/j.jim.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Li Y.; Srinivasan B.; Jing Y.; Yao X.; Hugger M. A.; Wang J. P.; Xing C. Nanomagnetic Competition Assay for Low-Abundance Protein Biomarker Quantification in Unprocessed Human Sera. J. Am. Chem. Soc. 2010, 132, 4388–4392. 10.1021/ja910406a. [DOI] [PubMed] [Google Scholar]