Abstract
While the formulation of nanoparticle (NP) suspensions has been widely applied in materials and life science, the recovery of NPs from such a suspension into a solid state is practically important to confer long-term storage stability. However, solidification, while preserving the original nanoscale properties, remains a formidable challenge in the pharmaceutical and biomedical applications of NPs. Herein we combined flash nanoprecipitation (FNP) and spray-drying as a nanofabrication platform for NP formulation and recovery without compromising the dissolution kinetics of the active ingredient. Clofazimine was chosen to be the representative drug, which has been recently repurposed as a potential treatment for cryptosporidiosis. Clofazimine was encapsulated in NPs with low-cost surface coatings, hypromellose acetate succinate (HPMCAS) and lecithin, which were required by the ultimate application to global health. Spray-drying and lyophilization were utilized to produce dried powders with good long-term storage stability for application in hot and humid climatic zones. The particle morphology, yield efficiency, drug loading, and clofazimine crystallinity in the spray-dried powders were characterized. The in vitro release kinetics of spray-dried NP powders were compared to analogous dissolution profiles from standard lyophilized NP samples, crystalline clofazimine powder, and the commercially available formulation Lamprene. The spray-dried powders showed a supersaturation level of up to 60 times the equilibrium solubility and remarkably improved dissolution rates. In addition, the spray-dried powders with both surface coatings showed excellent stability during aging studies with elevated temperature and humidity, in view of the dissolution and release in vitro. Considering oral delivery for pediatric administration, the spray-dried powders show less staining effects with simulated skin than crystalline clofazimine and may be made into minitablets without additional excipients. These results highlight the potential of combining FNP and spray-drying as a feasible and versatile platform to design and rapidly recover amorphous NPs in a solid dosage form, with the advantages of satisfactory long-term storage stability, low cost, and easy scalability.
Keywords: clofazimine, nanoparticles, flash nanoprecipitation, hypromellose acetate succinate, lecithin, spray-drying, storage stability
1. Introduction
Oral ingestion is the most convenient and commonly employed route of drug delivery because of the ease of administration, patient compliance, and flexibility in the design of the dosage form.1 Currently, up to 40% of new chemical entities discovered by the pharmaceutical industry are hydrophobic compounds in Biopharmaceutics Classification System class II, with low solubility.2,3 The low-solubility issue leads to inadequate and variable bioavailability, requiring very high dosing or multiple-dose treatment to achieve the desired concentration in systemic circulation and, therefore, the desired pharmacological response.4,5 Hence, a key goal in poorly soluble drug formulation is solubility enhancement, which, in turn, affords improved bioavailability and feasible dosage administration. Nanoparticle (NP) formulations can improve the bioavailability of hydrophobic drugs through two mechanisms. First, NPs can be formed via rapid precipitation processes to trap drug molecules in an amorphous state. The amorphous state results in a solubility of as much as 1000 times higher that of crystalline states.6,7 Second, the greater surface-to-volume ratio for NPs versus large drug crystals enhances the dissolution kinetics.
However, successful NP formation overcomes only the first challenge in the path to a successful oral dosage form. Equally important is the recovery of the NPs into a dry, solid form without compromising the enhanced dissolution kinetics and bioavailability. Common techniques for solvent removal include lyophilization (Lyo), salt flocculation, and spray-drying (SD).8 Lyo requires extremely long processing time, and its high cost generally makes it a last-resort solidification process. Additionally, the increase of the particle concentration and changes of the solvent compositions during freezing may induce various stresses that cause particle crystallization and growth. Salt flocculation, or flocculation initiated by an excipient to create a filterable solid that can be tray dried, has recently been advanced as a new method of making redispersible powders.8,9 In contrast, SD is a one-step, continuous, and scalable drying method and is therefore an attractive candidate for large-scale NP powder processing. Solidification is achieved by mixing a heated gas with an atomized liquid of fine droplets within a vessel, causing the solvent to evaporate quickly through direct contact. SD requires a shorter processing time than Lyo and ultrafiltration, with the cost of drying 30–50 times less than that of Lyo.10 Moreover, adjustment of the process parameters enables the direct manipulation of various particle properties, such as the powder size and morphology, which is particularly useful for meeting the specific requirements of various administration routes and delivery systems.11 Another advantage of SD is that it can be set up as an aseptic process for Good Manufacturing Practice production (GMP), with the use of filters on the gas flow, sterilization of the nozzle and chamber walls, and fast product collection.12
While SD of pharmaceutical solutions to produce powders is widely practiced13 and some studies have addressed the inclusion of NPs in the solvent phase,14−16 little is available to guide in the selection of spray-dried excipients appropriate for different NP surface stabilizers. Here we report on the SD of preformed NPs as a means to obtain dried powders that display rapid drug dissolution kinetics. We specifically focus on clofazamine (Cfz), a drug that is effective against Cryptosporidium infections, which are the second major cause of infant mortality in sub-Saharan Africa.17,18 The absorption of Cfz is dissolution-limited, and solubility enhancement is the focus of the NP formulation development. We previously showed that NPs stabilized by zein, hypromellose acetate succinate, or lecithin enhanced the dissolution of Cfz to high levels of supersaturation following Lyo,19 while in the current work, we particularly aim to explore the rapid recovery of drug-loaded NPs with an appropriate SD protocol, to maintain the fast dissolution kinetics. Furthermore, we have also demonstrated the potential of spray-dried NP powders as pediatric formulations in the field of global health.
Flash nanoprecipitation (FNP), a stabilizer-directed rapid precipitation process, is used to produce nanometer-sized particles. In FNP, amphiphilic stabilizers and hydrophobic drugs are molecularly dissolved in an organic phase and mixed rapidly with an antisolvent stream to drive controlled precipitation with tunable particle size (∼50–500 nm).20 In this study, instead of using a synthetic diblock copolymer, we performed FNP using Cfz and two stabilizers, HPMCAS and lecithin. After the NP formulation, Lyo and SD were both optimized to obtain dried NP powders, in order to produce a solid oral dosage form with high tolerance to temperature and humidity. The dried powders were further tested in simulated gastric and intestinal fluids for in vitro release to investigate the effect of different surface stabilizers and drying pathways on the dissolution kinetics of Cfz. Long-term stability studies were then performed with the spray-dried powders to demonstrate the resistance to degradation in harsh conditions. In addition, we examined both the spray-dried powders’ skin staining effects and compressibility into minitablets; both of these factors are relevant to the powders’ applicability as a pediatric administration in sub-Saharan Africa.
2. Materials and Methods
Materials
Cfz (Figure 1a), sucrose, trehalose, mannitol, and all solvents (HPLC grade) were purchased from Sigma-Aldrich (Milwaukee, WI) and used as received. Affinisol hypromellose acetate succinate (HPMCAS; USP grade) and MethocelTM HPMC E3 (viscosity of 2.4–3.6 mPa·s at 2% solution in water at 20 °C; Tg = 174 °C) were gifts from Dow Chemical Company (Midland, MI). l-α-Lecithin was purchased from Fisher Scientific (Waltham, MA). FaSSIF/FeSSIF/FaSSGF and FeSSIF-V2 powders were purchased from Biorelevant.com Ltd. (London, U.K.). Deionized (DI) water (18.2 MΩ·cm) was prepared by a NANOpure Diamond UV ultrapure water system (Barnstead International, Dubuque, IA).
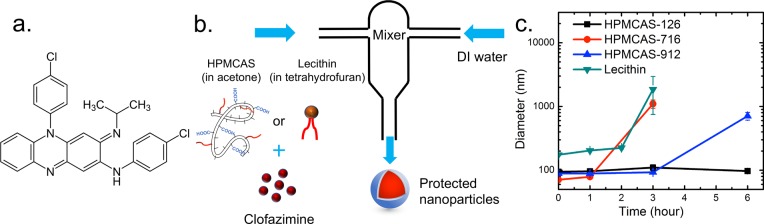
Figure 1.
(a) Chemical formula of clofazimine. (b) Schematic setup of the protected NPs made with the CIJ mixer. (c) Size stability of clofazimine NPs with various surface coatings in 10 vol % organics (HPMCAS-126, black ■; HPMCAS-716, red ●; HPMCAS-912, blue ▲; lecithin, cyan ▼).
NP Formulation and Characterization
NPs were created via FNP first using a CIJ jet mixer (Figure 1b) to optimize the formulations. Briefly, an organic stream of either acetone (for HPMCAS) or tetrahydrofuran (THF; for lecithin) with molecularly dissolved Cfz and stabilizers was rapidly mixed against a DI water stream into the mixing chamber of a CIJ mixer in a 1:1 volume ratio. The concentrations in the organic stream were 5 mg/mL Cfz and 5 mg/mL HPMCAS for the HPMCAS formulation and 10 mg/mL Cfz and 5 mg/mL lecithin for the lecithin formulation. The resulting mixed stream was collected in a quenching DI water bath to drop the final organic concentration to 10 vol %. NP formulations for SD were produced using a multi-inlet vortex mixer (MIVM; Figure 2a). The MIVM’s four-inlet geometry allows higher supersaturation during mixing than the two-inlet CIJ mixer and bypasses the secondary quenching step;21 thus, this mixer is preferred for the continuous and scalable production of NPs. In the MIVM setup, one organic stream containing Cfz and stabilizers and three other water streams were mixed together, with a volumetric flow rate of organic:water = 1:3, making the final organic concentration 10 vol %. Therefore, no further quenching is necessary to reach the same dilution as the CIJ formulations. All NP suspensions for the SD process were prepared from the MIVM. For the HPMCAS formulation, the concentration in the organic stream was 5 mg/mL for both Cfz and the stabilizer, with a total flow rate of 160 mL/min into the MIVM. For the lecithin formulation, the concentration in the organic stream was 50 mg/mL for Cfz and 25 mg/mL for lecithin, with a total flow rate of 120 mL/min for the MIVM. Accounting for the dimensions of the MIVM, the effective Reynolds numbers22 are 11280 and 7970 for HPMCAS and lecithin, respectively [see the Supporting Information (SI) for detailed calculations]. The NP diameter was determined from triplicate experiments by dynamic light scattering (DLS) at 25 °C with a Zetasizer Nano-ZS (Malvern Instruments, Southboro, MA), using a detection angle of 173°. DLS data were processed with Malvern’s software using a distribution analysis. The average size was given based on a cumulant model. The cumulant analysis is defined in International Organization for Standardization (ISO) standard document 13321. On the other hand, the intensity-weighted size distribution was obtained from a distribution analysis of the “General Purpose Mode” provided by the software.
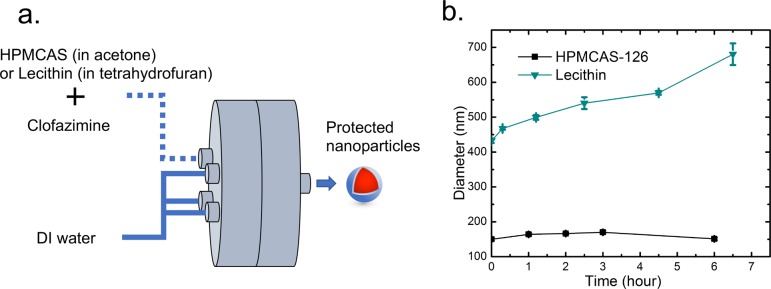
Figure 2.
(a) Schematic setup of the protected NPs made with a MIVM for the SD process. (b) Size stability of clofazimine NPs generated by a MIVM with HPMCAS-126 and lecithin in 10 vol % organics (HPMCAS-126, black ■; lecithin, cyan ▼).
NP Lyo
Lyo was carried out using a benchtop VirTis Advantage (Gardiner, NY) with cryoprotectants (i.e., mannitol or HPMC E3). NP solutions (0.5 mL) were mixed with 0.1 mL cryoprotectant solutions at different concentrations to afford various final NP/cryoprotectant mass ratios of up to 1:30. The mixtures were then flash-frozen by fast immersion in a dry ice/acetone cooling bath (−78 °C) for 1 min with mild agitation. The frozen samples were then immediately transferred to the lyophilizer with the shelf temperature at −20 °C under vacuum (<1 × 10–3 bar). After 2 days, the dried powders were removed, sealed, and stored at −20 °C. The effects of the different NP/cryoprotectant mass ratios were examined by reconstituting lyophilized NP powders in DI water at room temperature and subsequently analyzing by DLS. Sonication assistance was used when necessary to disperse powders for DLS measurements.
NP SD
A mini spray-drier B-290 (Büchi Corp., New Castle, DE), equipped with a two-fluid nozzle, was used for drying the NP suspension. The nozzle consists of a tip and a cap with diameters of 0.7 and 1.5 mm, respectively, and the drier is equipped with a high-performance cyclone provided by Büchi Corp. Compressed nitrogen at 80 kPa was used to disperse the liquid into droplets, with the flow rate controlled by a rotameter. The use of nitrogen also ensured that the oxygen concentration was below the explosive limits. The NP suspension was mixed with excipients and then fed by a peristaltic pump into the spray-drier. The spray-drier was run in an open mode, in which the outlet gas was vented into an exhaust hood after passing the outlet filter. SD parameters, including the inlet temperature, outlet temperature, drying gas flow rate, liquid feed rate, and gas flow rate of the aspirator were optimized for each NP suspension. After SD, only the powders at the bottom of the collector were considered as the spray-dried samples, and no further collection from the cyclone wall was performed. Then spray-dried powders were collected in scintillation vials, sealed, and stored in a vacuum desiccator at room temperature (20 °C) before use. To determine the particle size, the powders were deposited on a microscope slide and observed under a bright-field microscope (Nikon Eclipse E200, Minato, Tokyo, Japan). The loading capacity (LC) and mass yield efficiency (YE) were calculated with the following equations:
Karl Fischer Analysis for Moisture Content
The powder moisture content was measured using a V20S Compact volumetric KF titrator (Mettler Toledo, Columbus, OH). An Aquastar Titrant 5 and an Aquastar Combimethanol (EMD Millipore, Burlington, MA) were used as titrants with two-component reagents and solvent, respectively. A quantity of the sample powder (∼20–30 mg) was weighed accurately before being transferred quickly to the titration vessel to prevent moisture absorption or desorption. The mixture was stirred for 5 min before initiating the automatic titration process. The water content results obtained by the volumetric KF titrator were expressed in percent (w/w).
Powder X-ray Diffraction (PXRD)
PXRD was performed using a Bruker D8 Advance diffractometer with Ag Kα radiation (λ = 0.56 Å) and a LynxEye-Xe detector. For each test, ∼10 mg of powder sample was loaded into a polyimide capillary with an inner diameter of 1 mm that was sealed at both ends with quick setting epoxy and mounted on a capillary stage, which rotates at a speed of 60 rpm during operation. Data were collected, over a 2θ range of 3–20° (corresponding to a Cu Kα 2θ value of 8.2–57.0°), with a step size of 0.025° (0.067° for Cu Kα radiation) and a count rate of 5 s/step. Note that, in the following discussion, all PXRD results are presented with 2θ value corresponding to Cu Kα radiation.
Differential Scanning Calorimetry (DSC)
A TA Instruments Q200 (New Castle, DE) was used for all DSC measurements. Dried samples (5–10 mg) were placed in hermetically sealed aluminum pans and equilibrated at 20 °C under a dry N2 atmosphere (50 mL/min). Subsequently, the samples were heated from 20 to 250 °C at a heating rate of 5 °C/min. The scan was analyzed by TA Instruments Universal Analysis 2000 software.
Dissolution Test
The following in vitro dissolution test was performed to investigate the release kinetics of the samples. Fasted-state simulated gastric fluid (FaSSGF) and fasted/fed-state intestinal fluids (FaSSIF and FeSSIF) were prepared following the manufacturer’s instructions. Triplicate experiments were performed for each formulation with a release medium swap assay. In addition, dissolution tests were also evaluated with free Cfz powders and Lamprene as controls. For release under gastric conditions, dried samples were resuspended in prewarmed FaSSGF (37 °C) to achieve a drug concentration of 75 μg/mL by pipetting up and down vigorously multiple times. The samples were incubated at 37 °C (NesLab RTE-111 bath circulator, Thermo Fisher Scientific, Waltham, MA) for 30 min without agitation to mimic physiological gastric conditions and transition time in the stomach. Because the particles remained small and therefore Brownian motion kept them well dispersed, the effect of gastric mixing was not considered. Aliquots were taken at 1, 5, 10, 15, 20, and 30 min. Each aliquot was centrifuged at 20800g for 5 min to pellet NPs. The Cfz concentration in the supernatant was determined by UV–vis spectroscopy at 491 nm and a calibration curve. For release under intestinal conditions, after passing through the FaSSGF protocol, the solutions were further diluted 10 times with 1.1× FaSSIF (pH = 6.5) or FeSSIF (pH = 5.8), resulting in a final Cfz concentration lower than its solubility limit in both buffers. Aliquots were taken at 15, 30, 45, 60, 120, 240, and 360 min and were centrifuged at 28000g for 10 min. We note that these centrifugation conditions provide complete separation of the NPs from the supernatant, as confirmed by the lack of a DLS signal in the supernatant after centrifugation. The supernatant was then analyzed by UV–vis spectroscopy at 491 nm, and the Cfz concentration was calculated based on a calibration curve. All of the time points are defined as the incubation time from the assay start to the sampling.
Long-Term Storage Stability
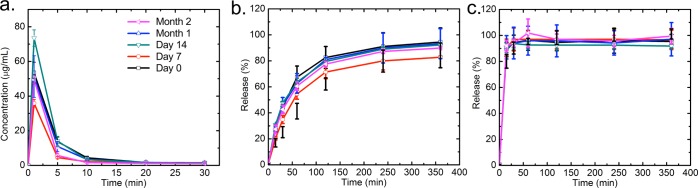
To determine the long-term stability of the spray-dried NPs under high temperature and humidity, the powders were placed in a capped scintillation vial, which was then placed in a desiccator (Nalgene, Thermo Fisher Scientific, Waltham, MA). The desiccator was sealed using high-vacuum grease (Dow Corning, Dow Chemical Company, Midland, MI), and the internal humidity was controlled using a beaker of a saturated aqueous solution of sodium chloride (>0.366 g/mL). The desiccator was placed inside a gravity convection general incubator (VWR, Radnor, PA) with a temperature control. The conditions inside the desiccator were kept as 40 °C and 70% relative humidity (RH), which was continuously monitored with a hygro/thermometer in the desiccator and a wireless reader outside the incubator (Traceable, Thermo Fisher Scientific, Waltham, MA). The dissolution tests described above were performed at day 0, 7, and 14 and month 1 and 2 to monitor the release kinetics of the powders.
Staining Test
A polyurethane membrane (Sigma-Aldrich, St. Louis, MO) was cut into 2 cm × 2 cm strips and adhered to the bottom of Fisherbrand Petri dishes (Fischer Scientific, Hampton, NH). NP powders were suspended in DI water at a concentration equivalent to that of Cfz at 3.33 mg/mL at room temperature for 30 min. A 1 mL aliquot of the NP suspension was then applied on top of the polyurethane membrane followed by incubation for 5 min. The NPs were removed via pipetting, and the membrane was rinsed by 1 mL of DI water three times to remove any loosely attached NPs. To quantify the Cfz staining on the membrane, the polyurethane membrane was peeled off and Cfz was extracted with 2 mL of acetone. The Cfz concentration was analyzed by a UV–vis spectrophotometer at 450 nm (Evolution 300 UV–vis, Thermo Electron, Waltham, MA).
Compressed Minitablet Test
Flat-surfaced 3 mm minitablets were prepared by direct compression using a Gamlen Tablet Press (GTP-1, Gamlen Tableting, Ltd., Nottingham, U.K.). The die was manually filled with preweighed NP powders. The compression pressure corresponded to a load of 100 kg.
3. Results and Discussion
NP Formulations by CIJ and MIVM
HPMCAS is a cellulose ester bearing acetyl and succinyl groups, which is synthesized by functionalizing HPMC with a mixture of monosuccinic acid and acetic acid esters.23 The dissolution behavior of this polymer in different pH buffers may be tuned by changing the ratio of the succinoyl and acetyl moieties. HPMCAS has been widely used to create stable amorphous solid dispersions with poorly soluble active pharmaceutical ingredients.24,25 Although several studies examined HPMCAS as a carrier in hot-melt extrusion and SD, formulating NPs with HPMCAS as a surface stabilizer has received little attention. In the current study, three standard commercial GMP grades of HPMCAS, HPMCAS-126, -716, and -912, were investigated. They are differentiated by the ratio of acetyl and succinyl substituents on the HPMC backbone.
Our previous work on nanoprecipitation had used a synthetic diblock copolymer, where the hydrophobic domain anchors on the NP surface and the hydrophilic (e.g., PEG) domain sterically stabilizes the NP. It was unclear whether the randomly hydrophobic HPMCAS would create stable NPs or the hydrophobic blocks would cause bridging between NPs. Our initial hypothesis was that the most hydrophobic HPMCAS would lead to the most unstable NP formulation, but discrepancy was observed in the experiments. Regarding the stability of Cfz NPs prepared with CIJ as shown in Figure 1c, the HPMCAS-126 NPs were the most stable formulation with no size change for at least 6 h, while the size of the HPMCAS-716 NPs increased significantly from 70 nm to around 1 μm in 3 h. With the highest acetyl substitution level, HPMCAS-126 is the most hydrophobic of the three HPMCAS polymers. This cellulose derivative may interact with the Cfz core more strongly than HPMCAS-716 and -912, resulting in longer NP stability. Therefore, HPMCAS-126 was selected for the remainder of this study. A MIVM was further used to provide a large batch of NP suspensions for SD. Because of the different mixer geometries and mixing solvent ratios, the size of the HPMCAS-126 NPs increased to 150 nm from 90 nm in a CIJ but again remained constant for 6 h (Figure 2b).
Lecithin designates the group of phospholipid substances occurring in animal and plant tissues. It is an essential component of the cell membrane and has been widely utilized as a stabilizer to form NPs for controlled drug delivery.26l-α-Lecithin derived from soybean was used in the current study. For Lyo trials, lecithin NPs produced with a CIJ mixer were 175 nm in diameter, and that size was maintained for around 3 h (Figure 1b). When this formulation was tested to produce a spray-dried powder, low YE was observed. To increase the YE in SD, 2.5 times higher concentrations of Cfz and lecithin were used in the MIVM, which greatly improved the SD yield efficiency as descried below. These MIVM-generated lecithin NPs had an average initial diameter of 432 nm, which grew to 540 nm in 3 h and then 680 nm in 6 h (Figure 2b). Lecithin NPs have a shorter stability window compared with the HPMCAS NPs. Polymeric stabilizers, because of multivalent surface attachment and thicker steric layers, are often more effective than single-tailed small molecular surfactants.23 All of the drying processes were performed immediately after the NP formulation to minimize the influence of the size growth on the in vitro release experiments.
Lyo of Clofazimine NPs
Lyo, also known as freeze-drying, is generally considered to be the “gold standard” for obtaining redispersible solid powders from NP suspensions. However, large-scale Lyo is prohibitively expensive for drugs intended for global health. Lyo consists of the removal of solvents from a frozen sample by sublimation and desorption under vacuum.27 Stresses induced by freezing and dehydration, such as the mechanical force exercised by the crystallization of ice, may induce irreversible aggregation of the NPs. Therefore, cryoprotectants are commonly required to preserve the NP integrity.
Sucrose, trehalose, mannitol, and HPMC E3 were screened at different NPs/cryoprotectant mass ratios. These cryoprotectants were selected because they rely on a different mechanism of protection. During freezing, sucrose and trehalose form glass phases with high Tg, while mannitol forms crystal domains. Such a matrix of the cryoprotectant immobilizes the NPs, preventing their aggregation and protecting them against the mechanical stress of ice crystals.28 HPMC E3 was chosen because its cellulose backbone is compatible with that of HPMCAS, and the lack of hydrophobic substitution should enable it to coat the NPs without bridging between NPs. Upon reconstitution with water, considerably large particles (on the order of microns) were obtained for both Cfz-loaded HPMCAS and lecithin NPs with all cryoprotectants. Additionally, HPMCAS NPs with sugar cryoprotectants generated densely packed lyophilized cakes, while fluffy lyophilized cakes were formed when using HPMC E3 with a mass ratio as low as 1:0.5. Because the high porosity and open structure of such fluffy lyophilized cakes increased the surface area and therefore facilitated reconstitution, HPMC E3 was chosen as the favorable cryoprotectant for HPMCAS NPs. Further increasing the HPMC E3 concentrations only marginally reduced the particle size and polydispersity. To maximize the drug loading in the final formulation while maintaining good redispersibility, HPMCAS NPs with the HPMC E3 cryoprotectant at a mass ratio of 1:0.5 were used for the following dissolution tests. Lecithin NPs lyophilized with mannitol with a mass ratio of 1:3 produced a less dense lyophilized cake compared with other cryoprotectants19 and was thus chosen as the cryoprotectant for further study.
The difference in the optimal cryoprotectants for the two NP types is related to the differences between HPMCAS and lecithin as surface stabilizers. The hydrophobic substitution on HPMCAS makes it strongly interact with the hydrophobic Cfz core. However, it is also interactive with other HPMCAS molecules if forced into contact. For example, if the Cfz NP suspension is centrifuged to pellet the NPs, the resulting pellet did not redisperse back by the addition of water. HPMC E3 is a less hydrophobic cellulose that is miscible with HPMCAS; however, it has a relatively weak interaction with itself or HPMCAS-126. Therefore, HPMC E3 acts as a protective layer around the NPs, which prevents direct NP contact during Lyo, enabling redispersion. In contrast, the lecithin-stabilizing layer is a very thin, zwitterionic layer that stabilizes by electrostatic repulsion. In this case, the mannitol sugar crystallizes around the NP, resulting in a crystalline shell that prevents particle aggregation during drying.14,28
SD of Clofazimine NPs
Because SD is a continuous processing operation involving a combination of several stages, including atomization, mixing of the spray with the drying gas, evaporation, and production separation, it is important to optimize the operating conditions based on the quality requirements for the final product.29 For instance, the inlet gas temperature significantly affects the physical state of the drug, residual moisture content, and particle morphology.30 In addition, the excipients found to be the most successful for Lyo, HPMC E3 and mannitol, were added into the NP suspension after FNP and prior to SD. These act as a coating material protecting the NPs against oxidation and irreversible particle–particle aggregation during drying.
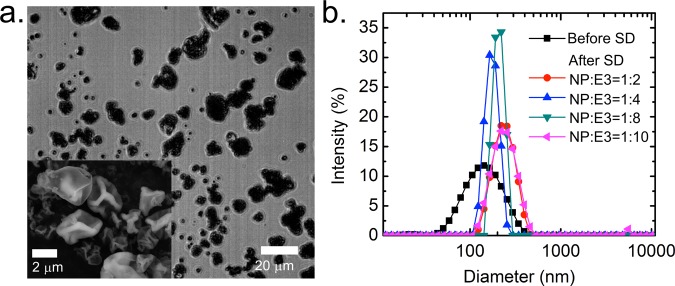
Table 1 summarizes the characterizations of the spray-dried powders, including the glass transition/melting temperature of the stabilizers, inlet gas temperature, particle diameter, LC, YE, residual moisture content, and redispersity in water. For HPMCAS NPs, the operating conditions were optimized to be an inlet temperature of 150 °C, a drying gas flow rate of 300 L/h (at the standard temperature and pressure), a feed rate of the NP suspension of 6 mL/min, and an aspirator setting of 90% (gas flow rate of 35 m3/h). Using microscopy, the volumetric diameters were determined to be d10% = 3.7 μm, d50% = 6.7 μm, and d90% = 9.1 μm based on image analysis for 200 particles. The outlet filter blocked all of these microparicles, and no individual NP was produced and released to the air during the SD. YEs for HPMCAS NPs were slightly lower than those of lecithin NPs because the dried powders were stickier and adhered more to the drying chamber of the spray-drier, resulting in a larger mass loss. On the other hand, the higher residual moisture content of spray-dried HPMCAS powders might come from the fact that the total solid concentration in the HPMCAS NP suspension was one-third of that in the lecithin NP suspension, so the HPMCAS powders were not completely dried because of the higher water content in the atomized droplets. Also, the morphology of the spray-dried powders was observed to be shriveled, instead of dense spheres (Figure 3a). Two characteristic times are critical in the drying process for the formation of such dimpled and hollow powders.15 The first is the time required for a droplet to dry, τdry, while the second is the time required for a solute or NP to diffuse from the surface of the droplet to its center, d2/4D, where d is the diameter of the droplet and D is the solute or NP diffusion coefficient. The ratio of these two characteristic times defines a dimensionless mass transport number as Pe = d2/(4Dτdry), which describes the relative importance of diffusion and convection dynamics. For the current SD condition, Pe ≈ O(10) for HPMC E3 and Pe ≈ O(103) for HPMCAS NPs (see the SI for detailed calculations). Therefore, the slower diffusion of the NPs allows them to accumulate on the free surface and form a shell during drying, but because the shell is further compressed by the large capillary forces of the shrinking droplet, the shell would buckle or fold, showing the final wrinkled morphology, as shown in Figure 3a.31
Table 1. Characterizations of Spray-Dried Powders with HPMCAS and Lecithin as the Stabilizers, Including the Glass Transition/Melting Temperature of the Stabilizers, SD Inlet Gas Temperature, SD Particle Diameter, LC, YE, Residual Moisture Content, and Redispersity in Water.
| sample | stabilizer Tg or Tm (°C) | Tinlet (°C) | diameter (d10%/d50%/d90%) (μm) | LC (wt %) | YE (wt %) | residual moisture (wt %) | size after redispersion |
|---|---|---|---|---|---|---|---|
| HPMCAS-126 NPs | Tg = 120a | 150 | 3.7/6.7/9.1 | 14.3 ± 0.2 vs 16.7% in formulation | 45 ± 9 | 3.1 ± 0.2 | 250 ± 5 nm |
| lecithin NPs | Tm = 45b | 125 | 3.5/5.9/8.4 | 17.8 ± 0.2 vs 16.7% in formulation | 60 ± 5 | 0.7 ± 0.1 | 1.5 ± 0.9 μm |
Figure 3.
(a) Bright-field microscopy image of the spray-dried HPMCAS NP powders (mass ratio of NP:HPMC E3 = 1:2). Inset: Scanning electron microscopy image of the powders. (b) Size distribution of the HPMCAS NP suspension before SD and the aqueous suspension after reconstituting spray-dried powders with DI water, for different mass ratios between NPs and HPMC E3 in the SD process.
Importantly, when the HPMCAS NPs were spray-dried along with HPMC E3 at a NP/HPMC E3 mass ratio of greater than 1:2, the resulting powders could be redispersed to the nanoscale size (Figure 3b). After SD, the microparticles were formed by the HPMCAS NPs in a polymeric matrix of HPMC E3. When redispersed with water, HPMC E3 was dissolved and the HPMCAS NPs were resuspended in the aqueous phase. Such an observation confirmed that the high-temperature SD did not induce a significant degree of aggregation.
We note that, with Lyo, the dried powders with the same mass ratio of excipient could not be readily redispersed to NPs with DI water and formed large microparticles instead, even upon sonication. For the HPMCAS NPs, it was unexpected that SD could give superior redispersion over Lyo.
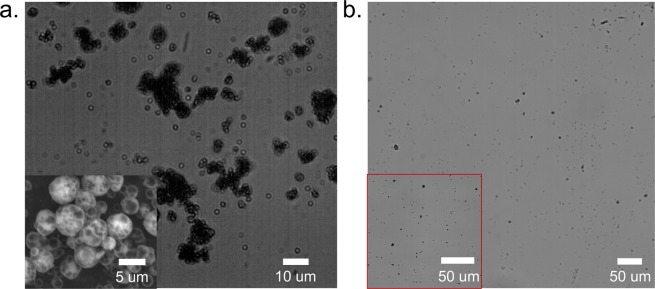
For lecithin NPs, the operating conditions were optimized to be an inlet temperature of 125 °C, a drying gas flow rate of 473 L/h (at the standard temperature and pressure), a feed rate of the NP suspension of 4 mL/min, and an aspirator setting of 90% (gas flow rate of 35 m3/h). The lower inlet temperature compared with HPMCAS was chosen because degradation of l-α-lecithin occurs at around 150 °C.19 The resulting powder’s volumetric diameter was determined to be d10% = 3.5 μm, d50% = 5.9 μm, and d90% = 8.4 μm based on image analysis for 200 particles (Figure 4a). Although Pe ≈ O(103) for lecithin NPs (see the SI for detailed calculations), the surface of the powder was much smoother compared with the case of the HPMCAS coating, indicating that mannitol was able to form a shell that could resist the capillary force during fast drying without buckling. In addition, it is known that mannitol added in the formulation promotes the formation of spherical and smooth-surfaced microparticles.32 Also, both the spray-dried and lyophilized lecithin powders could only be redispersed to micrometer-sized colloids, even when large amounts of mannitol were added as excipients (Figure 4b). Previously, we had shown that mannitol could be used as a spray-freeze-drying excipient for lecithin NPs and that the NPs could be redispersed from the powder.14 These results indicate that the much faster freezing conditions for spray-freeze-drying are required to prevent lecithin NP aggregation. However, as discussed below, the level of aggregation does not compromise the rapid dissolution of either lecithin formulation. Compared with polymeric HPMCAS, lecithin is a small-molecular-weight molecule with a short hydrophobic tail and a zwitterionic headgroup. Hence, it is expected that the thicker adsorbed polymer layer of HPMCAS is more effective at NP stabilization than lecithin, resulting in a better redispersity of spray-dried NPs stabilized by HPMCAS than lecithin.
Figure 4.
(a) Bright-field microscopy image of the spray-dried lecithin NP powders. Inset: Scanning electron microscopy image of the powders. The mass ratio between lecithin NPs and mannitol is 1:3. (b) Microscopy image of the aqueous suspension after reconstitution of spray-dried powders with DI water. Inset: Microscopy image with a larger magnification (40×).
PXRD and DSC
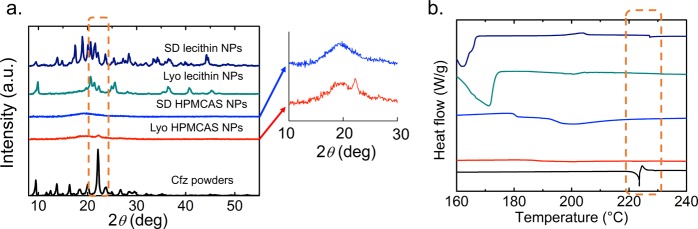
PXRD and calorimetric techniques have been used to identify the physical state of drug molecules in a complex polymeric matrix.33 They are useful to study changes in the crystallinity of the drug, which could be one of the mechanisms responsible for improved dissolution.34 With PXRD, the major diffraction peak in the signal of Cfz crystals appears at 2θ = 22.1° (Cu Kα radiation), which is in good agreement with previous studies.35 The reduction of the crystallinity of drugs encapsulated in the NPs was indicated by a decrease of the intensity, as well as peak broadening at 2θ = 22.1° for lyophilized and spray-dried NPs (highlighted by the dashed box in Figure 5a). In addition, DSC with Cfz crystals shows a sharp melting endotherm at 223.3 °C, followed by an exothermal peak due to degradation. However, the melting endotherms for all of the NP samples shifted to a lower temperature and had a broader peak width (highlighted by the dashed box in Figure 5b), confirming the lower crystallinity of the drug in the NPs. Hence, both PXRD and DSC results indicate a reduction in the level of crystallinity for Cfz encapsulated by FNP compared with raw Cfz powders. For HPMCAS coating, the PXRD signal of lyophilized NPs shows a relatively more pronounced peak at 2θ = 22.1° compared with spray-dried NPs, suggesting a higher crystallinity in the lyophilized powders. For the lecithin-coating NPs, new diffraction peaks for NPs appear as a result of crystallization of the excipient, mannitol, during the drying processes, which mask the Cfz peaks. Although it is difficult to directly compare the lyophilized and spray-dried lecithin samples using PXRD because mannitol is known to have different crystal states,36 the appearance of a small peak at around 227 °C in the DSC signal of the spray-dried lecithin NPs indicates a higher level of crystallinity compared with lyophilized lecithin NPs.
Figure 5.
(a) PXRD pattern and (b) heat flow from DSC for clofazimine powder and HPMCAS/lecithin NPs with different drying processes. Inset: Closeup of the PXRD patterns for spray-dried and lyophilized HPMCAS NPs.
Dissolution Tests
Pharmaceutical solid oral dosage forms must undergo dissolution in the intestinal fluids of the gastrointestinal tract before they can be absorbed and achieve the systemic circulation. Therefore, dissolution is a critical part of the drug-delivery process. The 24 h solubilities of Cfz in FaSSGF, FaSSIF, and FeSSIF were determined to be 0.36, 6.20, and 29.60 μg/mL, respectively. The enhanced solubilizing capacity of intestinal fluids compared with gastric fluids can be attributed to bile and pancreatic secretions and the presence of exogenous lipid products.37 We performed experiments to test the Cfz dissolution kinetics in vitro for powders using different stabilizers and drying processes following a previously established protocol.19
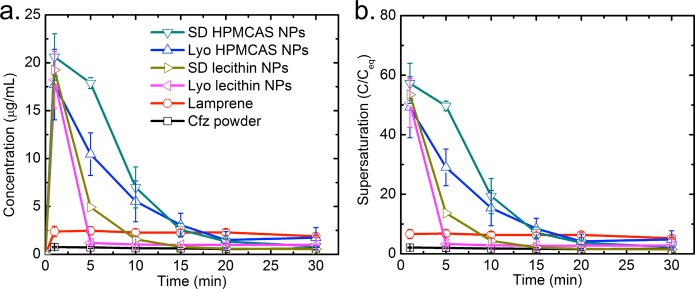
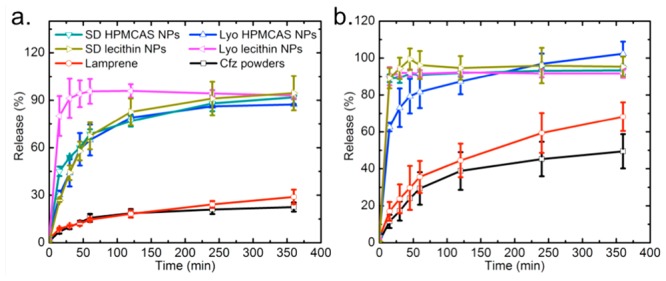
Lyophilized or spray-dried Cfz samples were dispersed in FaSSGF with an initial concentration of ∼208 times the equilibrium solubility of free Cfz powder. Cfz powders (raw drug crystalline) and the commercial product Lamprene were included as control samples. Lamprene is formulated as a microcrystalline suspension of Cfz in an oil-wax base in gelatin capsules. The evolution of the concentration of Cfz dissolved in FaSSGF from various samples during a 30 min exposure is shown in Figure 6, in which the supersaturation level is defined as the ratio between the dissolved Cfz concentration and the solubility of the Cfz crystals. The Cfz powder only reached the solubility limit of 0.36 μg/mL, while Lamprene showed a plateau at around 2.2 μg/mL (∼6× solubility) after 1 min of incubation. However, HPMCAS and lecithin NPs quickly achieved a much higher supersaturation right after dispersion, with the Cfz concentration up to 57× solubility for the case of spray-dried HPMCAS NPs. The supersaturation levels for the two coatings were not significantly different and were in the range of 49–57× solubility. The maximum supersaturation of lecithin NPs occurred within the time resolution of the first measurement, which was 1 min, while HPMCAS NPs showed a prolonged supersaturation; the latter can be explained by a previous study’s findings that HPMCAS inhibits crystallization from the supersaturated solution generated by dissolution of the amorphous material.38 Importantly, there was no significant difference in the supersaturation curves between SD and Lyo in the case of both HPMCAS and lecithin stabilizers, which indicates that the low levels of crystallinity of the encapsulated drugs were preserved well even in the high-temperature SD. The area under the curve (AUC) of Cfz over time for spray-dried NPs was ∼1.3 times that of Lyo for both HPMCAS and lecithin. Here AUC reflects the total drug exposure of Cfz in FaSSGF over the 30 min experimental period, and hence larger AUC demonstrates a higher bioavailability enhancement.
Figure 6.
(a) Dissolution kinetics and (b) supersaturation level of HPMCAS/lecithin NPs with different drying processes compared to the clofazimine powder and Lamprene in FaSSGF.
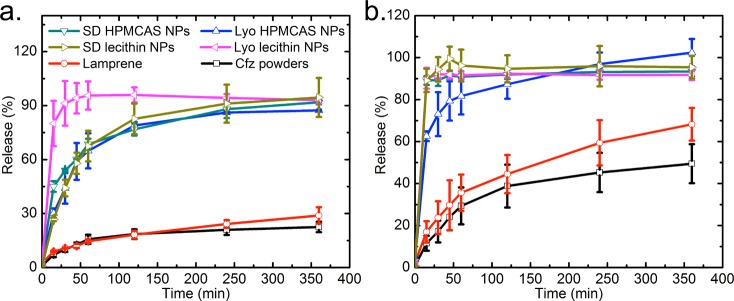
Next, after 30 min of incubation at 37 °C and pH = 1.6, an NP/gastric fluid solution was further diluted into FaSSIF and FeSSIF to simulate the fasted and fed conditions in the intestine. The release kinetics regarding the Cfz concentration at different time points are shown in Figure 7. Here the percentage of release is defined as the mass ratio between the dissolved drug and the total drug put into FaSSIF and FeSSIF. All samples show a faster dissolution rate in the fed state because the higher lecithin and bile salt concentration helps to solubilize the lipophilic drug.39 Within 6 h, the Cfz powder and Lamprene only demonstrated releases of 20–30% in FaSSIF and 50–70% in FeSSIF, while NP samples reached a much faster release in both simulated intestinal fluids, which is highly desirable because cryptosporidiosis infections mostly reside in the intestine.18 HPMCAS NPs have a slower release compared with lecithin NPs in these intestinal conditions. In simulated gastric conditions, the presence of a lecithin stabilizer may have modified the form of recrystallized Cfz, and, consequently, the drug was more susceptible to dissolution under intestinal fluid conditions. Compared with NPs solidified by Lyo, spray-dried NPs displayed similar fast-release behavior. The faster release of spray-dried HPMCAS NPs in FeSSIF demonstrates a lower crystallinity of Cfz encapsulated in spray-dried powders than in lyophilized NPs, as confirmed by the redispersity and PXRD observation (Figure 5a). On the other hand, consistent with the DSC results for lyophilized and spray-dried lecithin NPs, spray-dried lecithin NPs show a slower dissolution rate in FaSSIF because of the negative impacts of temperature elevation during SD for lecithin coating, but the release was still much faster than those of the Cfz powder and Lamprene. Therefore, a recovery process based on scalable and cost-effective SD is a viable route to maintaining the dissolution properties of NPs, which is crucial to enable easy access of the therapeutics to targeted patients in developing countries.
Figure 7.
Dissolution kinetics of HPMCAS/lecithin NPs with different drying processes compared to the clofazimine powder and Lamprene in (a) FaSSIF and (b) FeSSIF.
Long-Term Storage Stability
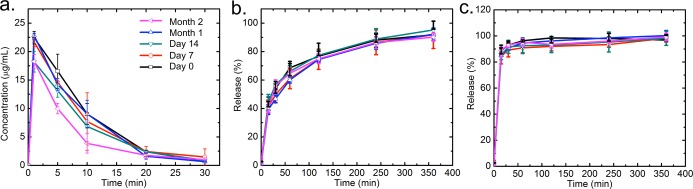
The long-term storage stability of dried powders is a critical property for medical applications of NPs. In particular, regarding this Cfz-loaded NP formulation for global health, the hot and humid climates in tropical and equatorial regions could affect the physical state of the drug.40 NPs may tend to aggregate, such that the drug may recrystallize and the dissolution kinetics could be compromised.41 Following the Food & Drug Administration long-term stability guide, harsh storage conditions are required (40 °C, 75% RH) to demonstrate the robustness of the NP formulation in those hot and humid regions. The spray-dried HPMCAS and lecithin NPs showed a long-term stability over 2 months under these harsh conditions by their dissolution kinetics in FaSSGF, FaSSIF, and FeSSIF, as shown in Figures 8 and 9. For both HPMCAS and lecithin NPs, no significant changes of release were observed between day 0 and month 2, showing that the dissolution properties of the NPs remained stable. The moisture contents of HPMCAS and lecithin samples also remained constant at around 3.1 and 0.7 wt %, respectively (see the SI). The sustained dissolution kinetics in high temperature and humidity highlights the potential of the spray-dried NPs for a feasible commercial drug product with good long-term storage stability in developing countries.
Figure 8.
Dissolution kinetics of spray-dried HPMCAS NPs under the accelerated aging conditions in (a) FaSSGF, (b) FaSSIF, and (c) FeSSIF.
Figure 9.
Dissolution kinetics of spray-dried lecithin NPs under the accelerated aging conditions in (a) FaSSGF, (b) FaSSIF, and (c) FeSSIF.
Staining Test
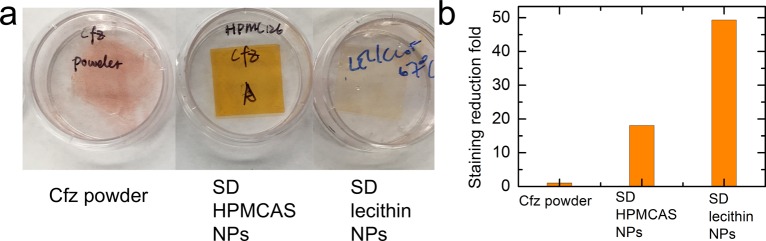
Cryptosporidiosis is the leading cause of diarrhea in infants and small children in the developing world. Therefore, it is essential to formulate an anti-cryptosporidium therapy for pediatric administration. Cfz has been widely recognized to cause exogenous pigmentation.42 It arises as a result of Cfz making contact with the oral mucosa and, hence, staining the mouth bright red, which has a negative impact on patient compliance because of social stigma associated with the red staining. In order to test the treatment compliance of our therapy, the staining effects of the dried powders were quantified using a polyurethane membrane to mimic human skin.43 As shown in Figure 10, compared with Cfz powders, the spray-dried NPs exhibit significant staining reduction of 18- and 49-fold for HPMCAS and lecithin, respectively. The surface coating of HPMCAS and lecithin significantly reduced mucosal adhesion; hence, less staining of the membrane was observed for NPs. The spray-dried HPMCAS powders display a weaker staining reduction than lecithin powders, possibly because of the carboxyl-substituted cellulose, which is known to be mucoadhesive.44,45
Figure 10.
(a) Photographs of the stained polyurethane membrane after treatments of Cfz powder and spray-dried HPMCAS/lecithin NPs. (b) Staining reduction with Cfz powders and spray-dried HPMCAS/lecithin NPs.
Compressed Minitablet Test
As a proof of concept, we tested the suitability of the spray-dried powders to be directly compressed into minitablets. A minitablet is typically defined as a tablet with a diameter of less than 3 mm46 and is desirable for pediatric and geriatric patients, providing improved swallowing, flexible dosing, and a high degree of dispersion in the gastrointestinal tract to minimize the risks of high local drug concentration. Minitablet production is often more demanding than that of large tablets, and it typically requires specialized excipients to obtain the targeted flow and compression properties, which may lower the drug loading and affect the dissolution kinetics of the final solid powders. However, without the addition of extra excipients, the spray-dried powders could be easily compressed into minitablets. Taking spray-dried HPMCAS NPs as an example, around 7 mg powders were manufactured into one 3-mm-diameter minitablet via simple tableting procedures (see the SI for the appearance of the mini-tablet). On average, 2 mL of the NP suspension is required and the time spent to produce one minitablet is approximately 1 min. The appropriateness of flow and high compactibility of the spray-dried powders further highlights the potential of these NP powders for minitablet oral drug delivery. Currently, it should be noted that the desired dosage form of the pediatric formulation is still under discussion; hence, we have not further explored the minitablet processing.
4. Conclusions
To demonstrate a versatile nanofabrication platform that can encapsulate drug molecules into NPs and rapidly recover them from solutions without compromising the fast dissolution kinetics, the FNP process was combined with SD to produce dried NP powders. Taking a poorly soluble, anti-Cryptosporidium drug, clofazimine, as the active ingredient, NP formulations were successfully developed with two biocompatible stabilizers as surface coatings, HPMCAS and lecithin. NPs with both coatings demonstrated a stability window of up to 6 h after the formulation. Solidification of preformed NP suspensions was accomplished by Lyo and SD with optimized protocols and excipients. In SD, HPMC E3 and mannitol were used for the HPMCAS and lecithin NP formulations, respectively, to avoid irreversible NP aggregations. The spray-dried HPMCAS NPs exhibited extraordinary redispersity to nanoscale size compared with the lyophilized samples, while both the spray-dried and lyophilized lecithin NPs showed some aggregation during reconstitution with particle sizes of O(1 μm). In addition, the spray-dried HPMCAS powders displayed a crumpled morphology, but the smooth shell was formed for spray-dried lecithin powders. The further characterization using PXRD and DSC demonstrated a lower order of crystallinity of Cfz in all dried powders compared with raw Cfz powders, and no significant differences were observed between spray-dried and lyophilized samples for both HPMCAS and lecithin coatings.
The dissolution behavior of Cfz NPs was evaluated by incubation in simulated gastric conditions, followed by a media swap into intestinal conditions. The newly developed Cfz formulations achieved significantly higher supersaturation levels (∼40–50×) in gastric fluid and faster dissolution kinetics in simulated fasted or fed fluid compared to raw Cfz powder or the commercial product Lamprene. Regarding two different drying methods, the spray-dried powders showed supersaturation and dissolution kinetics similar to those of the lyophilized samples for both coatings. Moreover, even stored in high temperature and humidity, the spray-dried Cfz NPs maintained complete and fast dissolutions for over 2 months, which highlights the excellent long-term storage stability of the NP powders. The formulations also significantly reduced the staining of the simulated skin samples and were easily compressed into minitablets, demonstrating the potential of these newly developed Cfz NPs as a feasible solid dosage form for pediatric administration in global health.
These results highlight the potential of an integrated nanofabrication platform using FNP, followed by spray-drying for the formulation and rapid recovery of NPs with respect to applications in life and materials science. In particular, such a cost-effective process will enable easy access of nanotherapeutics for targeted patients in developing countries. However, there are still questions to be further addressed. Regarding an integrated platform, an industrial-scale spray-drier, such as a GEA Niro Pharma PSD-3 spray-drier, can process up to 300 mL/min of liquid, which would match the production rate of 160 mL/min by our FNP process. This will enable a continuous process to directly produce NP powders. In addition, the difference in the gut transition time and intestinal fluid composition (such as pH and bile secretion)47 between pediatric and adult populations may significantly affect the dissolution kinetics and, hence, the bioavailability. For instance, a higher gastric pH in newborns compared with that in adults may reduce the dissolution of the weakly basic Cfz and, hence, the adsorption. Furthermore, understanding how the dissolution kinetics relates to the bioavailability will also help to find which stabilizer and processing is optimal for the desired pediatric formulations. These all require future in vivo tests.
Acknowledgments
The work was supported by the Bill and Melinda Gates Foundation (Grant OPP1150755) and the National Science Foundation Graduate Research Fellowship (Grant DGE-1656466) awarded to K.D.R. The authors thank Dr. Niya Bowers, Dr. Pius Tse, and Dr. Chih-Duen Tse for intellectual discussion.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsanm.8b00234.
Information of Affinsol HPMCAS, calculation of the effective Reynolds number for the MIVM, velocity and kinematic viscosity, calculation for Pe at the SD process for HPMCAS and lecithin NPs, clofazimine calibration curves in THF, FaSSGF, FaSSIF, and FeSSIF, residual moisture for spray-dried HPMCAS and lecithin NPs in the long-term storage stability study, appearance of lyophilized and spray-dried NPs, and a compressed minitablet test (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Savjani K. T.; Gajjar A. K.; Savjani J. K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, 195727. 10.5402/2012/195727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku M. S. Use of the Biopharmaceutical Classification System in Early Drug Development. AAPS J. 2008, 10, 208–212. 10.1208/s12248-008-9020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegemann S.; Leveiller F.; Franchi D.; de Jong H.; Linden H. When Poor Solubility Becomes an Issue: From Early Stage to Proof of Concept. Eur. J. Pharm. Sci. 2007, 31, 249–261. 10.1016/j.ejps.2007.05.110. [DOI] [PubMed] [Google Scholar]
- Gupta U.; Agashe H. B.; Asthana A.; Jain N. K. Dendrimers: Novel Polymeric Nanoarchitectures for Solubility Enhancement. Biomacromolecules 2006, 7, 649–658. 10.1021/bm050802s. [DOI] [PubMed] [Google Scholar]
- Abdelhamid D.; Arslan H.; Zhang Y. Y.; Uhrich K. E. Role of Branching of Hydrophilic Domain on Physicochemical Properties of Amphiphilic Macromolecules. Polym. Chem. 2014, 5, 1457–1462. 10.1039/C3PY01072D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P.; Chawla G.; Bansal A. K. Physical Stability and Solubility Advantage from Amorphous Celecoxib: The Role of Thermodynamic Quantities and Molecular Mobility. Mol. Pharmaceutics 2004, 1, 406–413. 10.1021/mp049938f. [DOI] [PubMed] [Google Scholar]
- Matteucci M. E.; Brettmann B. K.; Rogers T. L.; Elder E. J.; Williams R. O.; Johnston K. P. Design of Potent Amorphous Drug Nanoparticles for Rapid Generation of Highly Supersaturated Media. Mol. Pharmaceutics 2007, 4, 782–793. 10.1021/mp0700211. [DOI] [PubMed] [Google Scholar]
- Matteucci M. E.; Paguio J. C.; Miller M. A.; Williams R. O. III; Johnston K. P. Flocculated Amorphous Nanoparticles for Highly Supersaturated Solutions. Pharm. Res. 2008, 25, 2477–2487. 10.1007/s11095-008-9659-3. [DOI] [PubMed] [Google Scholar]
- D’Addio S. M.; Kafka C.; Akbulut M.; Beattie P.; Saad W.; Herrera M.; Kennedy M. T.; Prud’homme R. K. Novel Method for Concentrating and Drying Polymeric Nanoparticles: Hydrogen Bonding Coacervate Precipitation. Mol. Pharmaceutics 2010, 7, 557–564. 10.1021/mp900260q. [DOI] [PubMed] [Google Scholar]
- Desobry S. A.; Netto F. M.; Labuza T. P. Comparison of Spray-Drying, Drum-Drying and Freeze-Drying for Beta-Carotene Encapsulation and Preservation. J. Food Sci. 1997, 62, 1158–1162. 10.1111/j.1365-2621.1997.tb12235.x. [DOI] [Google Scholar]
- Beck-Broichsitter M.; Schweiger C.; Schmehl T.; Gessler T.; Seeger W.; Kissel T. Characterization of Novel Spray-Dried Polymeric Particles for Controlled Pulmonary Drug Delivery. J. Controlled Release 2012, 158, 329–335. 10.1016/j.jconrel.2011.10.030. [DOI] [PubMed] [Google Scholar]
- Sollohub K.; Cal K. Spray Drying Technique: Ii. Current Applications in Pharmaceutical Technology. J. Pharm. Sci. 2010, 99, 587–597. 10.1002/jps.21963. [DOI] [PubMed] [Google Scholar]
- Vehring R. Pharmaceutical Particle Engineering Via Spray Drying. Pharm. Res. 2008, 25, 999–1022. 10.1007/s11095-007-9475-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Addio S. M.; Chan J. G. Y.; Kwok P. C. L.; Benson B. R.; Prud’homme R. K.; Chan H. K. Aerosol Delivery of Nanoparticles in Uniform Mannitol Carriers Formulated by Ultrasonic Spray Freeze Drying. Pharm. Res. 2013, 30, 2891–2901. 10.1007/s11095-013-1120-6. [DOI] [PubMed] [Google Scholar]
- Tsapis N.; Bennett D.; Jackson B.; Weitz D. A.; Edwards D. A. Trojan Particles: Large Porous Carriers of Nanoparticles for Drug Delivery. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 12001–12005. 10.1073/pnas.182233999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaubal M. V.; Popescu C. Conversion of Nanosuspensions into Dry Powders by Spray Drying: A Case Study. Pharm. Res. 2008, 25, 2302–2308. 10.1007/s11095-008-9625-0. [DOI] [PubMed] [Google Scholar]
- Cholo M. C.; Steel H. C.; Fourie P. B.; Germishuizen W. A.; Anderson R. Clofazimine: Current Status and Future Prospects. J. Antimicrob. Chemother. 2012, 67, 290–298. 10.1093/jac/dkr444. [DOI] [PubMed] [Google Scholar]
- Love M. S.; Beasley F. C.; Jumani R. S.; Wright T. M.; Chatterjee A. K.; Huston C. D.; Schultz P. G.; McNamara C. W. A High-Throughput Phenotypic Screen Identifies Clofazimine as a Potential Treatment for Cryptosporidiosis. PLoS Neglected Trop. Dis. 2017, 11, e0005373. 10.1371/journal.pntd.0005373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Y.; Feng J.; McManus S. A.; Lu H. D.; Ristroph K. D.; Cho E. J.; Dobrijevic E. L.; Chan H. K.; Prud’homme R. K. Design and Solidification of Fast-Releasing Clofazimine Nanoparticles for Treatment of Cryptosporidiosis. Mol. Pharmaceutics 2017, 14, 3480–3488. 10.1021/acs.molpharmaceut.7b00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. K.; Prud’homme R. K. Flash Nanoprecipitation of Organic Actives and Block Copolymers Using a Confined Impinging Jets Mixer. Aust. J. Chem. 2003, 56, 1021–1024. 10.1071/CH03115. [DOI] [Google Scholar]
- Chow S. F.; Sun C. C.; Chow A. H. L. Assessment of the Relative Performance of a Confined Impinging Jets Mixer and a Multi-Inlet Vortex Mixer for Curcumin Nanoparticle Production. Eur. J. Pharm. Biopharm. 2014, 88, 462–471. 10.1016/j.ejpb.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Cheng C. Y.; Liu Y.; Prud’homme R. K.; Fox R. O. Mixing in a Multi-Inlet Vortex Mixer (Mivm) for Flash Nano-Precipitation. Chem. Eng. Sci. 2008, 63, 2829–2842. 10.1016/j.ces.2007.10.020. [DOI] [Google Scholar]
- Tanno F.; Nishiyama Y.; Kokubo H.; Obara S. Evaluation of Hypromellose Acetate Succinate (Hpmcas) as a Carrier in Solid Dispersions. Drug Dev. Ind. Pharm. 2004, 30, 9–17. 10.1081/DDC-120027506. [DOI] [PubMed] [Google Scholar]
- Tajarobi F.; Larsson A.; Matic H.; Abrahmsen-Alami S. The Influence of Crystallization Inhibition of Hpmc and Hpmcas on Model Substance Dissolution and Release in Swellable Matrix Tablets. Eur. J. Pharm. Biopharm. 2011, 78, 125–133. 10.1016/j.ejpb.2010.11.020. [DOI] [PubMed] [Google Scholar]
- Zhang K.; Xu Y. J.; Johnson L.; Yuan W. Q.; Pei Z. J.; Wang D. H. Development of near-Infrared Spectroscopy Models for Quantitative Determination of Cellulose and Hemicellulose Contents of Big Bluestem. Renewable Energy 2017, 109, 101–109. 10.1016/j.renene.2017.03.020. [DOI] [Google Scholar]
- Muller R. H.; Mader K.; Gohla S. Solid Lipid Nanoparticles (Sln) for Controlled Drug Delivery - a Review of the State of the Art. Eur. J. Pharm. Biopharm. 2000, 50, 161–77. 10.1016/S0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- Abdelwahed W.; Degobert G.; Stainmesse S.; Fessi H. Freeze-Drying of Nanoparticles: Formulation, Process and Storage Considerations. Adv. Drug Delivery Rev. 2006, 58, 1688–713. 10.1016/j.addr.2006.09.017. [DOI] [PubMed] [Google Scholar]
- D’Addio S. M.; Chan J. G. Y.; Kwok P. C. L.; Prud’homme R. K.; Chan H. K. Constant Size, Variable Density Aerosol Particles by Ultrasonic Spray Freeze Drying. Int. J. Pharm. 2012, 427, 185–191. 10.1016/j.ijpharm.2012.01.048. [DOI] [PubMed] [Google Scholar]
- Sham J. O. H.; Zhang Y.; Finlay W. H.; Roa W. H.; Lobenberg R. Formulation and Characterization of Spray-Dried Powders Containing Nanoparticles for Aerosol Delivery to the Lung. Int. J. Pharm. 2004, 269, 457–467. 10.1016/j.ijpharm.2003.09.041. [DOI] [PubMed] [Google Scholar]
- Santhalakshmy S.; Don Bosco S. J.; Francis S.; Sabeena M. Effect of Inlet Temperature on Physicochemical Properties of Spray-Dried Jamun Fruit Juice Powder. Powder Technol. 2015, 274, 37–43. 10.1016/j.powtec.2015.01.016. [DOI] [Google Scholar]
- Iskandar F.; Gradon L.; Okuyama K. Control of the Morphology of Nanostructured Particles Prepared by the Spray Drying of a Nanoparticle Sol. J. Colloid Interface Sci. 2003, 265, 296–303. 10.1016/S0021-9797(03)00519-8. [DOI] [PubMed] [Google Scholar]
- Bruschi M. L.; Cardoso M. L. C.; Lucchesi M. B.; Gremiao M. P. D. Gelatin Microparticles Containing Propolis Obtained by Spray-Drying Technique: Preparation and Characterization. Int. J. Pharm. 2003, 264, 45–55. 10.1016/S0378-5173(03)00386-7. [DOI] [PubMed] [Google Scholar]
- Chen K. J.; Fang T. H.; Hung F. Y.; Ji L. W.; Chang S. J.; Young S. J.; Hsiao Y. J. The Crystallization and Physical Properties of Al-Doped Zno Nanoparticles. Appl. Surf. Sci. 2008, 254, 5791–5795. 10.1016/j.apsusc.2008.03.080. [DOI] [Google Scholar]
- Narang A. S.; Srivastava A. K. Evaluation of Solid Dispersions of Clofazimine. Drug Dev. Ind. Pharm. 2002, 28, 1001–1013. 10.1081/DDC-120006431. [DOI] [PubMed] [Google Scholar]
- Keswani R. K.; Baik J.; Yeomans L.; Hitzman C.; Johnson A. M.; Pawate A. S.; Kenis P. J. A.; Rodriguez-Hornedo N.; Stringer K. A.; Rosania G. R. Chemical Analysis of Drug Biocrystals: A Role for Counterion Transport Pathways in Intracellular Drug Disposition. Mol. Pharmaceutics 2015, 12, 2528–2536. 10.1021/acs.molpharmaceut.5b00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A. I.; Akers M. J.; Nail S. L. The Physical State of Mannitol after Freeze-Drying: Effects of Mannitol Concentration, Freezing Rate, and a Noncrystallizing Cosolute. J. Pharm. Sci. 1998, 87, 931–935. 10.1021/js980001d. [DOI] [PubMed] [Google Scholar]
- Clarysse S.; Psachoulias D.; Brouwers J.; Tack J.; Annaert P.; Duchateau G.; Reppas C.; Augustijns P. Postprandial Changes in Solubilizing Capacity of Human Intestinal Fluids for Bcs Class Ii Drugs. Pharm. Res. 2009, 26, 1456–1466. 10.1007/s11095-009-9857-7. [DOI] [PubMed] [Google Scholar]
- Konno H.; Handa T.; Alonzo D. E.; Taylor L. S. Effect of Polymer Type on the Dissolution Profile of Amorphous Solid Dispersions Containing Felodipine. Eur. J. Pharm. Biopharm. 2008, 70, 493–499. 10.1016/j.ejpb.2008.05.023. [DOI] [PubMed] [Google Scholar]
- Thomas N.; Holm R.; Rades T.; Mullertz A. Characterising Lipid Lipolysis and Its Implication in Lipid-Based Formulation Development. AAPS J. 2012, 14, 860–871. 10.1208/s12248-012-9398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bott R. F.; Oliveira W. P. Storage Conditions for Stability Testing of Pharmaceuticals in Hot and Humid Regions. Drug Dev. Ind. Pharm. 2007, 33, 393–401. 10.1080/03639040600975022. [DOI] [PubMed] [Google Scholar]
- Kim K. R.; Ahn K. Y.; Park J. S.; Lee K. E.; Jeon H.; Lee J. Lyophilization and Enhanced Stability of Fluorescent Protein Nanoparticles. Biochem. Biophys. Res. Commun. 2011, 408, 225–229. 10.1016/j.bbrc.2011.03.123. [DOI] [PubMed] [Google Scholar]
- Muller S. Melanin-Associated Pigmented Lesions of the Oral Mucosa: Presentation, Differential Diagnosis, and Treatment. Dermatol Ther 2010, 23, 220–9. 10.1111/j.1529-8019.2010.01319.x. [DOI] [PubMed] [Google Scholar]
- Tas C.; Ozkan Y.; Savaser A.; Baykara T. In Vitro Release Studies of Chlorpheniramine Maleate from Gels Prepared by Different Cellulose Derivatives. Farmaco 2003, 58, 605–11. 10.1016/S0014-827X(03)00080-6. [DOI] [PubMed] [Google Scholar]
- Perioli L.; Ambrogi A.; Angelici F.; Ricci M.; Giovagnoli S.; Capuccella M.; Rossi C. Development of Mucoadhesive Patches for Buccal Administration of Ibuprofen. J. Controlled Release 2004, 99, 73–82. 10.1016/j.jconrel.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Y.; Chan J. W.; Moretti A.; Uhrich K. E. Designing Polymers with Sugar-Based Advantages for Bioactive Delivery Applications. J. Controlled Release 2015, 219, 355–368. 10.1016/j.jconrel.2015.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou H.; Liu M.; Wang L.; Mishra S. R.; Qu W.; Johnson J.; Brunson E.; Almoazen H. Development of a Mini-Tablet of Co-Grinded Prednisone-Neusilin Complex for Pediatric Use. AAPS PharmSciTech 2013, 14, 950–958. 10.1208/s12249-013-9981-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor H. K.; Marriott J. F. Paediatric Pharmacokinetics: Key Considerations. Br. J. Clin. Pharmacol. 2015, 79, 395–404. 10.1111/bcp.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.