Abstract
Purpose
To evaluate the alteration of pressure characteristics in the cerebral venous sinuses before and after venous sinus stenting (VSS) using mean sinus pressures (MSPs), sinus pressure gradient (SPG), and sinus pressure pulsatility (SPP) parameters among the idiopathic intracranial hypertension (IIH) patients.
Materials and Methods
Prospective evaluation of 45 consecutive IIH patients who underwent VSS at our institution. A written informed consent approved by the Weill Cornell Institutional Review Board was signed by the study participants. All patients (n = 45) were evaluated for MSPs and SPG. In a subgroup of 12 (n = 12) consecutive patients, SPP was measured. MSP was measured using microcatheter at superior sagittal sinus (SSS), transverse sinus (TS), and sigmoid sinus (SS). SPG was measured as trans-stenotic gradient and trans-torcular gradient. SPP was recorded in the dominant TS with a six French intermediate catheter. Statistical analysis was performed using paired student t-test and two sample t-tests tested for both equal and unequal variances. P values below 0.05 were considered significant.
Results
The mean age of the study population was 30.6 ± 10 years (7–59 years) and 43 out of 45 are female patients. The mean weight and BMI of the study population were 96 ± 24.7 kg (30.8–144 kg) and 35.6 ± 8.3 kg/M2 (16.4–51.4 kg/M2), respectively. VSS in IIH patients resulted in immediate reduction of MSP in the SSS {Δ Mean: −8.1 mm Hg [95% confidence interval (CI): −5.0–11.7 mm Hg], p < 0.001} and TS [Δ Mean: −11.8 mm Hg (95% CI: −7.5 to 13.4 mm Hg), p < 0.001] and increase of MSP in SS [Δ Mean: 7.5 mm Hg (95% CI: 6–10.1 mm Hg), p < 0.001]. Significant reduction of trans-stenotic SPG reduction [Δ Mean: −15.7 mm Hg (95% CI: −13.6–17.8 mm Hg), p < 0.001] and SPP [Δ Mean: −8 mm Hg (95% CI: −2.5–13.4 mm Hg), p < 0.05] was observed following VSS.
Conclusion
VSS resulted in immediate alteration of the cerebral venous sinus pressure measurements in patients with IIH.
INTRODUCTION
Venous sinus stenting (VSS) has evolved as an effective therapeutic strategy in patients with refractory idiopathic intracranial hypertension (IIH) [1]. Venous outflow obstruction from sinus stenosis or occlusive arachnoid granulations is central in the poorly understood pathophysiology of IIH [2, 3]. The outflow obstruction most commonly occurs in the transverse sinus (TS) or at the transverse-sigmoid sinus (SS) junction. Farb et al. [4] reported 93% incidence of bilateral transverse sinus (BTS) stenosis in IIH patients. On the contrary, the incidence of BTS stenosis in non-IIH group varies from 3% to 7% [4, 5].
Evidence of venous outflow obstruction in IIH is proven by elevated central cerebral venous pressure and trans-stenotic pressure gradient. A trans-stenotic pressure gradient of 8–10 mm Hg across an intracranial venous sinus stenosis has been the threshold for intervention in most series [6–8]. Effectiveness of VSS was reported based on the reduction of trans-stenotic gradient following stenting. The reported variation in pressure gradient is a reflection of overall changes across the cerebral venous sinuses. However, there is limited understanding of pressure changes in each of the cerebral venous sinuses and their variation following VSS in IIH patients.
The purpose of the study is to evaluate the average venous pressures and amplitude of pressure variations in individual cerebral venous sinuses before and after VSS among the IIH patients.
MATERIALS AND METHODS
Patient selection
We present prospectively collected data of the patients who underwent VSS at our institution. The patients were enrolled either as a part of ongoing FDA approved clinical trial VSS for IIH Refractory to Medical Therapy (ClinicalTrials.gov Identifier: NCT01407809) or in a prospective patient registry, both approved by our Institutional Review Board (IRB). A written informed consent approved by the Weill Cornell IRB was signed by the study participants. Forty-five consecutive IIH patients treated with VSS were prospectively evaluated. All patients (n = 45) were evaluated for mean sinus pressures (MSPs) and sinus pressure gradient (SPG) across the stenosis. In a subgroup of 12 (n = 12) consecutive patients, sinus pressure pulsatility (SPP) was measured to study the amplitude of pressure variations using large bore catheters.
Study parameters
The pressure characteristics of the cerebral venous sinuses were evaluated using:
(1) Mean sinus pressures:
These were measured (using microcatheters) at superior sagittal sinus (SSS), TS, and SS.
(2) Sinus pressure gradient:
Trans-stenotic gradient: pressure difference between the TS (proximal to the stenosis) and SS (distal to the stenosis).
Trans-torcular gradient: pressure difference between SSS and TS.
(3) Sinus pressure pulsatility:
The cyclical variation of the venous sinus pressures demonstrating the maximum (systolic) and minimum (diastolic) venous pressures were recorded in the dominant TS with a six French 0.72 inch Navien intermediate catheter (Covidien, Medtronic, Minneapolis, MN).
Mean venous pressure: [maximum pressure + 2 (minimum pressure)]/3
Venous pulse pressure (VPP): maximum pressure − minimum pressure
Data collection
Procedures were performed in an angiographic suite (GE Innova 2100). Patient demographics including age, gender, weight, and BMI were collected from the database. The pre-stent study parameters were evaluated to study the baseline cerebral venous sinus pressure characteristics of the untreated refractory IIH patients. Post-treatment parameters were studied to evaluate the impact of VSS in this patient population. Our venous manometry and VSS procedure have been previously described in detail [9].
Mean sinus pressures
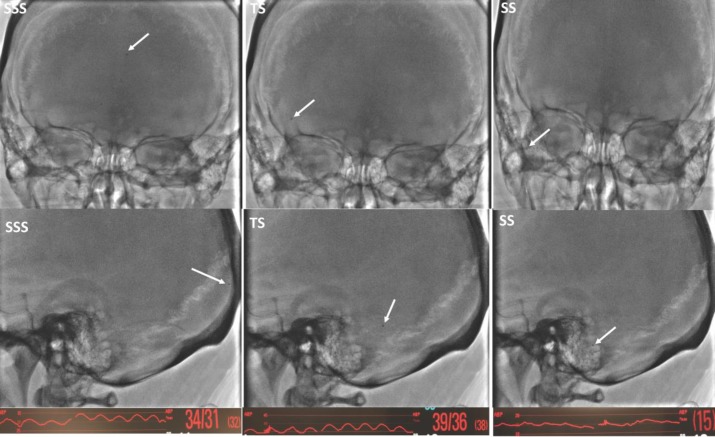
The MSP was recorded under local anesthesia before VSS and under general anesthesia following VSS. A five French Envoy (Codman Neurovascular, Raynham, MA) guide catheter was advanced over 0.035 inch Terumo guide wire (Terumo Medical Corporation, Somerset, NJ) via common femoral venous access into proximal jugular vein on the side of target stenosis. Then, a 2.7 French Headway-27 (MicroVention Inc., Tustin, CA) microcatheter was advanced over the Synchro-14 standard (Stryker Neurovascular, Fremont, CA) microwire via the Envoy guide catheter into the SSS. The microwire was then removed. The proximal end of the microcatheter was connected to a 60-inch long arterial line pressure cable including a disposable transducer, 3 cc squeeze flush device, and a macrodrip (Transpac IV monitoring kit, ICU medical, San Clemente, CA) via a 48-inch-long high-pressure (1200 psi) tubing (Merit Medical, South Jordan, UT). Continuous real-time venous pressures were measured at desired locations. The microcatheter was slowly retracted into the specific locations and cerebral venous pressures were measured at each location (Figure 1).
Figure 1. Frontal (top row) and latera (middle row) projections demonstrating the microcatheter position (white arrow) in the SSS, TS, and SS. Sinus pressure curves and MSP values at each location are shown in the bottom row.
Sinus pressure gradient
Pretreatment magnetic resonance venography (MRV) was reviewed by the authors Srikanth R. Boddu and Athos Patsalides to categorize the TS dominance pattern as co-dominant, dominant-hypoplastic, or dominant-aplastic systems [10]. A difference of >3 mm in the maximal antero-posterior diameter of the BTSes was used to differentiate unilateral dominant sinus versus co-dominant TS [11]. Trans-stenotic gradient was defined as a pressure difference between the TS and SS and used to study stenosis impact on cerebral venous pressure. Trans-torcular gradient was defined as the pressure difference between SSS and TS and used to understand the impact of contralateral TS and collateral venous channels on the dominant TS.
Sinus pressure pulsatility
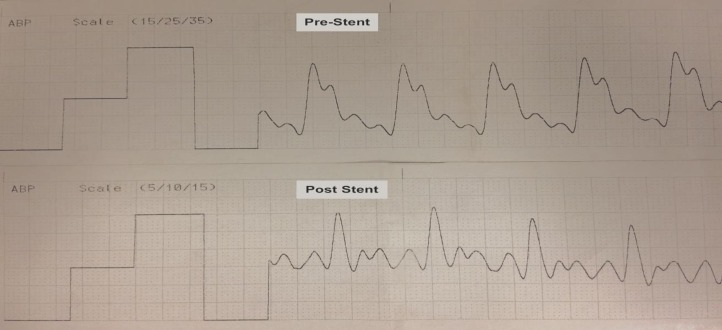
The SPP was measured in the dominant TS. Venous pulsatility measurements were performed under general anesthesia using a six French 0.72 inch Navien (Covidien, Medtronic, Minneapolis, MN) intermediate catheter. The 6F Navien was navigated over a combination of 2.7 French Headway-27 (MicroVention Inc., Tustin, CA) and Synchro standard microwire (Stryker Neurovascular, Fremont, CA) into the dominant TS. The microcather and microwire were then removed. The 6F Navien was connected to a pressure transducer and continuous real-time sinus pressure tracing with pulsatility was obtained. The sinus pressures were measured before and after VSS (Figure 2).
Figure 2. SPP recording using a six French intermediate catheter in dominant mid-TS before and after VSS. Note the reduced sinus pulsatility on post-stent recording compared to pre-stent recording (notice the variation in the scale between two recordings). The mean venous pressure and VPP were calculated from sinus pulsatility recordings.
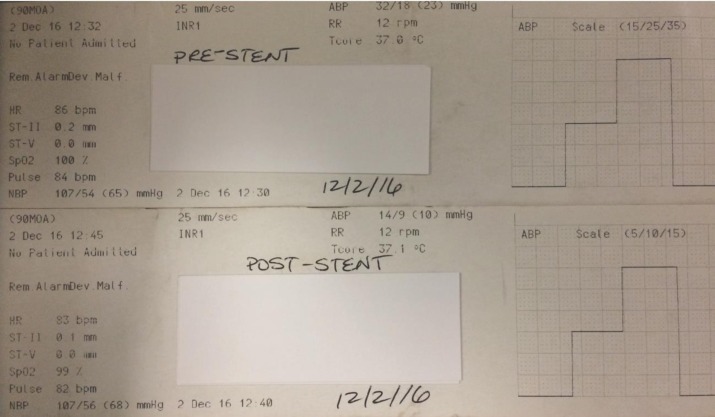
Based on the systolic and diastolic pressure reading, MSP was calculated as [systolic pressure + 2 (diastolic pressure)]/3. The VPP was calculated as maximum pressure − minimum pressure. Other hemodynamic parameters such as mean arterial blood pressure, heart rate, respiratory rate, temperature, and oxygen saturations were simultaneously measured to avoid systemic bias in the SPP measurement (Figure 3).
Figure 3. The systemic parameters are documented at the time of SPP recording before and after the venous sinus stent to avoid bias from a systemic parameter.
Statistical analysis
Statistical analysis was performed with SPSS version 21 (SPSS Inc., Chicago, IL). Patient demographics and study parameters (ISP, SPG, and SPP) were considered as continuous variables. TS dominance pattern was considered as categorical variable. Continuous variables were described with mean, range, and standard deviation (SD). Impact of VSS on pre- and post-stent MSP, SPG, and SPP was evaluated with paired student t-test. Impact of TS dominance pattern on SPG was calculated using two sample t-tests and tested for both equal and unequal variances. Precision of the diagnostic parameters was presented using a 95% confidence interval (CI). P values below 0.05 were considered significant.
RESULTS
The mean age of the study population was 30.6 ± 10 years (7–59 years) with 43 out of 45 being female patients. The mean weight and BMI of the study population were 96 ± 24.7 kg (30.8–144 kg) and 35.6 ± 8.3 kg/M2 (16.4–51.4 kg/M2), respectively.
Mean sinus pressures
The results of mean individual sinus pressures (measured with the microcatheter) are summarized in Table 1:
Table 1. Summary of the MSP before and after VSS.
| Location | Pre-stent pressure (mm Hg) ±SD (95% CI) | Post-stent pressure (mm Hg) ±SD (95% CI) | p-value (paired student t-test) |
|---|---|---|---|
| SSS (n = 39) | 35.4 ± 10.6 (32.3–38.6) | 27.3 ± 9.1 (24.4–30.2) | <0.001 |
| TS (n = 45) | 34.2 ± 9 (31.5–36.8) | 26.4 ± 8 (24–28.8) | <0.001 |
| SS (n = 45) | 16.8 ± 6.1 (15–18.6) | 24.7 ± 7.3 (22.5–26.9) | <0.001 |
SSS = Superior sagittal sinus; TS = Transverse sinus; SS = Sigmoid sinus
In patients with IIH, VSS resulted in significant reduction of sinus pressure was noted in the SSS [Δ Mean: −8.1 mm Hg (95% CI: −5.0–11.7 mm Hg), p < 0.001) and TS Δ Mean: −11.8 mm Hg (95% CI: −7.5–13.4 mm Hg), p < 0.001]. On the contrary, the SS pressure was significantly increased immediately after stenting [Δ Mean: 7.5 mm Hg (95% CI: 6–10.1 mm Hg), p < 0.001].
Sinus pressure gradient
The results of trans-stenotic and trans-torcular pressure gradients are summarized in Table 2.
Table 2. Summary of the mean SPG before and after VSS.
| Gradient | Pre-stent Δ mean (mm Hg) ±SD (95% CI) | Post-stent Δ mean (mm Hg) ±SD (95% CI) | p-value (paired student t-test) |
|---|---|---|---|
| Trans-stenotic (n = 45) (PTS–DSS) | 17.3 ± 7.4 (15.2–19.5) | 1.6 ± 1.5 (1.2–2.1) | <0.001 |
| Trans-torcular (n = 39) (SSS–DTS) | 1.2 ± 3.5 (0.2–2.3) | 1.1 ± 1.5 (0.7–1.5) | 0.825 |
PTS = proximal transverse sinus; DTS = distal transverse sinus; DSS = distal sigmoid sinus.
Significant reduction of the trans-stenotic SPG [Δ Mean: −15.7 mm Hg (95% CI: −13.6–17.8 mm Hg), p < 0.001] was noted after the VSS. The trans-torcular pressure gradient was unaffected with VSS in both the co-dominant (p = 0.65) and unilateral dominant (p = 0.72) TS systems.
Sinus pressure pulsatility
The results of SPP (n = 12) are summarized in Table 3.
Table 3. Summary of SPP before and after VSS in 12 patients.
| SPP | Pre-stent pressure (mm Hg) ±SD (95% CI) | Post-stent pressure (mm Hg) ±SD (95% CI) | p-value (paired student t-test) |
|---|---|---|---|
| Systolic pressure | 32 ± 18 (21.8–43.4) | 20.5 ± 7.1 (16.5–24.5) | 0.01 |
| Diastolic pressure | 21.4 ± 12.6 (14.3–28.5) | 15.3 ± 6.2 (11.9–18.9) | 0.03 |
| Mean venous pressure | 25 ± 14.6 (16.8–33) | 16.9 ± 6.4 (13.3–20.5) | 0.02 |
| VPP | 10.7 ± 6.9 (6.7–14.6) | 5.2 ± 1.9 (4.1–6.2) | 0.01 |
Significant reduction of systolic, diastolic, and mean venous pressure [Δ Mean: −8 mm Hg (95% CI: −2.5 to 13.4 mm Hg), p < 0.05] as well as VPP [Δ Mean: −5.5 mm Hg (95% CI: −1.8 to 9.1 mm Hg), p < 0.05] was observed following VSS. Statistical analysis of pre- and post-stent systemic parameters including systolic arterial pressure (p = 0.19), diastolic arterial pressure (p = 0.42), mean arterial pressure (p = 0.35), heart rate (p = 0.79), respiratory rate (p = 0.76), temperature (0.39), and oxygen saturation (0.55) showed no significant variation.
Discussion
Our study offers a prospectively collected dataset that provide baseline pressure characteristics of the cerebral venous sinuses in patients with IIH. We report the pressure variations (MSPs, SPG, and SPP) of the cerebral venous sinus before and after VSS. Mean pressures can be measured accurately even with a microcatheter. However, systolic and diastolic blood pressures are dampened when measured with microcatheters. Hence, we used a 6F catheter to have a more accurate reading of the pressure variations from pulsatile conditions in the venous sinus of 12 IIH patients. We found that, in our population of IIH patients, VSS significantly reduced the mean venous sinus pressure in the SSS (<0.001) and TS (<0.001), but increased the SS pressure (<0.001) resulting in overall reduction of trans-stenotic gradient (<0.001). A similar effect was demonstrated in SPP with significant reduction (<0.05) following VSS.
In patients with suspected IIH, MSP measurement at various locations of dural sinuses and calculation of trans-stenotic gradient are used to screen the patients suitable for VSS. A trans-stenotic pressure gradient of 8–10 mm Hg has been reported as threshold to pursue the VSS [6–8]. Recently, Raper et al. [12] compared the prestent MSP readings under conscious sedation and general anesthesia in patients suspected with IIH. The authors reported significantly higher maximum MSP in IIH subset. However, dedicated individual sinus pressure readings in an IIH subset is lacking. Fargen et al. [13] reported the percentage variation of the MSP in dural sinuses between awake and general anesthesia, but the information on individual sinus pressures was not reported. We report the MSP of individual sinuses (SSS, TS, and SS) of the dedicated IIH patient cohort before and after the VSS, which improves understanding of hemodynamic alteration in individual sinus rather than an overall pressure changes in cerebral venous sinuses.
Multiple prior studies reported the significant reduction of trans-stenotic gradient following VSS as treatment for IIH [6–8, 14]. Our findings of trans-stenotic gradient are in good concordance with the published literature, arguing in favor of VSS as an effective therapeutic option in the management of refractory IIH. Based on our findings, we would like to emphasize that the reduction of trans-stenotic gradient is not a reflection MSP reduction in individual sinuses; rather, it is a cumulative outcome of reduced MSP in SSS and TS and an increased MSP in SS, compared to the pre-stent baseline. This highlights the importance of understanding individual sinus pressure variations than just relying on trans-stenotic gradient. Considering post-stent manometry is performed immediately after stent deployment, the differential pressure changes in the sinuses (reduced MSP in SSS and TS with increased MSP in SS) may represent a transient phenomenon due to flow redistribution after the stenosis recanalization. The definitive outcome is expected to be the overall reduction of MSP in all dural sinuses including SS, as evidenced by the pattern of headache resolution in –two to four weeks (not immediately after VSS) and low opening pressure on the follow-up lumbar puncture [9].
The cerebral venous sinus system is essentially a low-pressure system with a pressure reflection of central venous pressure, that is, 2–6 mm Hg. The current selection of arterial stents with high radial force for VSS undoubtedly provides recanalization of the venous outflow occlusion. However, the concern will be over distention of the low-pressure venous. New ipsilateral focal retro-mastoid headaches subsequent to the VSS likely represent dural irritation from over dilatation. We have specifically evaluated the SPP which represents cyclical pressure changes at a given point in the dominant TS. We selected the proximal TS for SPP measurement based on its proximity to the stenosis and potential maximum sinus pressure in this location. We studied the maximum and minimum limits of the cyclical pressure changes to better understand the possible effect of VPP. Pulse pressure is defined as the difference between the systolic and diastolic pressures. Consistent elevation of the arterial pulse pressure is the most likely basis for stiffness of the major arteries and an established risk factor for atrial fibrillation [15]. A meta-analysis, which combined the results of several studies of 8000 elderly patients in all, found that a 10-mm Hg increase in arterial pulse pressure increased the risk of major cardiovascular complications and mortality by nearly 20% [16]. Perhaps, early intervention in IIH patients at lower VPP may avoid chronic changes and decompensation of the cerebral venous sinus. Larger prospective trials are required to study the impact of increased SPP and elevated VPP in chronic cerebrovascular changes and end-organ damage.
Considering the increase of VSS as a treatment option for refractory IIH, precise knowledge of the venous sinus pressure variation is crucial. The current use of carotid/peripheral arterial stents for VSS has inherent challenges such as navigation through sinus tortuosity, recurrent stenosis [8, 14], flow stagnation in vein of Labbe’ [17] and post stent headaches [9], etc. Perhaps, a new generation stent device tailored for the low pressure cerebral venous sinuses and early intervention at low VPP may enhance the role of VSS in IIH management to the next level that is, as a standard of care.
Limitations
The pre-stent individual sinus pressures and SPG were measured while patient was awake or on minimal sedation, while post stent measurements were performed with patient under general anesthesia. Recent studies showed that the maximum sinus venous pressure is significantly lower under general anesthesia than the conscious sedation or awake patients [12, 13]. Although this supports the accuracy of our baseline target sinus pressure and SPG, the precision of post VSS reading under general anesthesia are questionable. However, immediate resolution of the stenosis after VSS, immediate resolution of tinnitus, gradual improvement of headache and papilledema over days or weeks, persistent low opening pressure on lumbar puncture and wide patency of stent across stenosis at three-month follow-up MRV favor persistent reduction of sinus pressure and trans-stenotic gradient [9]. The number of patients with SPP measurements were relatively small (n = 12). The pre-and post VSS SPP was measured under general anesthesia. Although this may result in underestimation of SPP and VPP, it presents a more internally consistent parameter unbiased by anesthesia in the assessment of VSS impact.
CONCLUSION
VSS is an emerging therapeutic option for treatment of refractory IIH. In our experience, VSS significantly alters the pressure characteristics of the cerebral venous sinus as measured on MSP, SPG, and SPP. We provided baseline pressure measurements of the individual venous sinuses. Our data can be used towards better understanding of the pressure variations of the cerebral venous sinuses in IIH patients and new device innovation tailored for the needs of low-pressure cerebral venous system.
REFERENCES
- Albuquerque FC, et al. Time to re-assess the treatment of idiopathic intracranial hypertension. J NeuroInterventional Surg. 2016;8(6):549–550. doi: 10.1136/neurintsurg-2016-012460. [DOI] [PubMed] [Google Scholar]
- Friedman DI. Cerebral venous pressure, intra-abdominal pressure, and dural venous sinus stenting in idiopathic intracranial hypertension. J Neuro-Ophthalmol Off J North Am Neuro-Ophthalmol Soc. 2006;26(1):61–64. doi: 10.1097/01.wno.0000204663.33559.1e. [DOI] [PubMed] [Google Scholar]
- Johnston I, et al. Cranial venous outflow obstruction in the pseudotumour syndrome: incidence, nature and relevance. J Clin Neurosci Off J Neurosurg Soc Australas. 2002;9(3):273–278. doi: 10.1054/jocn.2001.0986. [DOI] [PubMed] [Google Scholar]
- Farb RI, et al. Idiopathic intracranial hypertension: the prevalence and morphology of sinovenous stenosis. Neurology. 2003;60(9):1418–1424. doi: 10.1212/01.wnl.0000066683.34093.e2. [DOI] [PubMed] [Google Scholar]
- Kelly LP, et al. Does bilateral transverse cerebral venous sinus stenosis exist in patients without increased intracranial pressure? Clin Neurol Neurosurg. 2013;115(8):1215–1219. doi: 10.1016/j.clineuro.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussière M, et al. Unilateral transverse sinus stenting of patients with idiopathic intracranial hypertension. AJNR Am J Neuroradiol. 2010;31(4):645–650. doi: 10.3174/ajnr.A1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed RM, et al. Transverse sinus stenting for idiopathic intracranial hypertension: a review of 52 patients and of model predictions. AJNR Am J Neuroradiol. 2011;32(8):1408–1414. doi: 10.3174/ajnr.A2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teleb MS, et al. Idiopathic intracranial hypertension. A systematic analysis of transverse sinus stenting. Interv Neurol. 2013;2(3):132–143. doi: 10.1159/000357503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkin MJ, Patsalides A. Venous sinus stenting for idiopathic intracranial hypertension: where are we now? Neurol Clin. 2017;35(1):59–81. doi: 10.1016/j.ncl.2016.08.006. [DOI] [PubMed] [Google Scholar]
- Boddu S, et al. Resolution of pulsatile tinnitus after venous sinus stenting in patients with idiopathic intracranial hypertension. PLoS ONE. 2016;11(10):e0164466. doi: 10.1371/journal.pone.0164466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, et al. CT arteriography and venography in pulsatile tinnitus: preliminary results. Am J Neuroradiol. 2006;27(8):1635–1638. [PMC free article] [PubMed] [Google Scholar]
- Raper DMS, et al. Intracranial venous pressures under conscious sedation and general anesthesia. J NeuroInterventional Surg. 2017;9(10):986–989. doi: 10.1136/neurintsurg-2017-012984. [DOI] [PubMed] [Google Scholar]
- Fargen KM, et al. Concomitant intracranial pressure monitoring during venous sinus stenting for intracranial hypertension secondary to venous sinus stenosis. J Neurointerventional Surg. 2013;5(4):e22. doi: 10.1136/neurintsurg-2012-010371. [DOI] [PubMed] [Google Scholar]
- Satti SR, et al. Meta-analysis of CSF diversion procedures and dural venous sinus stenting in the setting of medically refractory idiopathic intracranial hypertension. Am J Neuroradiol. 2015;36(10):1899–1904. doi: 10.3174/ajnr.A4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulse pressure important risk factor for the development of new-onset. [Apr 16;2017 ];2012 Dec 9; Available from: http://archive.is/GeM5.
- Blacher J, et al. Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch Intern Med. 2000;160(8):1085–1089. doi: 10.1001/archinte.160.8.1085. [DOI] [PubMed] [Google Scholar]
- Raper DMS, et al. Patency of the vein of Labbé after venous stenting of the transverse and sigmoid sinuses. J Neurointerventional Surg. 2017;9(6):587–590. doi: 10.1136/neurintsurg-2016-012903. [DOI] [PubMed] [Google Scholar]