Abstract
We report the palladium-catalyzed oxidation of hindered alkenes to form linear allylic esters. The combination of palladium(II) benzoate, 4,5-diazafluoren-9-one, and benzoquinone catalyzes the mild oxidation of terminal alkenes with tert-butyl benzoyl peroxide as an oxidant in the presence of diverse functional groups. Selective oxidation of terminal alkenes in the presence of trisubstituted and disubstituted alkenes has been achieved, and the ability to conduct the reaction on a gram scale has been demonstrated. The mild conditions and high tolerance for auxiliary functionality make this method suitable for the synthesis and derivatization of complex molecules.
Keywords: C–H activation, C–H bond functionalization, allylic oxidation, palladium catalysis, olefin functionalization
Graphical Abstract
C–H bond functionalization reactions could enable concise routes to complex molecules in diverse synthetic contexts, but many C–H bond functionalization reactions conducted on simple substrates do not translate to more-complex analogues.1 Among C–H bond functionalization reactions, the oxidation of allylic C–H bonds has been investigated by many groups because of the prevalence of olefins and alcohols in natural products and the application of allylic alcohols as synthetic intermediates.2 Although many catalytic methods for allylic oxidation have been reported,3 current systems do not generally address the challenge of conducting this class of reaction on hindered alkenes, such as those with fully substituted carbon atoms adjacent to the allylic unit. Such allyl units flanked by quaternary carbons are common substructures in natural products. Thus, synthetic methods for the functionalization of such allyl groups would be valuable for both the synthesis and direct functionalization of natural products.4
Since the initial reports on the catalytic allylic oxidation of olefins in the 1970s,5 selective palladium-catalyzed acetoxylations of allylic C–H bonds have been reported (Scheme 1A).6 Reactions with a variety of ligands, terminal oxidants, and solvents have been investigated.3 High selectivity for both the linear and branched allylic acetates has been achieved, and acid-sensitive substrates can be functionalized under certain conditions.6k,7,8 The acetoxylation of substrates containing amides, esters, and acetals has been accomplished in moderate to good yields.9 Many groups have investigated the mechanisms of these transformations in depth.6d,g,i,k,l,10 Recently, our group reported a system for the palladium-catalyzed oxidation of α-olefins to linear allylic benzoates with high selectivity for the linear ester under neutral conditions in combination with iridium-catalyzed allylic substitution.11 This process was used to prepare synthetically valuable enantioenriched products containing new C–O, C–N, and C–C bonds in one pot.
Scheme 1.
C–H Oxidation of α-Olefins
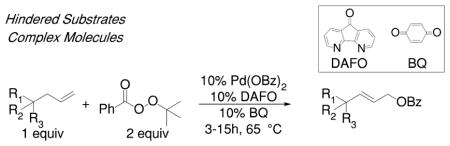
Despite this progress, current methods for the oxidation of allylic C–H bonds have significant limitations. Most relevant to the present investigation, hindered alkenes tend to resist oxidation at the allylic position. Stoltz et al. recently highlighted these constraints (Scheme 1B).12 The oxidation of substrates containing α-allyl lactams with quaternary centers next to the allyl group occurred to low conversions under both classical and modern catalytic conditions.
Although the authors subsequently devised conditions for the selective oxidation of α-allyl lactams, only trace yields were obtained when other classes of hindered substrates were evaluated.
With these challenges in mind, we assessed whether our conditions for the oxidation of alkenes to terminal allylic esters could be adapted to achieve the oxidation of sterically hindered alkenes with a high tolerance of functional groups and selectivity for linear products (Scheme 1C). Herein, we report the development of conditions for the palladium-catalyzed oxidation of hindered alkenes to form linear allylic benzoates. The ability to oxidize these substrates and the high tolerance of the system for functional groups makes this process particularly valuable for the functionalization of complex natural products containing quaternary carbons at the position α to the allyl unit.
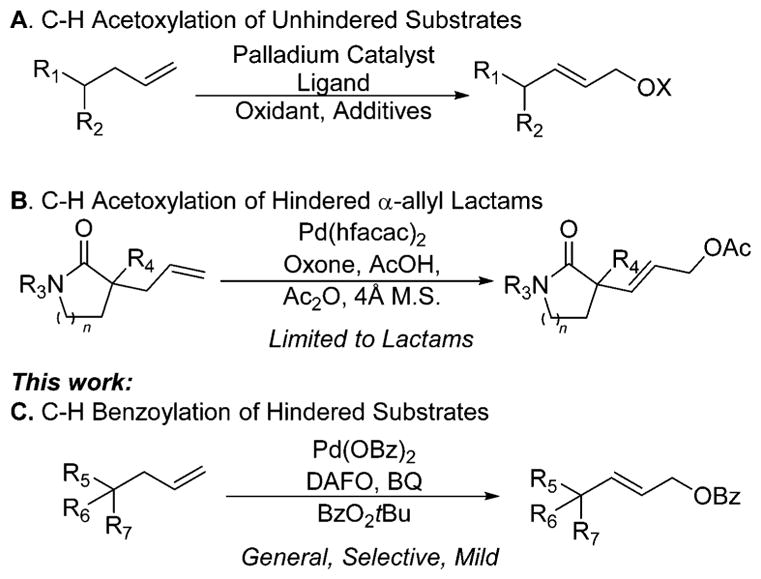
To develop a system capable of functionalizing compounds containing α-quaternary centers, we investigated the oxidation of model substrate 1b by modifying our previously reported conditions (Table 1). We found that the combination of Pd(OBz)2 and 4,5-diazafluoren-9-one (DAFO)6l catalyzes the reaction of 1b with tert-butyl benzoyl peroxide (BzO2tBu) to form 2b in 59% yield (Table 1, entry 3). No product was formed when either Pd(OBz)2 or DAFO was omitted from the reaction (Table 1, entries 1 and 2). The high conversion of 1b in the absence of DAFO suggests that unligated Pd(OBz)2 catalyzes the decomposition of 1b in the presence of tert-butyl benzoyl peroxide (Table 1, entry 2). The yield of the reaction conducted with 4 equiv of oxidant was similar to that obtained when the reaction was conducted with 2 equiv of oxidant (Table 1, entries 3 and 4). Neither increasing the loading of palladium and ligand to 20 mol % (Table 1, entry 5), nor increasing the loading of ligand to 20 mol % (while maintaining the loading of palladium at 10 mol %) increased the yield of 2b (Table 1, entry 6). However, the yields of reactions conducted with benzoquinone (BQ) as an additive were higher than those conducted without benzoquinone (Table 1, entries 7–9).
Table 1.
Evaluation of Conditions for the Oxidation of Model Ketone 1ba
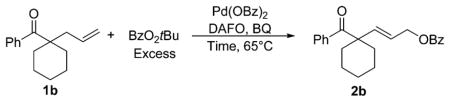
| |||||
|---|---|---|---|---|---|
| entry | %Pd(OBz)2 | %DAFO | %BQ | yield (%) | unreacted 1b (%) |
| 1 | 0 | 10 | 0 | 0 | quant |
| 2 | 10 | 0 | 0 | 0 | 22 |
| 3 | 10 | 10 | 0 | 59 | 0 |
| 4b | 10 | 10 | 0 | 58 | 0 |
| 5 | 20 | 20 | 0 | 55 | 0 |
| 6 | 10 | 20 | 0 | 51 | 21 |
| 7 | 10 | 10 | 10 | 74 | 0 |
| 8 | 10 | 10 | 50 | 78 | 0 |
| 9c | 10 | 10 | 10 | 79 | 0 |
Conditions: Reactions were conducted with 0.1 mmol (1 equiv) of 1b and 2.0 equiv of BzO2tBu. The ligand, 4,5-diazafluoren-9-one, is abbreviated as DAFO. Benzoquinone is abbreviated as BQ. The reaction duration was 20 h. Yields were determined by 1H NMR.
Reaction was conducted with 4.0 equiv of BzO2tBu.
Average yield of two reactions; reaction duration was 10 h.
The presence of BQ in the reaction mixture also increased the yields for the oxidation of unprotected homoallylic alcohols. The Sakurai–Hosomi reaction and other reactions of allyl nucleophiles with ketones have been developed for the synthesis of enantioenriched homoallylic alcohols, but the functionalization of tertiary, homoallylic alcohols is challenging because of the electron-withdrawing property of the alcohol and the steric hindrance at the allylic C–H bond.13–16 In addition, terpenoids containing homoallylic alcohols, such as linalool, have been observed to undergo unproductive side reactions under oxidative conditions resulting in multiple products.17
Reactions of model substrate 1m were conducted in the presence and absence of BQ (Table 2). In the absence of BQ, only low to moderate conversions of 1m and yields of 2m were obtained at 1 and 3 h (Table 2, entries 1 and 2). A high conversion of 1m was observed when the reaction time was extended to 48 h, but a low yield of 2m was obtained (Table 2, entry 3). In contrast, reactions conducted with added BQ fully converted 1m within an hour (Table 2, entries 4–6). The yield of 2m from the reaction with 5 mol % of benzoquinone was the same as that with 10 mol % BQ (Table 2, entries 4 and 5). Reactions containing 10 mol % of other quinones or metal salts gave lower conversions of 1m at 1 h than did the reaction conducted with 10 mol % BQ (see the Supporting Information).
Table 2.
Evaluation of Conditions for the Oxidation of Homoallylic Alcohol 1ma

| ||||
|---|---|---|---|---|
| entry | %BQ | time (h) | yield (%) | unreacted 1m (%) |
| 1 | 0 | 1 | 28 | 46 |
| 2 | 0 | 3 | 28 | 47 |
| 3 | 0 | 48 | 25 | 0 |
| 4 | 5 | 1 | 70 | 0 |
| 5 | 10 | 1 | 70 | 0 |
| 6 | 50 | 1 | 69 | 0 |
| 7 | 10 | 3 | 69 | 0 |
Conditions: Reactions were conducted with 0.1 mmol (1 equiv) of 1m and 2.0 equiv of BzO2tBu. Yields were determined by 1H NMR.
The origin of the higher rate and higher yield that are observed when the benzoylation reaction is conducted in the presence of BQ than in the absence of BQ is unclear. However, based on numerous precedents, we suggest that BQ could serve as a ligand that promotes the reductive elimination of an allyl palladium intermediate (see the Supporting Information).6j,k,10c,18 Alternatively, BQ could prevent catalyst decomposition.
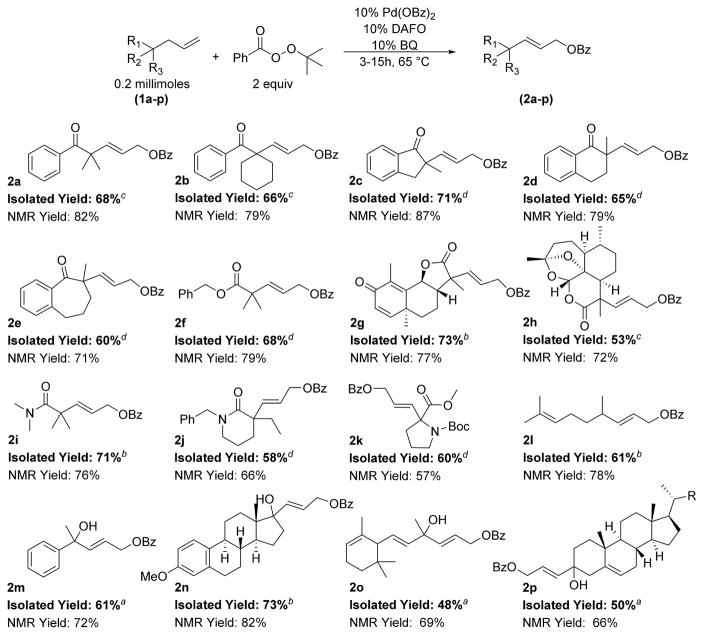
Having achieved high yields for the oxidation of two different classes of model substrates, we evaluated our conditions for the oxidation of allylic C–H bonds in a range of compounds (Scheme 2). Many of the products derived from these alkenes could serve as valuable synthetic intermediates (Scheme 2, entries 2a–2f, 2i–2m). In addition, derivatives of natural products were oxidized to provide complex precursors for further diversification (Scheme 2, entries 2g, 2h, 2k, 2l, 2n–2p). Generally, 1H NMR yields were higher than isolated yields by 10%–20% because of the need to separate the major product from minor byproducts having similar polarity to the major species. A representative 1H NMR spectrum of the crude product derived from the oxidation of 1m is included in the Supporting Information.
Scheme 2. Scope of C–H Benzoylation of Hindered and Complex Substrates*.
*Conditions: Reactions were conducted with 0.2 mmol (1 equiv) of substrate and 2.0 equiv of BzO2tBu. Yields were determined by 1H NMR prior to isolation. aReaction duration was 3 h. bReaction duration was 5 h. cReaction duration was 10 h. dReaction duration was 15 h. R = (CH2)3i-Pr.
Published attempts to conduct the acetoxylation of C–H bonds of α-allyl ketones resulted in low conversion.12 We found that reactions of acyclic ketones 1a and 1b occurred to full conversion in 10 h, despite the difference in the steric properties of the quaternary centers in these two ketones. Cyclic ketones containing 5-, 6-, and 7-membered rings underwent oxidation in high yields in 15 h (1c, 1d, 1e). The synthesis of enantioenriched variants of these substrates by asymmetric Tsuji allylic alkylation has been extensively developed because of their value as synthetic intermediates; our functionalization methodology provides products that could be further elaborated.6l,12,19
In addition to hindered ketones, hindered α-allyl esters and lactones also reacted to full conversion within <24 h. The model ester, 1f, was oxidized to 2f in good yield. Product 2f is valuable because of its similarity to methyl (E)-5-hydroxy-2,2-dimethylpent-3-enoate, which is an intermediate in the synthesis of bryostatin.20 Furthermore, the selective oxidation of 2f demonstrates that benzyl esters are compatible with our oxidative reaction conditions.
With these results in hand, we evaluated the allylic oxidation of more complex substrates. We found that a derivative of santonin, which is the five-membered α-allyl lactone 1g, underwent benzoylation under the conditions we developed in high yield. The cyclohexadiene moiety of 1g, which is responsible for the biological activity of santonin, did not undergo further oxidation.21 The six-membered lactone derived from deoxy-artemisnin, 1h, also underwent oxidation to furnish 2h in moderate yield.
High product yields were also obtained when linear amide 1i and lactam 1j were oxidized under the conditions we developed. Previous attempts to functionalize hindered, linear amides like 1i resulted in low conversions; lactams were the only class of hindered carbonyl compound that underwent oxidation in synthetically relevant yields.12 The oxidation of substrate 1k to 2k indicates that homoallylic carbamates are also compatible with our reaction conditions.
The benzoylation of homoallylic alcohol 1m led us to evaluate the oxidation of more complex homoallylic alcohols. Homoallylic alcohols derived from estrone and cholesterone underwent selective allylic oxidation (1n, 1p). These results confirm that alcohols derived from 5- and 6-membered cyclic ketones can be oxidized in moderate to good yields. Products derived from the oxidation of the trisubstituted olefin 1p did not form in significant quantities, suggesting that this system selectively oxidizes terminal olefins. This observation is corroborated by the selective oxidation of citronellal derivative 1l and α-ionone derivative 1o. The selective oxidation of the terminal olefin of 1o occurs in the presence of trisubstituted and disubstituted alkenes. This selectivity is valuable for the functionalization of terpenoid natural products, which often contain multiple olefins.
The benzoylation of compounds derived from natural products, such as santonin, deoxy-artmesinin, proline, citronellal, estrone, cholesterone, and α-ionone, establishes the utility of our method for the functionalization of complex molecules. To further demonstrate the synthetic value of our benzoylation procedure, 2g was prepared on a 1 g scale. The selective benzoylation of 1g is challenging because it contains multiple alkenes. This substrate underwent selective oxidation catalyzed by 5 mol % Pd(OBz)2, 5 mol % DAFO, and 20 mol % BQ. After 9 h at 65 °C, a 75% yield of 2g was determined by 1H NMR spectroscopy, and an isolated yield of 70% of 2g was obtained. These results closely match those obtained when 1g was benzoylated on a 0.2 mmol scale with a 10 mol % loading of palladium (Scheme 3).
Scheme 3.
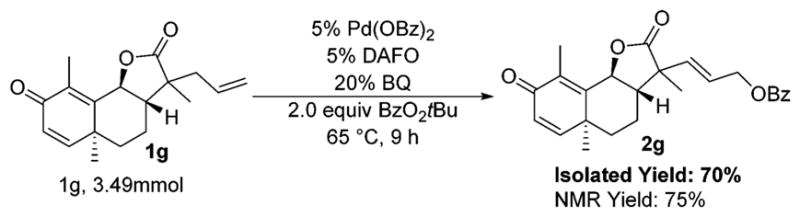
Gram-Scale Oxidation of Allyl–Santonin
In conclusion, we have demonstrated that the combination of palladium(II) benzoate, 4,5-diazafluoren-9-one, and benzoquinone catalyzes the benzoylation of hindered, terminal olefins in the presence of tert-butyl benzoyl peroxide. In many cases, the inclusion of benzoquinone in our reaction conditions led to higher reaction rates and product yields than were obtained in its absence. Under these new conditions, hindered substrates undergo rapid and selective functionalization. The application of this method to natural products provides intermediates for further diversification. Finally, the synthetic relevance of this method has been validated by the gram-scale oxidation of a santonin derivative.
Supplementary Material
Acknowledgments
We thank the NIH (No. GM 55382) for support of this work and Johnson–Matthey for a gift of Pd(OAc)2.
Footnotes
Notes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscatal.6b03648.
Experimental details for all reactions and analytical details of all relevant materials (PDF)
References
- 1.(a) Lyons TW, Sanford MS. Chem Rev. 2010;110:1147–1169. doi: 10.1021/cr900184e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wencel-Delord J, Dröge T, Liu F, Glorius F. Chem Soc Rev. 2011;40:4740–4761. doi: 10.1039/c1cs15083a. [DOI] [PubMed] [Google Scholar]; (c) Kuhl N, Hopkinson MN, Wencel-Delord J, Glorius F. Angew Chem, Int Ed. 2012;51:10236–10254. doi: 10.1002/anie.201203269. [DOI] [PubMed] [Google Scholar]; (d) Wencel-Delord J, Glorius F. Nat Chem. 2013;5:369–375. doi: 10.1038/nchem.1607. [DOI] [PubMed] [Google Scholar]; (e) Davies HML, Morton D. J Org Chem. 2016;81:343–350. doi: 10.1021/acs.joc.5b02818. [DOI] [PubMed] [Google Scholar]
- 2.Lumbroso A, Cooke ML, Breit B. Angew Chem, Int Ed. 2013;52:1890–1932. doi: 10.1002/anie.201204579. [DOI] [PubMed] [Google Scholar]
- 3.For a review of allylic acetoxylation, see: Liron F, Oble J, Lorion MM, Poli G. Eur J Org Chem. 2014;2014:5863–5883.
- 4.Kim KE, Li J, Grubbs RH, Stoltz BM. J Am Chem Soc. 2016;138:13179–13182. doi: 10.1021/jacs.6b08788. [DOI] [PubMed] [Google Scholar]
- 5.(a) Walker WE, Manyik RM, Atkins KE, Farmer ML. Tetrahedron Lett. 1970;11:3817–3820. [Google Scholar]; (b) Atkins KE, Walker WE, Manyik RM. Tetrahedron Lett. 1970;11:3821–3824. [Google Scholar]; (c) Hata G, Takahashi K, Miyake A. J Chem Soc D. 1970:1392–1393. [Google Scholar]
- 6.(a) Vargaftik MN, Moiseev II, Syrkin YK. Izv Akad Nauk SSSR. 1962;5:930–931. [Google Scholar]; (b) Anderson CB, Winstein S. J Org Chem. 1963;28:605–606. [Google Scholar]; (c) Wolfe S, Campbell PGC. J Am Chem Soc. 1971;93:1497–1499. [Google Scholar]; (d) Tsuji J, Sakai K, Nagashima H, Shimizu I. Tetrahedron Lett. 1981;22:131–134. [Google Scholar]; (e) Uemura S, Fukuzawa S-i, Toshimitsu A, Okano M. Tetrahedron Lett. 1982;23:87–90. [Google Scholar]; (f) McMurry JE, Kočotovský P. Tetrahedron Lett. 1984;25:4187–4190. [Google Scholar]; (g) Hansson S, Heumann A, Rein T, Aakermark B. J Org Chem. 1990;55:975–984. [Google Scholar]; (h) Larock RC, Hightower TR. J Org Chem. 1993;58:5298–5300. [Google Scholar]; (i) Grennberg H, Simon V, Bäckvall JE. J Chem Soc, Chem Commun. 1994:265–266. [Google Scholar]; (j) Chen MS, White MC. J Am Chem Soc. 2004;126:1346–1347. doi: 10.1021/ja039107n. [DOI] [PubMed] [Google Scholar]; (k) Chen MS, Prabagaran N, Labenz NA, White MC. J Am Chem Soc. 2005;127:6970–6971. doi: 10.1021/ja0500198. [DOI] [PubMed] [Google Scholar]; (l) Campbell AN, White PB, Guzei IA, Stahl SS. J Am Chem Soc. 2010;132:15116–15119. doi: 10.1021/ja105829t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Delcamp JH, White MC. J Am Chem Soc. 2006;128:15076–15077. doi: 10.1021/ja066563d. [DOI] [PubMed] [Google Scholar]; (b) Vermeulen NA, Delcamp JH, White MC. J Am Chem Soc. 2010;132:11323–11328. doi: 10.1021/ja104826g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Covell DJ, Vermeulen NA, Labenz NA, White MC. Angew Chem, Int Ed. 2006;45:8217–8220. doi: 10.1002/anie.200603321. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Liu Z, Bittman R. Org Lett. 2012;14:620–623. doi: 10.1021/ol2032448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henderson WH, Check CT, Proust N, Stambuli JP. Org Lett. 2010;12:824–827. doi: 10.1021/ol902905w. [DOI] [PubMed] [Google Scholar]
- 10.(a) Aakermark B, Larsson EM, Oslob JD. J Org Chem. 1994;59:5729–5733. [Google Scholar]; (b) Grennberg H, Bäckvall JE. Chem—Eur J. 1998;4:1083–1089. [Google Scholar]; (c) Lin BL, Labinger JA, Bercaw JE. Can J Chem. 2009;87:264–271. [Google Scholar]; (d) Diao T, Stahl SS. Polyhedron. 2014;84:96–102. doi: 10.1016/j.poly.2014.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) White PB, Jaworski JN, Fry CG, Dolinar BS, Guzei IA, Stahl SS. J Am Chem Soc. 2016;138:4869–4880. doi: 10.1021/jacs.6b01188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma A, Hartwig JF. J Am Chem Soc. 2013;135:17983–17989. doi: 10.1021/ja409995w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xing X, O’Connor NR, Stoltz BM. Angew Chem, Int Ed. 2015;54:11186–11190. doi: 10.1002/anie.201504007. [DOI] [PubMed] [Google Scholar]
- 13.(a) Hosomi A. Acc Chem Res. 1988;21:200–206. [Google Scholar]; (b) Yamasaki S, Fujii K, Wada R, Kanai M, Shibasaki M. J Am Chem Soc. 2002;124:6536–6537. doi: 10.1021/ja0262582. [DOI] [PubMed] [Google Scholar]; (c) Wadamoto M, Yamamoto H. J Am Chem Soc. 2005;127:14556–14557. doi: 10.1021/ja0553351. [DOI] [PubMed] [Google Scholar]; (d) Yus M, González-Gómez JC, Foubelo F. Chem Rev. 2013;113:5595–5698. doi: 10.1021/cr400008h. [DOI] [PubMed] [Google Scholar]
- 14.Wada R, Oisaki K, Kanai M, Shibasaki M. J Am Chem Soc. 2004;126:8910–8911. doi: 10.1021/ja047200l. [DOI] [PubMed] [Google Scholar]
- 15.(a) Casolari S, D’Addari D, Tagliavini E. Org Lett. 1999;1:1061–1063. [Google Scholar]; (b) Waltz KM, Gavenonis J, Walsh PJ. Angew Chem, Int Ed. 2002;41:3697–3699. doi: 10.1002/1521-3773(20021004)41:19<3697::AID-ANIE3697>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]; (c) Cunningham A, Woodward S. Synlett. 2002;2002:0043–0044. [Google Scholar]; (d) Kii S, Maruoka K. Chirality. 2003;15:68–70. doi: 10.1002/chir.10163. [DOI] [PubMed] [Google Scholar]; (e) Kim JG, Waltz KM, Garcia IF, Kwiatkowski D, Walsh PJ. J Am Chem Soc. 2004;126:12580–12585. doi: 10.1021/ja047758t. [DOI] [PubMed] [Google Scholar]
- 16.Gormisky PE, White MC. J Am Chem Soc. 2011;133:12584–12589. doi: 10.1021/ja206013j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Speziali MG, Robles-Dutenhefner PA, Gusevskaya EV. Organometallics. 2007;26:4003–4009. [Google Scholar]
- 18.(a) Bäckvall JE, Bystroem SE, Nordberg RE. J Org Chem. 1984;49:4619–4631. [Google Scholar]; (b) Bäckvall JE, Nystroem JE, Nordberg RE. J Am Chem Soc. 1985;107:3676–3686. [Google Scholar]; (c) Bäckvall JE, Gogoll A. Tetrahedron Lett. 1988;29:2243–2246. [Google Scholar]; (d) Szabó KJ. Organometallics. 1998;17:1677–1686. [Google Scholar]
- 19.(a) Behenna DC, Stoltz BM. J Am Chem Soc. 2004;126:15044–15045. doi: 10.1021/ja044812x. [DOI] [PubMed] [Google Scholar]; (b) Trost BM, Xu J, Schmidt T. J Am Chem Soc. 2009;131:18343–18357. doi: 10.1021/ja9053948. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Liu Y, Han SJ, Liu WB, Stoltz BM. Acc Chem Res. 2015;48:740–751. doi: 10.1021/ar5004658. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Craig RA, Loskot SA, Mohr JT, Behenna DC, Harned AM, Stoltz BM. Org Lett. 2015;17:5160–5163. doi: 10.1021/acs.orglett.5b02376. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Kita Y, Numajiri Y, Okamoto N, Stoltz BM. Tetrahedron. 2015;71:6349–6353. doi: 10.1016/j.tet.2015.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Numajiri Y, Jiménez-Osés G, Wang B, Houk KN, Stoltz BM. Org Lett. 2015;17:1082–1085. doi: 10.1021/ol503425t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keck G, Kraft M, Truong A, Sanchez C, Li W, Covel J, Welch D, Poudel Y, Peterson M. Bryostatin Analogs and Use Thereof as Antiviral Agents. WO2016025363 (A1) International Patent. 2016
- 21.Adekenov SM, Gafurov NM. Chem Nat Compd. 1992;28:452–455. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.