Abstract
The goal of our study was to compare the impact of pancreaticogastrostomy (PG) versus pancreaticojejunostomy (PJ) on the incidence of complications after pancreaticoduodenectomy. A systematic search was performed using RevMan 5.3 software. A meta-analysis showed that PG was not superior to PJ in terms of postoperative pancreatic fistula (POPF). In multicenter randomized controlled trials, the incidence of POPF was lower in patients undergoing PG than in those undergoing PJ. However, PG was associated with an increased risk of postoperative intraluminal hemorrhage, but no significant difference was observed between 2-layer PG and PJ. No significant differences were found in the rate of overall delayed gastric emptying, biliary fistula, reoperation, mortality, and morbidity. PG and PJ have similar incidences of POPF, but PG could be slightly superior to PJ in multicenter trials. However, this analysis verifies that PG has a higher rate of postpancreatectomy hemorrhage. Of note, a 2-layer anastomosis could reduce the occurrence of postpancreatectomy hemorrhage.
Key Words: meta-analysis, pancreaticojejunostomy, pancreaticogastrostomy, postoperative pancreatic fistula, randomized control trial
Pancreaticoduodenectomy (PD) is a complex, high-risk surgical procedure that is indicated primarily for neoplasms. PD may also be needed to manage pancreatic or duodenal trauma and chronic pancreatitis. Although operative mortality in patients undergoing PD has decreased to <5%, the incidence of postoperative morbidity remains high at 35% to 60%.1–6 Postoperative pancreatic fistula (POPF), with a prevalence of 5% to 30% after PD,7,8 still represented a clnically relevant problem, which could lead to many other postoperative complications and death.9 Therefore, the prevention and treatment of POPF is of the utmost importance. Before 2005, POPF was defined in a variety of manners, with >20 different definitions for clinical research exchange and the comparison of difficulties.10–12 The International Study Group on Pancreatic Fistula (ISGPF) organized experts from well-known European, Japanese, Australian, North American, and South American centers in 2005 to establish the definition and classification system of pancreatic fistula.13 In 2016, the ISGPS changed Grade A POPF to “biochemical leak,” which did not affect the clinical course.14
Pancreaticogastrostomy (PG) and pancreaticojejunostomy (PJ) are 2 widely undertaken surgical reconstruction techniques after PD. PG was first described by Waugh and Clagett15 in 1946 and has gained popularity in recent years as a result of a possible reduction in the occurrence of POPF. The optimal method of pancreatic-enteric anastomosis is not clear. Some studies comparing PJ with PG showed no difference between these methods.16–18 However, others showed that fewer postoperative fistulas develop after PG.19–21 Therefore, we performed an up-to-date meta-analysis of randomized controlled trials (RCTs) to summarize the currently available evidence on PG versus PJ according to the newest 2016 ISGPS criteria.
METHODS
Search Strategy
Two authors independently conducted a comprehensive search of sources including PubMed, Embase, Web of Science, and Cochrane Library Central from August 1, 1990 to August 1, 2017. The English search terms included the following: “pancreaticogastrostomy,” “pancreaticgastrostomies,” “pancreatogastrostomy,” “pancreatogastrostomies,” “pancreaticogastricanastomosis,” “pancreaticojejunostomy,” “pancreaticojejunostomies,” “pancreatojejunostomy,” “pancreatojejunostomies,” “pancreaticojejunal anastomosis,” “pancreaticoduodenectomy,” “pancreatoduodenectomy,” and “Whipple.” Only RCTs that reported POPF as the primary endpoint were included. The references of the articles identified after an initial search were also manually reviewed.
Inclusion and Exclusion Criteria
The following inclusion criteria were applied: (1) RCTs comparing the incidence of POPF after PG and PJ; (2) the definition of POPF conformed to the ISGPS (2016) guidelines; and (3) clinical studies using only human subjects.
We excluded studies that (1) were non-RCTs, retrospective studies, review articles, case reports, abstracts, editorials, and letters to the editor; (2) were published by the same author or agency repeatedly; and (3) had insufficient data on outcome measures of POPF.
Outcomes of Interest
The primary outcome measure was POPF. Secondary endpoints included postoperative delayed gastric emptying (DGE), postpancreatectomy hemorrhage (PPH), biliary fistula, and overall morbidity, mortality, and reoperation rates.
Data Extraction
To ensure the homogeneity of the extracted data, 2 authors independently extracted the original data in the literature onto a standardized form, including the first author, year of publication, type of study, country where the study was conducted, study design, and occurrence of POPF, PPH, DGE, and postoperative complications. If necessary, the author or authors of the study were contacted to obtain study data. Conflicts in data abstraction were resolved by a consensus and by referring to the original article.
Risk of Bias Assessment
The authors independently assessed the quality of the literature in accordance with the Cochrane Collaboration Handbook.22 The scoring system included the following criteria: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of the result assessment, incomplete data in the results, selective reporting, and other sources of bias.
Study quality was also assessed with the Jadad scale for RCTs.23 Two reviewers independently assessed the quality of the included studies, and discrepancies were resolved by discussion in plenum. One point was given for each of the 5 criteria the study fulfilled, resulting in a score ranging from 0 to 5. Studies receiving total scores between 0 and 2 were considered to be of poor methodological quality, whereas studies with a total score between 3 and 5 were considered to be of high methodological quality.
Statistical Analysis
All statistical analyses were performed using the Review Manager (RevMan) version 5.3 software (The Cochrane Collaboration, Oxford, UK). Risk ratios (RRs) with a 95% confidence interval (CI) were used to describe dichotomous outcomes. The publication bias was evaluated by a χ2 test and funnel plots. The heterogeneity among studies was evaluated by a χ2 test. A 2-tailed p-value of <0.05 was considered statistically significant. We also assessed the potential for publication bias through a visual inspection of funnel plot asymmetry. The meta-analysis was conducted according to the PRISMA statement.
RESULTS
Selected Studies and Characteristics of the Trials
On the basis of our search criteria, we identified 450 papers from the respective search engines, of which 360 duplicate articles were excluded. The remaining 150 studies were retrieved to examine their titles and abstracts, resulting in 9 articles that appeared to meet our selection criteria. Among these articles, 2 were excluded because they were retrospective studies. Finally, 7 RCTs with 1184 participants were included in the meta-analysis.1,2,24–28 A detailed flowchart of the selection process is shown in Figure 1.
FIGURE 1.
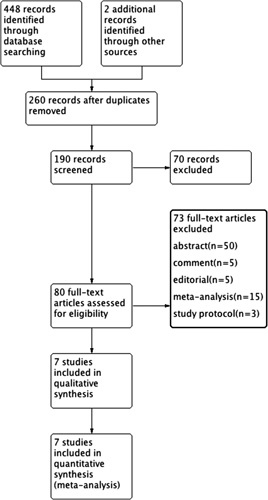
Flow diagram of the published articles evaluated for inclusion in this meta-analysis.
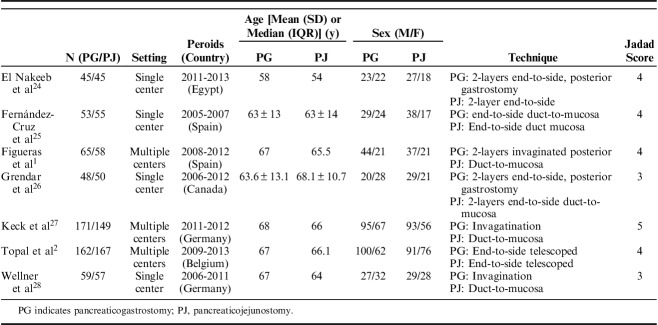
The 1184 patients were randomized to either the PG (n=603) or PJ (n=581) groups. Sample sizes ranged from 90 to 329, and the incidence rate of POPF varied from 11.11% to 21.14%. Three RCTs were multicenter trials, and 4 were single-center trials. All RCTs were published between 2008 and 2016. The indications for a surgical procedure included pancreatic ductal adenocarcinoma, duodenal cancer, ampullary carcinoma, distal bile duct cancer, and others. The texture of the pancreas was described in 6 studies. Six studies reported the prophylactic use of octreotide, and the data were comparable between the 2 intervention groups. The main characteristics of the studies included in this meta-analysis are presented in Table 1. Figure 2 presents an overview of the methodological quality of the studies included in the review.
TABLE 1.
Characteristics of Studies Included in the Systematic Review
FIGURE 2.
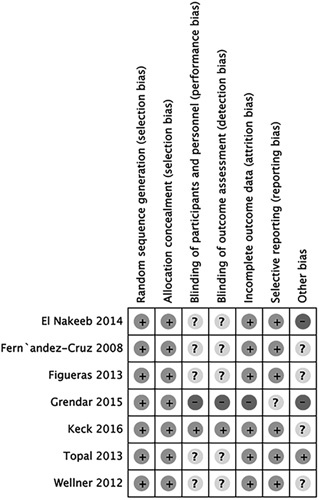
Consensus risk of bias assessment of the included studies.
Primary Outcome
All 7 studies compared the 2 anastomotic techniques with regard to the POPF rate (RR, 0.61; 95% CI, 0.34-1.09; P=0.09) (Fig. 3). The rate of POPF in the 3 multicenter studies was lower in the PG group (RR, 0.45; 95% CI, 0.21-0.98; P=0.04) (Fig. 4A), but no significant difference was found in single-center studies (RR, 0.87; 95% CI, 0.35-2.17; P=0.76) (Fig. 4B). The incidence of POPF after 2-layer PG was not significantly different from that of PJ (RR, 0.8; 95% CI, 0.22-3.18; P=0.80) (Fig. 4C). No statistically significant differences were observed in the incidence rates of POPF in 4 of 7 RCTs, and the incidence after single-layer PG was not significantly different from that of PJ (RR, 0.53; 95% CI, 0.27-1.02; P=0.06) (Fig. 4D). The meta-analysis of 7 studies also showed no significant difference in the rate of POPF in the PG group versus the duct-to-mucosa or telescope PJ group (RR, 0.63; 95% CI, 0.33-2.18; P=0.15 vs. RR, 0.74; 95% CI, 0.15-3.79; P=0.72) (Figs. 5E, F).
FIGURE 3.
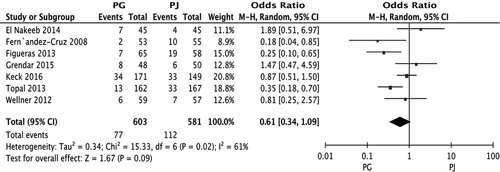
Forest plot of the meta-analysis comparing PG and PJ with regard to the incidence of POPF. CI indicates confidence interval; PG, pancreaticogastrostomy; PJ, pancreaticojejunostomy; POPF, postoperative pancreatic fistula.
FIGURE 4.
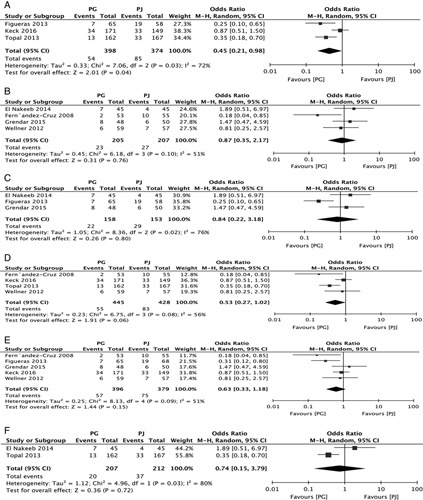
Forest plot of the subgroup meta-analysis of the incidence of POPF according to the type of center (A and B); based on the type of PG (C and D); based on the type of PJ (E and F). CI indicates confidence interval; PG, pancreaticogastrostomy; PJ, pancreaticojejunostomy; POPF, postoperative pancreatic fistula.
FIGURE 5.
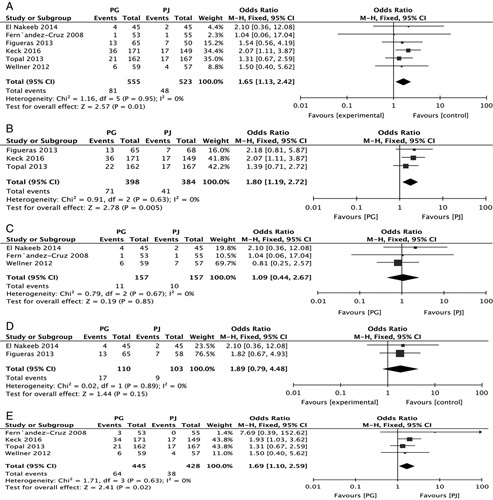
Forest plot of the subgroup meta-analysis of the incidence of PPH (A); according to type of center (B and C); according to the type of PG (D and E). CI indicates confidence interval; PG, pancreaticogastrostomy; PJ, pancreaticojejunostomy; PPH, postpancreatectomy hemorrhage.
Secondary Outcomes
PPH
Six RCTs reported the overall PPH incidence (81/555 vs. 48/523) in the PG and PJ groups, respectively. A meta-analysis of the 6 studies, using a fixed-effect model, demonstrated that PJ was significantly superior to PG (RR, 1.65; 95% CI, 1.13-2.42, P=0.01) (Fig. 5A). Three RCTs that reported the overall PPH incidence were multicenter trials. A meta-analysis of these 3 studies demonstrated that PJ was significantly superior to PG (RR, 1.80; 95% CI, 1.19-2.72; P=0.005) (Fig. 5B). Four RCTs reported the overall PPH incidence in single centers. A meta-analysis of these 4 studies showed that PJ was not significantly superior to PG (RR, 1.09; 95% CI, 0.44-2.67; P=0.85) (Fig. 5C). Two RCTs reported the overall PPH incidence after 2-layer PG. A meta-analysis of these 2 studies demonstrated that PJ was not significantly superior to PG (RR, 1.89; 95% CI, 0.79-4.48; P=0.15) (Fig. 5D). Four RCTs reported the overall PPH incidence after single-layer PG. A meta-analysis of these 4 studies demonstrated that PJ was significantly superior to PG (RR, 1.69; 95% CI, 1.10-2.59; P=0.02) (Fig. 5D).
DGE
Six RCTs reported the overall DGE incidence (13/555 vs. 116/531) in the PG and PJ groups. A meta-analysis of these 6 studies demonstrated that PG was not significantly superior to PJ (RR, 1.10; 95% CI, 0.82-1.48; P=0.50) (Fig. 6A).
FIGURE 6.
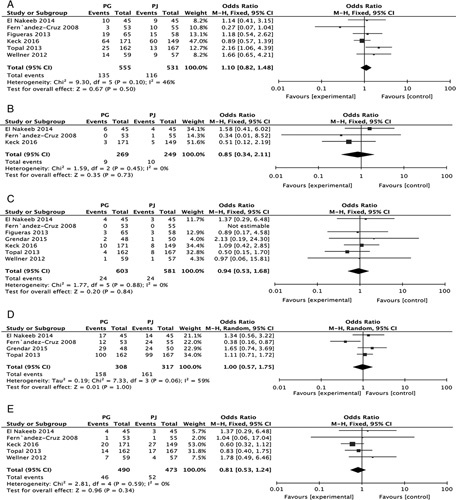
Forest plot of the subgroup meta-analysis of the incidence of DGE (A); bile fistula (B); mortality (C); morbidity (D) and reoperation (E). CI indicates confidence interval; DGE, delayed gastric emptying; PG, pancreaticogastrostomy; PJ, pancreaticojejunostomy.
Bile Fistula
Three RCTs reported the bile fistula incidence (9/269 vs. 10/249) in the PG and PJ groups. A meta-analysis of 6 studies demonstrated that PG was not significantly superior to PJ (RR, 0.85; 95% CI, 0.34-2.11; P=0.73) (Fig. 6B).
Mortality
Seven RCTs reported mortality rates (24/603 vs. 24/581) in the PG and PJ groups. A meta-analysis of these 7 studies demonstrated that PG was not significantly superior to PJ (RR, 0.84; 95% CI, 0.53-1.68; P=0.84) (Fig. 6C).
Morbidity
Four RCTs reported morbidity rates (158/308 vs. 161/317) in the PG and PJ groups. A meta-analysis of 4 studies demonstrated that PG was not significantly superior to PJ (RR, 1.00, 95% CI, 0.57-1.72, P=1.00) (Fig. 6D).
Reoperation
Five RCTs reported reoperation rates (46/490 vs. 52/473) in the PG and PJ groups. A meta-analysis of 5 studies demonstrated that PG was not significantly superior to PJ (RR, 0.81; 95% CI, 0.53-1.24, P=0.34) (Fig. 6E).
DISSCUSSION
The results of this meta-analysis show that PG and PJ are comparable anastomotic techniques following PD. Previous studies have suggested that PG is superior to PJ. Three RCTs,10,11,29 2 from high-volume centers and 1 multicenter trial,10,11 found no differences in terms of reconstruction techniques. Some differences are present in these studies, largely because of the lack of a uniform definition of POPF. Compared with previous meta-analyses on the same topic, our study included RCTs on the basis of the newest definition of POPF by the ISGPS, which increased its statistical strength. Of note, our analysis showed no significant difference in terms of POPF after PG compared with PJ. Most previous studies that have compared PG with PJ with regard to POPF were single-center trials. The strengths of single-center RCTs might be insufficient compared with multicenter RCTs. Subgroup analysis showed that the incidence of POPF in the PG group was lower than that of the PJ group in multicenter trials, but no significant difference was found in the single-center trials. Therefore, PG is slightly superior to PJ in terms of POPF occurrence. Some potential advantages of PG include that the presence of gastric acid can effectively inhibit the activation of pancreatic enzymes,2 the tension between the stomach and the pancreatic stump is minimized, and the abundant stomach wall vascularization decreases the chance of anastomotic ischemia.19 The occurrence of POPF after pancreatic surgery is related to the normal texture of the pancreas and the diameter of the pancreatic duct.30–32 Some studies included a description of the pancreatic texture.33,34 The texture of the pancreas and the diameter of the pancreatic ducts were different among the 7 included studies. These differences might have affected the outcome. Therefore, subgroup analysis of risk factors of different types of pancreatic fistula is necessary.
The surgical reconstruction techniques of PG include single-layer or double-layer PG with or without anterior gastrotomy. Few studies have examined the effect of different PG reconstruction techniques on POPF occurrence. This study demonstrated no difference in the incidence of POPF between 2-layer and single-layer techniques. Similar results were obtained in another study.16 Two predominant methods of PJ reconstruction exist: duct-to-mucosa anastomosis and invagination of the pancreatic remnant. No conclusive evidence to favor 1 method over the other exists. The subgroup analysis of the duct-to-mucosa and telescope techniques showed no difference between PG and PJ. These studies may indicate that specific surgical approaches in PG and PJ have little impact on POPF occurrence. The choice is determined by surgeon preference and the familiarity with different surgical approaches. However, it remains debatable which is the better reconstruction method after PD. However, the details of specific surgical methods were varied in these studies, and this conclusion remains to be further studied.
Another complication after PD is PPH. In the previous RCTs, the incidence of PPH was significantly higher in the PG group than in the PJ group.1,28,29 One potential reason is the abundant blood supply. A similar conclusion is also shown in the present study.35 The different PG anastomotic techniques include the single-layer or double-layer technique. For this reason, we have provided a subgroup analysis. In the 2-layer subgroup, the incidence of PPH in the PG group was similar to that of the PJ group. However, the rate of PPH was higher in the single-layer PG subgroup. A possible explanation is that double-layer anastomosis reduces the incidence of gastrointestinal tract bleeding after PG. PPH includes gastrointestinal bleeding and intraperitoneal hemorrhage. Among the 7 included RCTs, some studies did not distinguish between gastrointestinal bleeding and intraperitoneal hemorrhage. Further study on bleeding due to different causes is needed.
No differences were observed in DGE after PD between the 2 anastomosis groups. High heterogeneity was found between studies because of the lack of a standard definition of DGE among the studies. As previously described, some factors such as old age, early enteral nutrition and resection techniques, including PD and pylorus preserving pancreatoduodenectomy (PPPD), are the major risk factors of DGE after PD. Therefore, more RCTs on this subject are needed. No differences were observed between groups in the incidence of other site-related complications such as enteric or biliary fistula, mortality, morbidity, and reoperation, although these factors were only reported in a few studies.
Some limitations should be considered with regard to this review. First, the details of the surgical technique, such as the use of pancreatic stents and different types of sutures, are highly heterogeneous. In addition, because it was not possible to perform subgroup analyses according to pancreatic duct size, pancreatic texture or pancreatic pathology, it is unclear whether the potential advantages of PG are applicable to all subgroups of patients. Third, the use of prophylactic somatostatin or somatostatin analogs may also contribute to reducing the risk of POPF.
CONCLUSIONS
In summary, the findings of the present meta-analysis of randomized controlled studies demonstrate that PG and PJ exhibit similar incidences of POPF, but PG could be slightly superior to PJ. However, this study confirms that PG has a higher rate of PPH than PJ. Of note, 2-layer anastomosis could reduce the occurrence of PPH. Although this evidence was obtained from up-to-date randomized trials, PG cannot be considered superior to PJ in the incidence of DGE, bile fistula overall morbidity, reoperations, and mortality. Given the limitations of the current study, more large-scale, high-quality RCTs are required.
ACKNOWLEDGMENTS
We would like to acknowledge and thank the native English-speaking biologists who provided medical writing services on behalf of American Journal Experts.
Footnotes
All the data used in the study can be obtained from the original articles.
The author(s) received no financial support for the research, authorship, and/or publication of this article. The authors declare no conflicts of interest.
REFERENCES
- 1.Figueras J, Sabater L, Planellas P, et al. Randomized clinical trial of pancreaticogastrostomy versus pancreaticojejunostomy on the rate and severity of pancreatic fistula after pancreaticoduodenectomy. Br J Surg. 2013;100:1597–1605. [DOI] [PubMed] [Google Scholar]
- 2.Topal B, Fieuws S, Aerts R, et al. Pancreaticojejunostomy versus pancreaticogastrostomy reconstruction after pancreaticoduodenectomy for pancreatic or periampullary tumours: a multicentre randomised trial. Lancet Oncol. 2013;14:655–662. [DOI] [PubMed] [Google Scholar]
- 3.Addeo P, Delpero JR, Paye F, et al. Pancreatic fistula after a pancreaticoduodenectomy for ductal adenocarcinoma and its association with morbidity: a multicentre study of the French Surgical Association. HPB. 2014;16:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muscari F, Suc B, Kirzin S, et al. Risk factors for mortality and intra-abdominal complications after pancreatoduodenectomy: multivariate analysis in 300 patients. Surgery. 2006;139:591–598. [DOI] [PubMed] [Google Scholar]
- 5.Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–1211. [DOI] [PubMed] [Google Scholar]
- 6.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bassi C, Butturini G, Molinari E, et al. Pancreatic fistula rate after pancreatic resection. The importance of definitions. Dig Surg. 2004;21:54–59. [DOI] [PubMed] [Google Scholar]
- 8.Schäfer M, Müllhaupt B, Clavien PA. Evidence-based pancreatic head resection for pancreatic cancer and chronic pancreatitis. Ann Surg. 2002;236:137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paye F. The pancreatic stump after pancreatoduodenectomy: the “Achille’s heel” revisited…. J Visceral Surg. 2010;147:e13–e20. [DOI] [PubMed] [Google Scholar]
- 10.Bassi C, Falconi M, Molinari E, et al. Reconstruction by pancreaticojejunostomy versus pancreaticogastrostomy following pancreatectomy: results of a comparative study. Ann Surg. 2005;242:767–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeo CJ, Cameron JL, Maher MM, et al. A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg. 1995;222:580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duffas J-P, Suc B, Msika S, et al. A controlled randomized multicenter trial of pancreatogastrostomy or pancreatojejunostomy after pancreatoduodenectomy. Am J Surg. 2005;189:720–729. [DOI] [PubMed] [Google Scholar]
- 13.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an International Study Group (ISGPF) definition. Surgery. 2005;138:8–13. [DOI] [PubMed] [Google Scholar]
- 14.Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161:584–591. [DOI] [PubMed] [Google Scholar]
- 15.Waugh JM, Clagett OT. Resection of the duodenum and head of the pancreas for carcinoma; an analysis of thirty cases. Surgery. 1946;20:224–232. [PubMed] [Google Scholar]
- 16.Crippa S, Cirocchi R, Randolph J, et al. Pancreaticojejunostomy is comparable to pancreaticogastrostomy after pancreaticoduodenectomy: an updated meta-analysis of randomized controlled trials. Langenbecks Arch Surg. 2016;401:427–437. [DOI] [PubMed] [Google Scholar]
- 17.Wente MN, Shrikhande SV, Müller MW, et al. Pancreaticojejunostomy versus pancreaticogastrostomy: systematic review and meta-analysis. Am J Surg. 2007;193:171–183. [DOI] [PubMed] [Google Scholar]
- 18.Perivoliotis K, Sioka E, Tatsioni A, et al. Pancreatogastrostomy versus pancreatojejunostomy: an Up-to-Date Meta-Analysis of RCTs. Int J Surg Oncol. 2017;2017:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin H, Luo L, Zhu Z, et al. Pancreaticogastrostomy has advantages over pancreaticojejunostomy on pancreatic fistula after pancreaticoduodenectomy. A meta-analysis of randomized controlled trials. Int J Surg. 2016;36:18–24. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Ma L, Gao X, et al. Pancreaticogastrostomy versus pancreaticojejunostomy reconstruction after pancreaticoduodenectomy: a meta-analysis of randomized controlled trials. Surg Today. 2015;45:585–594. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y, Yu J, Wu L, et al. Meta-analysis of pancreaticogastrostomy versus pancreaticojejunostomy on occurrences of postoperative pancreatic fistula after pancreaticoduodenectomy. Asian J Surg. 2015;38:155–160. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- 24.El Nakeeb A, Hamdy E, Sultan AM, et al. Isolated Roux loop pancreaticojejunostomy versus pancreaticogastrostomy after pancreaticoduodenectomy: a prospective randomized study. HPB. 2014;16:713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernández-Cruz L, Cosa R, Blanco L, et al. Pancreatogastrostomy with gastric partition after pylorus-preserving pancreatoduodenectomy versus conventional pancreatojejunostomy: a prospective randomized study. Ann Surg. 2008;248:930–938. [DOI] [PubMed] [Google Scholar]
- 26.Grendar J, Ouellet J, Sutherland F, et al. In search of the best reconstructive technique in the whipple operation pancreaticojejunostomy versus pancreaticogastrostomy. A randomized clinical trial. Official J Int Hepato Pancreato Biliary Assoc. 2014;16:1. [Google Scholar]
- 27.Keck T, Wellner U, Bahra M, et al. Pancreatogastrostomy versus pancreatojejunostomy for RECOnstruction after PANCreatoduodenectomy (RECOPANC, DRKS 00000767): perioperative and long-term results of a multicenter randomized controlled trial. Ann Surg. 2016;263:440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wellner UF, Sick O, Olschewski M, et al. Randomized controlled single-center trial comparing pancreatogastrostomy versus pancreaticojejunostomy after partial pancreatoduodenectomy. J Gastrointest Surg. 2012;16:1686–1695. [DOI] [PubMed] [Google Scholar]
- 29.Duffas JP, Suc B, Msika S, et al. A controlled randomized multicenter trial of pancreatogastrostomy or pancreatojejunostomy after pancreatoduodenectomy. Am J Surg. 2005;189:720–729. [DOI] [PubMed] [Google Scholar]
- 30.Gouma DJ, van Geenen RC, van Gulik TM, et al. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg. 2000;232:786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pratt WB, Callery MP. Risk prediction for development of pancreatic fistula using the ISGPF classification scheme. World J Surg. 2008;32:419–428. [DOI] [PubMed] [Google Scholar]
- 32.Wellner UF, Kayser G, Lapshyn H, et al. A simple scoring system based on clinical factors related to pancreatic texture predicts postoperative pancreatic fistula preoperatively. HPB. 2010;12:696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murakami Y, Uemura K, Hayasidani Y, et al. A soft pancreatic remnant is associated with increased drain fluid pancreatic amylase and serum crp levels following pancreatoduodenectomy. J Gastrointest Surg. 2008;12:51–56. [DOI] [PubMed] [Google Scholar]
- 34.Shinchi H, Takao S, Maemura K, et al. A new technique for pancreaticogastrostomy for the soft pancreas: the transfixing suture method. J Hepatobiliary Pancreat Surg. 2006;13:212–217. [DOI] [PubMed] [Google Scholar]
- 35.He T, Zhao Y, Chen Q, et al. Pancreaticojejunostomy versus pancreaticogastrostomy after pancreaticoduodenectomy: a systematic review and meta-analysis. Dig Surg. 2013;30:56–69. [DOI] [PubMed] [Google Scholar]