Abstract
Background and Objective:
Allitridin [diallyl trisulfide (DATS)] is an extract from garlic (Allium sativum) that putatively improves endothelial function and is protective against cardiovascular diseases. Endothelial dysfunction after tissue ischemia in diabetic patients is partially due to poor angiogenic response. This study investigated whether DATS may improve angiogenesis in a diabetic mouse model with hind limb ischemia.
Methods:
Streptozotocin was administered by intraperitoneal injection to establish the model of diabetes in male C57BL/6 mice. After 14 days, nondiabetic and diabetic mice (n = 24, each) underwent unilateral hind limb ischemia by femoral artery ligation. The mice were apportioned to 4 groups: nondiabetic treated (or not) with DATS and diabetic treated (or not) with DATS. DATS treatment consisted of a single daily intraperitoneal injection of 500 μg·kg−1·d−1 for 14 days, beginning on the day of induced ischemia. Ischemia was scored by standard criteria. Blood perfusion was determined using thermal infrared imaging. Tissue capillary density and oxidative stress levels were measured by immunohistochemistry and immunofluorescence, respectively. Serum lipids were measured by enzymatic colorimetric assay. Fasting serum insulin was detected using an insulin enzyme-linked immunosorbent assay kit. Nitric oxide (NO) metabolites and protein carbonyls in tissues were determined by enzyme-linked immunosorbent assay. Targeted protein concentrations were measured by western blotting.
Results:
At 14 days after ligation, the ischemic skeletal muscle of the streptozotocin-induced diabetic mice had lower levels of endothelial NO synthase, phosphorylated endothelial NO synthase, and vascular endothelial growth factor compared with nondiabetic group. In addition, the hind limb blood perfusion, capillary density, and NO bioactivity were lower in the diabetic group, whereas oxidative stress and protein carbonyl levels were higher. These changes were ameliorated by DATS treatment.
Conclusions:
DATS treatment of diabetic mice promoted revascularization in ischemic tissue.
Key Words: diallyl trisulfide, angiogenesis, peripheral artery disease, diabetes
INTRODUCTION
Peripheral artery disease is a chronic arterial occlusive disorder of the extremities. It is caused by atherosclerosis, and the rates of morbidity and mortality are extremely high.1 The progression of atherosclerosis is exacerbated in diabetes mellitus, with accompanying endothelial damage, extracellular matrix protein glycosylation, and vascular denervation.2 Diabetic complications include impaired vascular remodeling and collateral formation after ischemic attack.3,4 Diabetes mellitus has also been shown to suppress endothelial nitric oxide synthase (eNOS) levels and nitric oxide (NO) bioactivity, resulting in impaired vascular function with concomitant elevated oxidative stress levels.5,6 The needed therapeutic modality for peripheral artery disease would promote angiogenesis with collateral vessel formation and revascularization. However, after much investigation in diabetic humans with peripheral artery disease, or animal models, such agents remain lacking.7
Allitridin [diallyl trisulfide (DATS)] is an organosulfur extract from garlic (Allium sativum).8 DATS has been demonstrated to have beneficial effects in cardiovascular diseases, including atherosclerosis9,10 and ischemia-reperfusion injury.11,12 These beneficial effects may be due to multitudinous biological activities, including antiplatelet aggregation and antioxidative stress.13,14 A recent study revealed that the antioxidative stress activity of DATS could attenuate diabetes and endothelial cell injury associated with high glucose levels.15 In addition, DATS was associated with higher eNOS levels that thereby upregulated NO activity to improve endothelial function.16 However, it remains unknown whether DATS may improve vascular disease or augment angiogenesis after ischemia complicated by diabetes.
This study investigated DATS for potential protective or angiogenic effects after ischemia in a mouse model of diabetes and the underlying mechanism(s).
METHODS
This study conformed to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (NIH Publication No. 85-23). The Animal Care Committee at Jiangsu University approved all the surgical protocols.
Animals and Diet
C57BL/6 mice (male, 8 week old) were purchased from SLAC Animal Co. (Shanghai, China) and housed in an environmentally controlled room (temperature at 22 ± 2°C with a 12-hour light–dark cycles). All mice had free access to water and food.
Establishment of Diabetic Mouse Model
The diabetic mouse model was established as previously described.17,18 Briefly, the mice were intraperitoneally (IP) injected with streptozotocin (STZ; 200 mg/kg) in a vehicle (0.1 mol/L sodium citrate buffer, pH 4.5) or citrate buffer (control). Blood glucose was measured 72 hours after the injection. A second injection of STZ (100 mg/kg) was given to mice with a blood glucose level <300 mg/dL. Four days after the second STZ injection, the blood glucose was again measured. Mice with blood glucose levels >300 mg/dL were accepted as diabetic mice.
Mouse Model of Hind Limb Ischemia
The mouse model of unilateral hind limb ischemia was created as previously described.19,20 Briefly, 2 weeks after, diabetes was successfully induced, with the mouse under anesthesia (chloral hydrate, 400 mg/kg, IP), the left femoral artery was ligated and cut (between the inguinal ligament proximally and popliteal fossa distally) to generate a model of severe ischemia.
DATS Treatments
After induction of ischemia, all animals were randomly allocated to 4 groups of 12 each: nondiabetic treated (or not) with DATS and diabetic treated (or not) with DATS. DATS treatment consisted of a single daily IP injection of 500 μg·kg−1·d−1 (based on previous literature21) for 14 days, beginning on the day of induced ischemia.
Necrosis Assay
The hind limb ischemia was scored as previously described.7 Briefly, on day 14 postligation, all mice were anesthetized (chloral hydrate, 400 mg/kg, IP), and ischemia of the foot was scored by gross examination as follows: (1) no necrosis; (2) slight necrosis, only in the nail bed; (3) necrosis in almost all digits; (4) ≥1 digit lost; and (5) severe ankle amputation.
Thermal Infrared Imaging Analysis
The blood perfusion of the ischemic/nonischemic hind limbs was determined by a thermal infrared imaging analyzer (Prism-DS 50137, FLIR Systems). In this method, blood perfusion in the ischemic hind limbs is indirectly represented as changes in color-coded images, in which dark-to-violet denotes low perfusion, and red-to-white indicates greater perfusion. The blood flow perfusion image was spotted and analyzed by computer-assisted analyses. Blood perfusion was calculated as a ratio of ischemic-to-nonischemic limb perfusion to minimize interference from surrounding light.20,22
Immunohistochemistry and Immunofluorescence Analysis
Twenty-four hours after the last IP injection of DATS, all mice were euthanized and the ischemic gastrocnemius muscles were fixed with 4% paraformaldehyde. For the immunohistochemistry assay, 3-μm thick muscle sections were stained with anti-CD31 antibody (Abcam, Cambridge, United Kingdom). For each animal, 8 fields chosen at random were counted, and the microvessel density was represented as the number of capillaries in each high field.23 To estimate the oxidative stress levels in ischemic tissues, antinitrotyrosine antibody (Upstate, Lake Placid, NY) was used for immunofluorescent staining, and the fluorescence intensity was quantified using Image Pro Plus software.20
Biochemical Analysis
Fasting blood glucose levels of the mice were obtained through tail vein measurement with a One Touch Glucometer (Johnson, NJ). Blood specimens for triglyceride and high-density lipoprotein analyses were obtained from the retro-orbital plexus and measured using an automatic biochemical analyzer and enzymatic colorimetric assay. The levels of fasting serum insulin were obtained with a mouse insulin enzyme-linked immunosorbent assay kit (ALPCO Diagnostics, Windham, NH), and the homeostasis model assessment of insulin resistance (HOMA-IR) was calculated and expressed by the formula, FINS × FBG/22.5.24,25
Measurement of Tissue Contents of Nitric Oxide Metabolite (NOx)
Two weeks after ligation of the left femoral artery, gastrocnemius ischemic skeletal muscles were collected. Equal amounts of muscle tissue were, respectively, homogenized and centrifuged to obtain protein supernatants. The total tissue NO (NOx) bioactivity was tested in accordance with the manufacturer's instructions (Beyotime, Haimen, China).
Measurement of Tissue Protein Carbonyl
Equal amounts of gastrocnemius ischemic skeletal muscle specimens were, respectively, homogenized and centrifuged to collect protein supernatants. Tissue protein carbonyls were detected by protein carbonyl enzyme-linked immunosorbent assay kit (Cell Biolabs, San Diego, CA) in accordance with the manufacturer's instructions. The tissue protein carbonyl concentration was normalized to the total protein contents and expressed as nmol/mg of protein.
Western Blotting
The concentration of total protein was measured by protein bicinchoninic acid assay kit (Beyotime). Equal amounts of protein specimens were resolved through sodium dodecyl sulfate polyacrylamide gel electrophoresis, then electrotransferred and immunoblotted with anti-eNOS, antiphosphorylated eNOS (anti-p-eNOS; Cell Signaling Technology, Danvers, MA), anti–vascular endothelial growth factor (anti-VEGF; Beyotime), and anti-β-actin (Proteintech Group, Chicago, IL). The membranes were incubated with IRDye 800CW conjugated secondary antibody. The bands of interest were captured with an Odyssey Imaging System (LI-COR Biosciences, Lincoln, NE). In our study, β-actin was used as an internal control to standardize the values.
Statistical Analysis
Results are shown as mean ± SE of the mean. Statistical analyses were conducted using 1-way analysis of variance and then Tukey's post hoc test. Differences were considered significant at P < 0.05.
RESULTS
Biochemical Parameters of the Treatment Groups
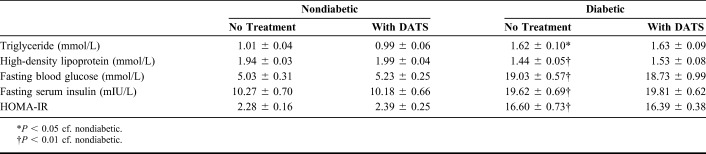
In the mouse groups not treated with DATS, the triglyceride, fasting blood glucose, fasting serum insulin, and HOMA-IR of the diabetic mice were significantly higher than those of the nondiabetic mice, whereas the high-density lipoprotein levels were significantly lower (Table 1). In the mouse groups treated with DATS, the diabetic and nondiabetic groups were statistically similar regarding triglyceride, high-density lipoprotein, and fasting blood glucose levels.
TABLE 1.
Biochemical Parameters of the Treatment Groups
Ischemic Damage and Blood Perfusion in Ischemic Limbs of the Treatment Groups
In the untreated mice, gross examination showed that the damage due to ischemia was significantly greater in the diabetic mice compared with the nondiabetic mice (Fig. 1A). Similarly, in the groups treated with DATS, ischemia damage was greater in the diabetic mice compared with the nondiabetic. In the respective diabetic and nondiabetic groups, by gross examination, mice treated with DATS showed significantly less ischemic damage compared with their nontreated counterparts.
FIGURE 1.
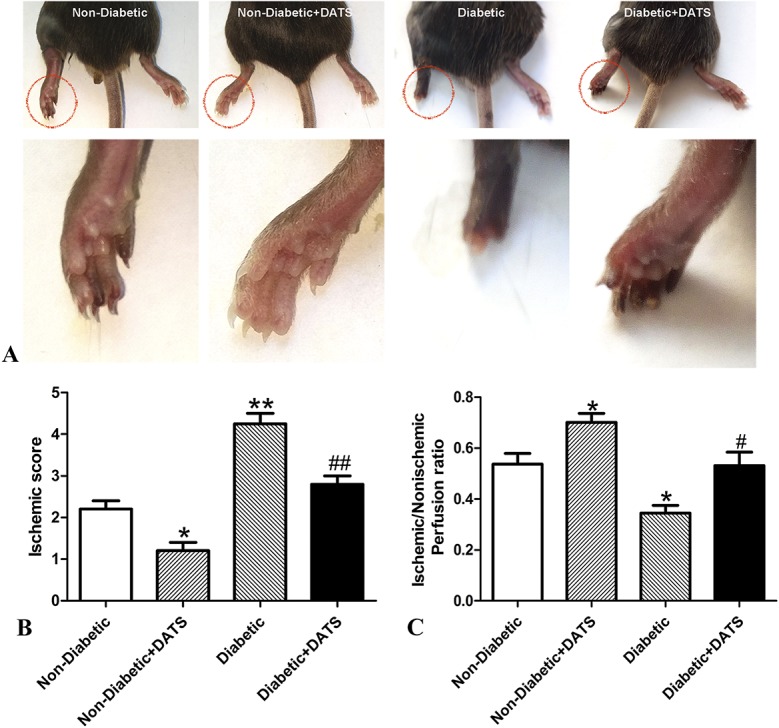
Ischemic damage and blood perfusion in treated and nontreated diabetic and control mice 14 days after ligation. Ischemic damage is greater (and blood perfusion is less) in diabetic mice than in nondiabetic mice. However, in the diabetic and nondiabetic groups, ischemic damage is less (and blood perfusion is higher) in mice treated with DATS. A, Representative photographs of hind limbs. B, Ischemic scores. C, Blood perfusion. *P < 0.05 cf. nondiabetic; **P < 0.01 cf. nondiabetic; #P < 0.05 cf. diabetic; ##P < 0.01 cf. diabetic.
In the untreated mice, the scores for ischemia of the diabetic mice were significantly higher than those of the nondiabetic mice (Fig. 1B). In the groups treated with DATS, ischemia scores of the diabetic mice were significantly higher than those of the nondiabetic mice. In the respective diabetic and nondiabetic groups, the ischemia scores of mice treated with DATS were significantly lower than those of their nontreated counterparts.
In the untreated mice, the blood perfusion ratio of the diabetic mice was significantly lower than those of the nondiabetic mice (Fig. 1C). In the groups treated with DATS, the blood perfusion ratio of the diabetic mice was significantly lower than those of the nondiabetic. However, in the respective diabetic and nondiabetic groups, the mean blood perfusion ratios of mice treated with DATS were significantly higher than those of their nontreated counterparts.
Angiogenesis in Ischemic Limbs of the Treatment Groups
To determine the relative regeneration of capillaries among the treatment groups, CD31 immunohistochemistry staining of muscle sections was performed 14 days after ligation (Fig. 2A). In both the untreated and treated mice, the capillary density of the diabetic mice and presence of CD31 were obviously less than those of the nondiabetic mice (Figs. 2A, B) in the corresponding groups. However, the capillary density and CD31 levels of mice treated with DATS were greater than those of their nontreated counterparts.
FIGURE 2.
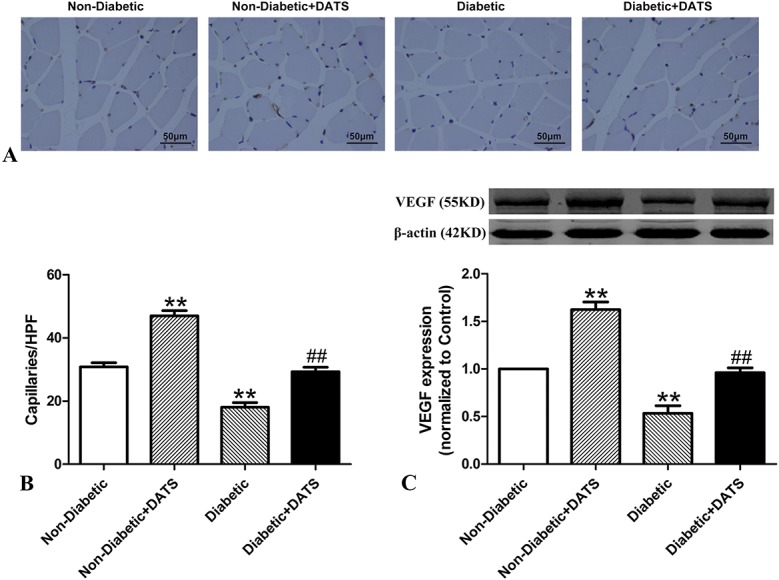
Capillary density and VEGF levels in ischemic hind limbs of treated and nontreated diabetic and control mice 14 days after ligation. Capillary density and VEGF levels are lower in diabetic mice than in nondiabetic mice. However, in the diabetic and nondiabetic groups, capillary density and VEGF levels are higher in mice treated with DATS. A and B, Anti-CD31 immunohistochemistry staining. C, Western blot of normalized VEGF. Scale bar = 50 μm. **P < 0.01 cf. nondiabetic. ##P < 0.01 cf. diabetic.
Several studies have reported that VEGF is a potent angiogenic molecule.26,27 Thus, in this study, the VEGF levels in the ischemic limbs were determined at day 14 postligation (Fig. 2C). The normalized VEGF levels of the diabetic rats were significantly lower than those of the nondiabetic mice in both treated and nontreated groups. However, mice treated with DATS showed significantly higher levels of VEGF in both the diabetic and nondiabetic mice compared with their nontreated counterparts.
NO Bioavailability in Ischemic Limbs of the Treatment Groups
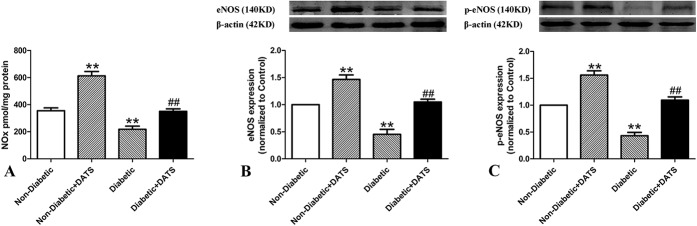
The NOx, eNOS, and p-eNOS levels of the diabetic mice were significantly lower than those of the nondiabetic mice, in both the treated and nontreated groups (Fig. 3). However, these levels in the treated mice were significantly higher than those of the nontreated in both the diabetic and nondiabetic groups.
FIGURE 3.
Tissue NOx levels and eNOS, p-eNOS protein levels in ischemic hind limbs of treated and nontreated diabetic and control mice 14 days after ligation. NOx, eNOS, and p-eNOS levels are lower in diabetic mice than in nondiabetic mice. However, in the diabetic and nondiabetic groups, NOx, eNOS, and p-eNOS levels are higher in mice treated with DATS. A, NOx (B), eNOS, and (C) p-eNOS levels. **P < 0.01 cf. nondiabetic. ##P < 0.01 cf. diabetic.
Indicators of Oxidative Stress in Ischemic Limbs of the Treatment Groups
In this study, ischemic tissues were stained with antinitrotyrosine antibody for an immunofluorescence analysis of oxidative stress (Fig. 4A, B). The fluorescence and nitrotyrosine levels of the ischemic tissues of the diabetic mice were significantly greater than those of the nondiabetic mice in both the treated and nontreated groups. However, the fluorescence and nitrotyrosine levels of the mice treated with DATS were significantly less than those of the nontreated mice, in both the diabetic and nondiabetic groups.
FIGURE 4.
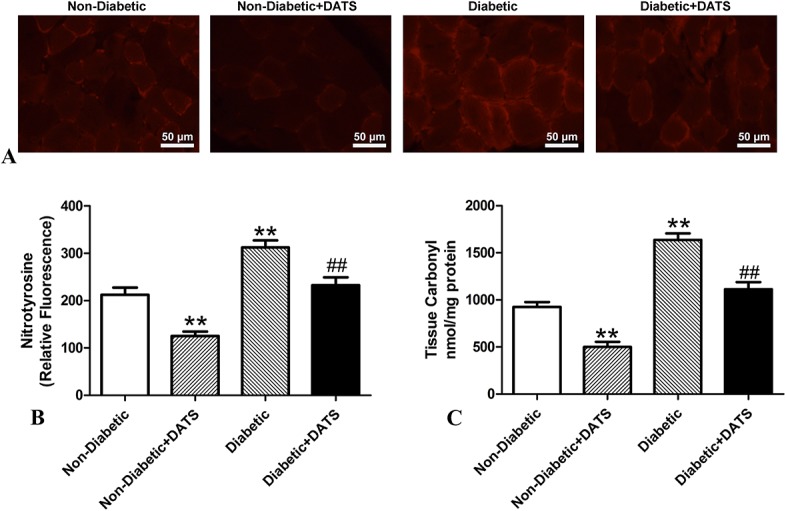
Analyses indicating oxidative stress in ischemic limbs of treated and nontreated diabetic and control mice 14 days after ligation. Oxidative stress levels are higher in diabetic mice than in nondiabetic mice. However, in the diabetic and nondiabetic groups, oxidative stress levels are lower in mice treated with DATS. A, Representative photographs of nitrotyrosine immunostaining; scale bar = 50 μm. B, Quantitative analysis of nitrotyrosine immunostaining. C, Protein carbonyl levels. **P < 0.01 cf. nondiabetic. ##P < 0.01 cf. diabetic.
The protein carbonyl levels of the ischemic tissues of the diabetic mice were significantly greater than those of the nondiabetic mice in both the treated and nontreated groups (Fig. 4C). However, the protein carbonyl levels of the mice treated with DATS were significantly less than those of the nontreated mice, in both the diabetic and nondiabetic groups.
DISCUSSION
This study investigated the potential angiogenic benefits of DATS treatment after ischemia in a mouse model of diabetes and underlying mechanisms. Diabetes was established in 24 mice by IP injection of STZ, whereas an equal number of mice remained nondiabetic. The establishment of the diabetic model was verified by biochemical parameters. All mice were subjected to hind limb ischemia through ligation of the left femoral artery. In both the diabetic and nondiabetic groups, 12 mice were administered DATS through IP injection. After treatment, the biochemical parameters of the diabetic and nondiabetic groups were similar. Between the untreated groups, relative to the nondiabetic mice, the diabetic mice showed greater ischemic damage; lower blood perfusion ratio, capillary density, and VEGF levels in ischemic tissues; and lower NOx, eNOS, and p-eNOS levels. However, in both the diabetic and nondiabetic groups, these effects in mice given DATS were significantly ameliorated.
Impairment of angiogenesis in response to tissue ischemia may account for the poor prognosis of patients with cardiovascular diseases in diabetes mellitus.28 Previous literature reported that, compared with nondiabetic mice, diabetic mice with hind limb ischemia showed impaired recovery of perfusion and limited microvasculature density.29 Our current data are consistent with previous results that ameliorating diabetes-related damage may contribute to collateral vessel formation and recovery of perfusion in response to hind limb ischemia. Indeed, as illustrated here, DATS was associated with greater capillary density and blood perfusion in response to hind limb ischemia in the diabetic mice, compared with the nondiabetic. This may be attributed to the stimulating effect of DATS on angiogenesis after ischemia.
Angiogenesis is a complicated process, involving the participation of many cytokines. VEGF is a robust angiogenic factor, with a vital role in angiogenesis. Previous literature reported that diabetes is associated with lower VEGF levels.30,31 Recently, it was demonstrated that DATS can increase VEGF expression in mice in chronic heart failure.32 Our current study results are consistent with previous investigations and revealed for the first time that DATS was associated with higher VEGF levels in diabetic hind limb ischemic tissue. It seems that the improvement of diabetes-related impairment may stimulate the expression of VEGF in the setting of tissue ischemia.
A fully functioning endothelium is a crucial precondition to the process of angiogenesis,33 and endothelial cell dysfunction is a hallmark of vascular damage in diabetes.34 A typical feature of endothelial dysfunction is impairment of NO activity in diabetes.35 A recent study revealed that diabetic mice had lower NO levels than did nondiabetic mice.2 A previous investigation showed that depletion of NO, through eNOS gene knockout, can suppress angiogenesis in a hind limb ischemic mouse model,36 whereas repletion of NO bioavailability through eNOS overexpression improved blood perfusion.37 Another investigation showed that DATS could activate eNOS and increase NO bioavailability, exerting a cardioprotective effect in a myocardial ischemia-reperfusion injury mouse model.38 Our preset results are consistent with previous investigations that diabetic mice have depressed NO bioavailability, whereas DATS treatment seemed to enhance eNOS expression and NO bioavailability in diabetic mice.
Reactive oxygen species (ROS) can impair eNOS function and suppress NO bioactivity, ultimately leading to tissue damage. Moreover, surplus oxidative stress may retard angiogenesis and revascularization of ischemic tissues in diabetes.39 In type 2 diabetic animals, ROS are significantly higher, and eNOS and NO bioactivity are lower in vascular tissue, relative to the controls.40 It has also been demonstrated that DATS can attenuate doxorubicin-induced cardiomyocyte apoptosis by inhibiting the generation of ROS.41 Our current results are in agreement with these observations and suggest, for the first time, that DATS can attenuate oxidative stress levels in diabetic hind limb ischemic tissues. In addition, DATS may attenuate oxidative stress levels in diabetic mice through a number of possible mechanisms, including antioxidant effects, and inhibition of intracellular ROS. Further investigations are required to confirm the role of DATS in mitigating oxidative stress.
Studies have reported that DATS possesses antidiabetic effects.42,43 Our current research indicates that DATS ameliorates diabetes-mediated impairment of hind limb ischemia and angiogenesis. The findings are supported by previous reports showing that DATS augmented ischemia-induced angiogenesis and rescued cardiac dysfunction after heart failure by improving angiogenesis.21,32 However, these investigations were not performed in the clinically relevant context of hyperglycemia, as high blood glucose is a pivotal contributor to diabetes-impaired vascular complications. Our current investigation showed that the effects of DATS were independent of changes in blood glucose, serum insulin, and blood lipids. This indicates that the mode of action of DATS on diabetes-impaired angiogenesis is not related to glucose stress but to mechanisms that are conducive to diabetes-impaired angiogenesis, including VEGF production and signaling, eNOS activation, and the reduction of oxidative stress.
Overall, our current data indicate that DATS treatment can improve angiogenic potential, tissue viability, and ischemic tissue blood perfusion and mitigate oxidative stress, in a diabetic mouse model with peripheral artery disease. These findings warrant clinical confirmation that DATS treatment may promote angiogenesis and tissue reperfusion in diabetic patients with peripheral artery disease and ischemia.
Footnotes
Supported by the China National Natural Science Foundation (81670405, 81370409 and 81400269), the Natural Science Foundation of Jiangsu Province (BK20161355), the social development Foundation of Zhenjiang (SH2015041) and Shanxi provincial Commission of Health and Family Planning (2017053).
The authors report no conflicts of interest.
H.-B. Yang and H.-M. Liu authors have contributed equally.
Z.-Y. Lu and J.-C. Yan designed the research work; H.-B. Yang and H.-M. Liu performed the research experiments; H.-B. Yang contributed new reagents/analytic tools; H.-M. Liu and J.-C. Yan analyzed data; Z.-Y. Lu and J.-C. Yan wrote the paper.
REFERENCES
- 1.Aronow WS. Peripheral arterial disease in the elderly. Clin Interv Aging. 2007;2:645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pattillo CB, Bir SC, Branch BG, et al. Dipyridamole reverses peripheral ischemia and induces angiogenesis in the Db/Db diabetic mouse hind-limb model by decreasing oxidative stress. Free Radic Biol Med. 2011;50:262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kajiwara H, Luo Z, Belanger AJ, et al. A hypoxic inducible factor-1 alpha hybrid enhances collateral development and reduces vascular leakage in diabetic rats. J Gene Med. 2009;11:390–400. [DOI] [PubMed] [Google Scholar]
- 4.van Golde JM, Ruiter MS, Schaper NC, et al. Impaired collateral recruitment and outward remodeling in experimental diabetes. Diabetes. 2008;57:2818–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin KY, Ito A, Asagami T, et al. Impaired nitric oxide synthase pathway in diabetes mellitus: role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation. 2002;106:987–992. [DOI] [PubMed] [Google Scholar]
- 6.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. [DOI] [PubMed] [Google Scholar]
- 7.Bir SC, Pattillo CB, Pardue S, et al. Nitrite anion therapy protects against chronic ischemic tissue injury in db/db diabetic mice in a NO/VEGF-dependent manner. Diabetes. 2014;63:270–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo WW, Wang WJ, Tsai CY, et al. Diallyl trisulfide (DATS) suppresses high glucose-induced cardiomyocyte apoptosis by inhibiting JNK/NFkappaB signaling via attenuating ROS generation. Int J Cardiol. 2013;168:270–280. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee SK, Maulik SK. Effect of garlic on cardiovascular disorders: a review. Nutr J. 2002;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi R, Liao F, Inoue K, et al. Inhibition by diallyl trisulfide, a garlic component, of intracellular Ca(2+) mobilization without affecting inositol-1,4, 5-trisphosphate (IP(3)) formation in activated platelets. Biochem Pharmacol. 2000;60:1475–1483. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Y, Bhushan S, Yang C, et al. Controllable hydrogen sulfide donors and their activity against myocardial ischemia-reperfusion injury. ACS Chem Biol. 2013;8:1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Sun X, Zhang H, et al. Controlled release hydrogen sulfide delivery system based on mesoporous silica nanoparticles protects graft endothelium from ischemia-reperfusion injury. Int J Nanomedicine. 2016;11:3255–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan KC, Hsu CC, Yin MC. Protective effect of three diallyl sulphides against glucose-induced erythrocyte and platelet oxidation, and ADP-induced platelet aggregation. Thromb Res. 2002;108:317–322. [DOI] [PubMed] [Google Scholar]
- 14.Kim SH, Lee IC, Ko JW, et al. Diallyl disulfide prevents cyclophosphamide-induced hemorrhagic cystitis in rats through the inhibition of oxidative damage, MAPKs, and NF-kappaB pathways. Biomol Ther (Seoul) 2015;23:180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu LL, Yan L, Chen YH, et al. A role for diallyl trisulfide in mitochondrial antioxidative stress contributes to its protective effects against vascular endothelial impairment. Eur J Pharmacol. 2014;725:23–31. [DOI] [PubMed] [Google Scholar]
- 16.Nie XM, Zhou YJ, Xie Y, et al. Effect of stent coated with diallyl trisulfide on endothelial structure and function after coronary injury: experiment with dogs [in Chinese]. Zhonghua Yi Xue Za Zhi. 2006;86:1125–1128. [PubMed] [Google Scholar]
- 17.Iwabayashi M, Taniyama Y, Sanada F, et al. Role of serotonin in angiogenesis: induction of angiogenesis by sarpogrelate via endothelial 5-HT1B/Akt/eNOS pathway in diabetic mice. Atherosclerosis. 2012;220:337–342. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki N, Yamashita T, Takaya T, et al. Augmentation of vascular remodeling by uncoupled endothelial nitric oxide synthase in a mouse model of diabetes mellitus. Arterioscler Thromb Vasc Biol. 2008;28:1068–1076. [DOI] [PubMed] [Google Scholar]
- 19.Lu ZY, Li RL, Zhou HS, et al. Therapeutic ultrasound reverses peripheral ischemia in type 2 diabetic mice through PI3K-Akt-eNOS pathway. Am J Transl Res. 2016;8:3666–3677. [PMC free article] [PubMed] [Google Scholar]
- 20.Qi J, Wang JJ, Duan JL, et al. Leonurine improves age-dependent impaired angiogenesis: possible involvement of mitochondrial function and HIF-1alpha dependent VEGF activation. Front Pharmacol. 2017;8:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashida R, Kondo K, Morita S, et al. Diallyl trisulfide augments ischemia-induced angiogenesis via an endothelial nitric oxide synthase-dependent mechanism. Circ J. 2017;81:870–878. [DOI] [PubMed] [Google Scholar]
- 22.Hao C, Huang ZH, Song SW, et al. Arterial baroreflex dysfunction impairs ischemia-induced angiogenesis. J Am Heart Assoc. 2014;3:e000804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu ZY, Li RL, Zhou HS, et al. Rescue of hypertension-related impairment of angiogenesis by therapeutic ultrasound. Am J Transl Res. 2016;8:3087–3096. [PMC free article] [PubMed] [Google Scholar]
- 24.Lee S, Muniyappa R, Yan X, et al. Comparison between surrogate indexes of insulin sensitivity and resistance and hyperinsulinemic euglycemic clamp estimates in mice. Am J Physiol Endocrinol Metab. 2008;294:E261–E270. [DOI] [PubMed] [Google Scholar]
- 25.Muniyappa R, Chen H, Muzumdar RH, et al. Comparison between surrogate indexes of insulin sensitivity/resistance and hyperinsulinemic euglycemic clamp estimates in rats. Am J Physiol Endocrinol Metab. 2009;297:E1023–E1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrikopoulos P, Eccles SA, Yaqoob MM. Coupling between the TRPC3 ion channel and the NCX1 transporter contributed to VEGF-induced ERK1/2 activation and angiogenesis in human primary endothelial cells. Cell Signal. 2017;37:12–30. [DOI] [PubMed] [Google Scholar]
- 27.Molina F, Del Moral ML, Peinado MA, et al. Angiogenesis is VEGF-independent in the aged striatum of male rats exposed to acute hypoxia. Biogerontology. 2017;18:759–768. [DOI] [PubMed] [Google Scholar]
- 28.Yetkin E, Topal E, Erguzel N, et al. Diabetes mellitus and female gender are the strongest predictors of poor collateral vessel development in patients with severe coronary artery stenosis. Angiogenesis. 2015;18:201–207. [DOI] [PubMed] [Google Scholar]
- 29.Landazuri N, Joseph G, Guldberg RE, et al. Growth and regression of vasculature in healthy and diabetic mice after hindlimb ischemia. Am J Physiol Regul Integr Comp Physiol. 2012;303:R48–R56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hazarika S, Dokun AO, Li Y, et al. Impaired angiogenesis after hindlimb ischemia in type 2 diabetes mellitus: differential regulation of vascular endothelial growth factor receptor 1 and soluble vascular endothelial growth factor receptor 1. Circ Res. 2007;101:948–956. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Hazarika S, Xie D, et al. In mice with type 2 diabetes, a vascular endothelial growth factor (VEGF)-activating transcription factor modulates VEGF signaling and induces therapeutic angiogenesis after hindlimb ischemia. Diabetes. 2007;56:656–665. [DOI] [PubMed] [Google Scholar]
- 32.Polhemus D, Kondo K, Bhushan S, et al. Hydrogen sulfide attenuates cardiac dysfunction after heart failure via induction of angiogenesis. Circ Heart Fail. 2013;6:1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun L, Bai Y, Du G. Endothelial dysfunction–an obstacle of therapeutic angiogenesis. Ageing Res Rev. 2009;8:306–313. [DOI] [PubMed] [Google Scholar]
- 34.Dhananjayan R, Koundinya KS, Malati T, et al. Endothelial dysfunction in type 2 diabetes mellitus. Indian J Clin Biochem. 2016;31:372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Napoli C, Ignarro LJ. Nitric oxide and pathogenic mechanisms involved in the development of vascular diseases. Arch Pharm Res. 2009;32:1103–1108. [DOI] [PubMed] [Google Scholar]
- 36.Takata K, Imaizumi S, Kawachi E, et al. The ApoA-I mimetic peptide FAMP promotes recovery from hindlimb ischemia through a nitric oxide (NO)-related pathway. Int J Cardiol. 2016;207:317–325. [DOI] [PubMed] [Google Scholar]
- 37.Brevetti LS, Chang DS, Tang GL, et al. Overexpression of endothelial nitric oxide synthase increases skeletal muscle blood flow and oxygenation in severe rat hind limb ischemia. J Vasc Surg. 2003;38:820–826. [DOI] [PubMed] [Google Scholar]
- 38.Predmore BL, Kondo K, Bhushan S, et al. The polysulfide diallyl trisulfide protects the ischemic myocardium by preservation of endogenous hydrogen sulfide and increasing nitric oxide bioavailability. Am J Physiol Heart Circ Physiol. 2012;302:H2410–H2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paravicini TM, Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care. 2008;31(suppl 2):S170–S180. [DOI] [PubMed] [Google Scholar]
- 40.Silva MA, Bruder-Nascimento T, Cau SB, et al. Spironolactone treatment attenuates vascular dysfunction in type 2 diabetic mice by decreasing oxidative stress and restoring NO/GC signaling. Front Physiol. 2015;6:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wen SY, Tsai CY, Pai PY, et al. Diallyl trisulfide suppresses doxorubicin-induced cardiomyocyte apoptosis by inhibiting MAPK/NF-kappaB signaling through attenuation of ROS generation. Environ Toxicol. 2018;33:93–103. [DOI] [PubMed] [Google Scholar]
- 42.Padiya R, Banerjee SK. Garlic as an anti-diabetic agent: recent progress and patent reviews. Recent Pat Food Nutr Agric. 2013;5:105–127. [DOI] [PubMed] [Google Scholar]
- 43.Kook S, Kim GH, Choi K. The antidiabetic effect of onion and garlic in experimental diabetic rats: meta-analysis. J Med Food. 2009;12:552–560. [DOI] [PubMed] [Google Scholar]