Abstract
Transcranial magnetic stimulation (TMS) over the cerebellum facilitates the spinal reflex in healthy humans. The aim of this study was to investigate whether such cerebellar spinal facilitation (CSpF) appears in patients with spinocerebellar ataxia (SCA) presenting with atrophy in the cerebellar gray matter and dentate nucleus. One patient with SCA type 6 and another with SCA type 31 participated in this study. TMS over the right primary motor cortex was used to induce motor-evoked potentials in the right first dorsal interosseous muscle, which were detected using electromyography. Conditioning TMS using interstimulus intervals of 1–8 ms was performed over the right cerebellum as a test to measure cerebellar brain inhibition (CBI). To assess the H-reflex and the M-wave recruitment curve of the right soleus muscle, we performed electrical stimulation of the right tibial nerve. The stimulation intensity was set to that at the center of the H-reflex curve of the ascending limb. To measure CSpF, we delivered TMS over the right cerebellum 100, 110, 120, and 130 ms before the right tibial nerve stimulation. Voxel-based morphometry was used to verify the presence of atrophy in the cerebellar gray matter and dentate nucleus. CBI was absent in both cases. However, a significant facilitation of the H-reflex occurred with an interstimulus interval of 120 ms in both cases. These findings indicate that the pathways associated with the induction of CSpF and CBI are different, and that the cerebellar gray matter and dentate nucleus are not needed for the induction of CSpF. The possible origin of CSpF may be examined by stimulation of other cerebellar deep nuclei or the brainstem.
Keywords: cerebellar brain inhibition, cerebellar spinal facilitation, cerebellum, H-reflex, spinal reflex, spinocerebellar ataxia, transcranial magnetic stimulation, voxel-based morphometry
Introduction
Cerebellar transcranial magnetic stimulation (TMS) is often used during clinical examination to probe the functional connectivity between the cerebellum and the contralateral motor cortex in patients with ataxia 1. Motor-evoked potentials (MEPs) induced by TMS over the primary motor cortex (M1) are inhibited by cerebellar TMS in healthy humans 2. However, such cerebellar brain inhibition (CBI) is absent in patients with cerebellar ataxia with deficits in the cerebellar cortex 3 or dentatothalamocortical pathways 1,3. Thus, CBI is often used to evaluate the functional connectivity between the cerebellum and the contralateral M1 1,4.
In addition, cerebellar TMS allows one to probe the functional connectivity between the cerebellum and the spinal motoneuron pool. We have recently reported a method to evaluate this connectivity using cerebellar TMS, followed by examination of the H-reflex, which reflects the excitability of the spinal motoneuron pool 5,6. Using this method, the H-reflex of the right soleus muscle was shown to be facilitated by cerebellar TMS 110–130 ms before the tibial nerve stimulation used to induce this reflex 5,6. This cerebellar spinal facilitation (CSpF) was modulated when performing a sensory motor synchronization task involving finger tapping, which requires increased cerebellar activity 7. This indicates that CSpF can be affected by cerebellar activity. Therefore, similar to CBI, CSpF may be useful for probing the function of the cerebellum.
This new technique, however, raises some questions. First, it is unclear whether, like CBI, CSpF is induced by stimulation of Purkinje fibers in the cerebellar gray matter 3. If that is the case, similar to CBI 3, CSpF should be absent in patients with cerebellar gray matter atrophy, such as those with spinocerebellar ataxia (SCA) type 6 or type 31 8,9. In contrast, the presence of CSpF and absence of CBI in these patients would suggest that CSpF induction by cerebellar TMS may be mediated by the dentatothalamocortical pathway, which is necessary for CBI 2. We investigated this subject in patients with SCA 6 or 31 in whom CBI was absent.
To achieve this goal, we selected patients diagnosed with SCA 6 or 31 in whom we confirmed the presence of cerebellar gray matter atrophy using MRI 10. Recently, a voxel-based lesion-symptom mapping study using MRI showed that voxel-based morphometry (VBM) allows for an unbiased in-vivo whole-brain quantitative analysis of differences in the volumes of gray and white matter and of cerebrospinal fluid 11. Limb ataxia has been shown to significantly correlate with lesions in the interposed nucleus and part of the dentate nucleus, whereas ataxic posture and gait have been shown to be correlated with lesions in the fastigial and interposed nuclei 12. We used VBM to examine the atrophy in the cerebellar gray matter and dentate nuclei.
The cortical silent period (cSP) in electromyograms (EMGs) of hand muscles appears after the induction of MEP by TMS over the M1 during small voluntary muscle contraction 13. This cSP, which reflects the excitability of the γ-aminobutyric acid-mediated inhibitory neural circuit in M1 14, was found to be expanded in patients with cerebellar ataxia 15, suggesting an impairment in this circuit. To confirm that this is the case, we measured cSP in our patients.
Participants and methods
Participants
Two individuals with SCA type 6 and 31, respectively, participated in this study. Eighty-five healthy adults (mean age: 23.7±3.7 years) with no history of epilepsy were also enrolled to obtain control data for CSpF. The experimental protocol was explained to the participants, who provided written informed consent to participate in the experiments. The Ethics Committee of Mihara Memorial Hospital approved the experimental procedures (approval number: 079-03) and the study was carried out according to the principles and guidelines of the Declaration of Helsinki.
Case 1 was a 79-year-old male patient. He had been diagnosed with SCA 6 on the basis of genetic testing 22 years ago. Case 2 was a 57-year-old female patient diagnosed with SCA 31 six years ago. Both patients had undergone physical therapy following their diagnoses. The Scale for the Assessment and Rating of Ataxia 16 score was 23 in case 1 and 10 in case 2. Gait without support was not possible in daily living in case 1, but was possible in case 2. Muscle weakness and sensory disturbance were not observed in either case. Brain MRI indicated atrophy of the anterior lobe of the cerebellum in both cases (Fig. 1).
Fig. 1.
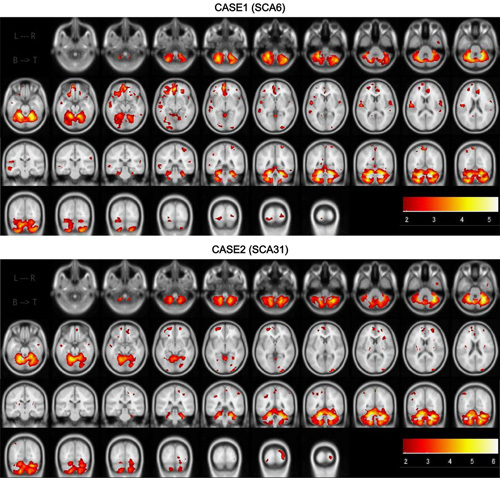
z-Score mapping. The upper 2 lines indicate the axial plane and the lower 2 lines indicate the coronal plane in cases 1 and 2. The lower scale indicates the z-score.
Image processing
We used Discovery MR750w on a 3.0 T system (GE Healthcare, Waukesha, Wisconsin, USA) for MRI acquisition. Axial three-dimensional T1-weighted turbo gradient echo images (sequence: three-dimensional spoiled gradient-recalled, slice resolution: 100%, frequency encoding: 256, matrix size: 256×256, slice thickness: 1 mm, flip angle: 14°, and field of view: 25.6) were obtained.
VBM was performed to identify differences in the regional volume between the two patients with SCA. Structural brain data were obtained from Information eXtraction from Images (IXI), which is a database including a large set of MRI data (N=546; 241 men, 304 women, and one unknown; age, 20–86 years). This database has been used previously in a study using the open-access software BAAD (Brain Anatomical Analysis using diffeomorphic deformation, version 4.1; Shiga University of Medical Science, Otsu, Japan) for MRI processing 17. The T1-weighted image data from the two patients were converted into Digital Imaging and Communications in Medicine files and segmentation analysis was carried out to separate gray and white matter data. Regions of interest (ROIs) were set according to the Automated Anatomical Labeling, Brodmann, and Laboratory of Neuro Imaging Probabilistic Brain Atlas atlases on the basis of the Montreal Neurological Institute coordinate system. The z-score for regional volume, which indicates the number of SDs from the mean, was calculated on the basis of age-matched standard brain templates in the IXI database. For example, a z-score of 2 refers to the mean volume of an ROI of the standard brain plus 2 SD.
Cerebellar brain inhibition
The participants were seated in a chair, and their heads, trunks, and forearms were fixed. Motor cortex TMS was delivered using a figure-of-eight coil connected to a magnetic stimulator (The Magstim Co. Ltd, Whitland, UK). The maximum magnetic flux density of this coil was 1.2 T. Cerebellar TMS was delivered using a double-cone coil (YM-133B; Nihon Kohden, Tokyo, Japan) connected to a magnetic stimulator (SMN-1200; Nihon Kohden). The maximum magnetic flux density of this double-cone coil was 0.96 T. The figure-of-eight coil was then positioned at the hotspot for MEPs on the right first dorsal interosseous (FDI) muscle and the current in the coil was directed in the anterior to posterior direction. The center of the junction of the double-cone coil was placed 1 cm below and 3 cm to the right of the inion to stimulate the right cerebellum 4,18 and the current in the coil was directed downward. This induced an upwardly traveling current in the brain.
To record EMG signals, two surface-recording electrodes were placed 2 cm apart on the right FDI. The signals were amplified through an EMG amplifier (house-made) with a pass-band filter of 0.3 Hz–1 kHz and converted into digital signals at a sampling rate of 1 kHz using an analog-to-digital converter (USB-6008; National Instruments Co. Ltd, Austin, Texas, USA).
To calculate CBI, unconditioned and conditioned MEPs induced by cerebellar TMS were measured. In conditioning trials, cerebellar TMS was delivered before the test TMS over the contralateral M1. Interstimulus intervals (ISIs) of 1–8 ms were used 19. The intensity of the conditioning cerebellar TMS was set to 90% of the maximum output of the magnetic stimulator 18,20. To obtain a test MEP size of ∼1 mV 2, the intensity of the stimulator was adjusted before the examination and applied during the examination. Ten trials were conducted for each ISI. The intertest intervals were 5 s.
Cortical silent period
To measure cSP duration, TMS was delivered to the right FDI muscle during slight muscle contraction with the same intensity as that used for the CBI measurements. Ten trials were conducted in the same position as that used for the CBI measurements.
Cerebellar spinal facilitation
The participants with SCA were seated in a chair in a position similar to that used during the CBI measurements. Both ankles of the participants were fixed using a brace to prevent changes in ankle angle 5,6. Test posterior tibial nerve electrical stimulation was delivered using an electrical isolator (MEB-2306; Nihon Kohden) over the right popliteal fossa. To record EMG signals, two Ag/AgCl surface-recording electrodes were placed 2 cm apart on the right soleus muscle. The EMG signals were amplified and stored in a measuring device (MEB-2306; Nihon Kohden). The recruitment curve of the H-reflex and M-wave amplitude were calculated before the examination. A stimulation intensity sufficient to induce an amplitude approximately at the center of the H-reflex curve of the ascending limb was used for the test H-reflex 21. The test H-reflex was calculated on the basis of the stimulation intensity.
Conditioning cerebellar TMS was performed as described for the CBI measurements 5,18,20. Tibial nerve electrical stimulation evoking the H-reflex from the right soleus muscle was delivered 100, 110, 120, and 130 ms after the conditioning cerebellar TMS 5. Background EMG activity was monitored during the measurement and the absence of activity in the tested muscle was confirmed. The test was repeated nine times for each ISI. The test intervals were longer than 7 s.
Data from the healthy participants were acquired in exactly the same manner as that used in a previous study of CSpF 5. The healthy participants were placed in the prone position, whereas the patients with SCA were seated on a reclining seat as they could not maintain the prone position for long periods. The ISI for the healthy participants was 110 ms, which is known to produce the greatest facilitation 5.
Analysis
CBI was calculated as the ratio of the amplitude of the conditioned MEP to that of the unconditioned MEP 3,4. CSpF was calculated by dividing the amplitude of the conditioned H-reflex by that of the unconditioned H-reflex 5,6. We used the one-sample t-test to measure differences in CBI or CSpF for each ISI. We used a significance level (α value) of 0.05 and Bonferroni’s correction. We used one-way analyses of variance (ANOVAs) with post-hoc Bonferroni’s correction for multiple comparisons to evaluate differences in the means for all ISIs (α=0.05).
Results
None of the participants showed any side effects during any of the examinations. Figure 1 shows the z-score maps obtained on the basis of the VBM analysis for cases 1 and 2, indicating significant atrophy in all cerebellar areas. In case 1, the z-scores for the total volumes of the cerebellum, brainstem, and right and left dentate nuclei were 4.4, 0.8, 4.3, and 3.1, respectively. In case 2, the z-scores were 3.6, 0.6, 3.2, and 3, respectively. In case 1, the z-scores of the total volumes of the precentral gyrus, supplementary motor area, putamen, thalamus, and red nucleus (right/left) were −1.2/−1.2, −0.5/−0.1, −0.8/−0.7, −0.6/−0.3, and −0.7/−1.4, respectively. The z-scores for the same areas in case 2 were −0.8/0.3, 0.8/0.8, −1.8/−1.1, −0.2/−0.4, and −1.4/−2.1, respectively.
Figures 2a and b show CBI in cases 1 and 2. A one-sample t-test showed no significant differences for any of the ISIs in either case (P>0.05). A one-way ANOVA did not show significant differences for any of the ISIs in case 1 [F(7, 79)=0.23, P=0.98] or case 2 [F(7, 79)=0.2, P=0.98]. Figure 2c and d show the recruitment curves of the H-reflex and M-wave amplitudes for both patients. The H-reflex recruitment curve for the ascending limb was centered at 10 mV in both cases. This intensity was used for our experiments. Figure 2e and f show CSpF in both cases. A one-sample t-test showed significant facilitation, with ISIs of 100, 110, 120, and 130 ms for case 1, and with an ISI of 120 ms in case 2. A one-way ANOVA showed a significant difference between ISIs, both in case 1 [F(3, 35)=3.1, P=0.04] and in case 2 [F(3, 35)=3.9, P=0.018]. The post-hoc Bonferroni’s multiple comparison test indicated that CSpF with an ISI of 120 ms was significantly higher than that with an ISI of 100 ms in case 1 (P=0.04) and that with an ISI of 110 ms in case 2 (P=0.03). The CSpF means for an ISI of 120 ms were 1.7±0.6 and 2.3±0.5 for cases 1 and 2, respectively. The mean CSpF in the healthy participants was 1.7±0.5. The mean cSP durations in cases 1 and 2 were 217.6±12 and 103±13, respectively.
Fig. 2.
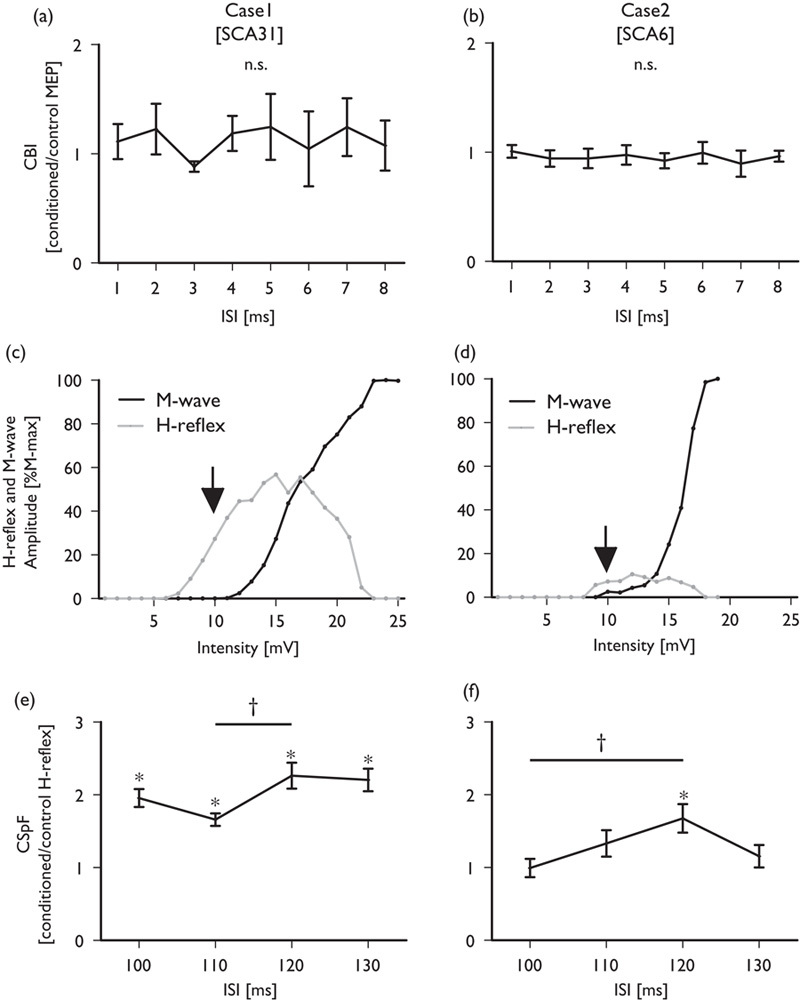
Time course of the mean conditioned MEP amplitude compared with that for the control condition in case 1 (a) and case 2 (b). The H-reflex and M-wave recruitment curves for cases 1 and 2 are shown in (c) and (d), respectively. The arrows indicate the center of the H-reflex recruitment curve for the ascending limb. The time courses for the mean conditioned H-reflex amplitude compared with that of the control condition are shown for case 1 (e) and case 2 (f). Error bars represent the SEM. *P<0.05 (one-sample t-test), †P<0.05 (paired t-test).
Discussion
The aim of this study was to determine whether CSpF is present in patients with atrophy of the cerebellar gray matter and dentate nucleus in whom CBI is absent. VBM analysis showed that both patients presented with extended atrophy in the cerebellar gray matter and dentate nucleus, which are regions needed for the induction of CBI. CBI was indeed absent in these patients, but CSpF was observed in both cases at the same level as that observed in healthy participants. These findings indicate that the pathway underlying CSpF is different from that underlying CBI.
The MEP induced by TMS over the M1 is inhibited by contralateral conditioning cerebellar TMS applied 5–7 ms before the TMS over the M1 1,2. No such CBI was observed in patients with neocerebellar atrophy, such as SCA, dentatorubral-pallidoluysian atrophy 3, or infarction of the cerebellar outflow to the thalamus 22. These findings indicate that the traveling time, number of synapses, and action potentials induced by conditioning TMS in Purkinje fibers within the cerebellar gray matter are considered the origin of CBI 19 and that the cerebellar gray matter, dentate nucleus, and thalamus are needed for CBI induction 3,19. However, in our study, VBM analysis indicated that the sites of atrophy were the cerebellar gray matter and the dentate nucleus, but not the thalamus. Therefore, the absence of CBI can be explained by the atrophy of the cerebellar gray matter, which is in agreement with the findings of previous studies 3. In other words, CBI is caused by TMS-induced action potentials in cerebellar Purkinje fibers, whereas this cannot be the case for CSpF.
The conditioning stimulation on the cerebellar hemisphere not only inhibits the test MEP induced by TMS over the contralateral M1, but may also facilitate the MEP when an ISI of 3 ms is used 19. This facilitation is believed to occur because of stimulation of output fibers from the dentate nucleus 3,19. In our study, the facilitation induced by cerebellar TMS with an ISI of 3 ms was absent in both patients. Therefore, the dentate nucleus may not be needed for the induction of CSpF.
In addition to the dentate nucleus, cerebellar deep nuclei including the fastigial and interposed nuclei have projections to the spinal motoneuron pool through the vestibulospinal or reticulospinal tracts 23,24. Furthermore, the vestibulospinal and reticulospinal tracts may contribute toward the induction of CSpF 6. Therefore, it is possible that the CSpF is because of stimulation of the fastigial or interposed nuclei in the cerebellum. However, in this study, it was not clear whether these nuclei were intact or atrophied. This is because the above nuclei were not available for VBM analysis in the BAAD software. We also could not create ROIs at the fastigial and interposed nuclei. Therefore, further studies are needed to investigate whether these nuclei are associated with CSpF.
The CSpF may alternatively be because of the direct stimulation of the corticospinal tract or the extrapyramidal tract in the brainstem as cerebellar TMS using a double-cone coil has been shown to stimulate the brainstem 25. In this study, VBM analysis indicated no atrophy in the brainstem in either of the patients. Therefore, it is possible that the CSpF was because of stimulation of the brainstem. However, the cervicomedullary MEP with short latency 25 was not found in not only healthy participants but also both SCA patients in EMG of the soleus muscle. This indicates that although the corticospinal tract in the brainstem was not adequately stimulated to induce action potentials following cerebellar TMS, CSpF could still be induced. Nevertheless, we cannot reject the possibility that the CSpF was because of the stimulation of extrapyramidal tracts such as the vestibulospinal, rubrospinal, or reticulospinal tracts in the brainstem. This is because there is currently no non-invasive method for the detection of the potential induced by TMS in the extrapyramidal tract in the brainstem. Therefore, further studies are needed to probe whether CSpF is induced in patients with atrophy or damage only in the brainstem.
The durations of cSP in cases 1 and 2 were ∼200 and 100 ms, respectively. A previous study reported that the duration of cSP in a patient with cerebellar ataxia was about 200 ms, whereas it was ∼100 ms in healthy adults 15. cSP duration reflects the excitability of the inhibitory neural circuit in M1, which is mediated by γ-aminobutyric acid 14. These findings indicate that this circuit might be impaired in case 1, but not in case 2. However, CBI was absent and CSpF was induced in both cases. Therefore, the excitability of this neural circuit was not associated with the induction of CBI or CSpF.
Conclusion
Significant atrophy in the cerebellar gray matter and dentate nuclei was confirmed using VBM. In addition, the absence of CBI and the induction of CSpF were confirmed using established neurophysiological methods. These findings indicate that the pathway associated with the induction of CSpF is different from that associated with the induction of CBI, and that the cerebellar gray matter and dentate nucleus may not be required for the induction of CSpF. CSpF may be because of the stimulation of two other cerebellar deep nuclei, namely, the fastigial and interposed nuclei, or of extrapyramidal tracts, such as the vestibulospinal, rubrospinal, or reticulospinal tracts in the brainstem.
Acknowledgements
This work was supported by JSPS KAKENHI (grant number: 25870951).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Grimaldi G, Argyropoulos GP, Boehringer A, Celnik P, Edwards MJ, Ferrucci R, et al. Non-invasive cerebellar stimulation – a consensus paper. Cerebellum 2014; 13:121–138. [DOI] [PubMed] [Google Scholar]
- 2.Pinto AD, Chen R. Suppression of the motor cortex by magnetic stimulation of the cerebellum. Exp Brain Res 2001; 140:505–510. [DOI] [PubMed] [Google Scholar]
- 3.Groiss SJ, Ugawa Y. Cerebellum. Handb Clin Neurol 2013; 116:643–653. [DOI] [PubMed] [Google Scholar]
- 4.Matsugi A, Okada Y. Cerebellar transcranial static magnetic field stimulation transiently reduces cerebellar brain inhibition. Funct Neurol 2017; 32:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsugi A, Mori N, Uehara S, Kamata N, Oku K, Mukai K, et al. Task dependency of the long-latency facilitatory effect on the soleus H-reflex by cerebellar transcranial magnetic stimulation. Neuroreport 2014; 25:1375–1380. [DOI] [PubMed] [Google Scholar]
- 6.Matsugi A, Mori N, Uehara S, Kamata N, Oku K, Okada Y, et al. Effect of cerebellar transcranial magnetic stimulation on soleus Ia presynaptic and reciprocal inhibition. Neuroreport 2015; 26:139–143. [DOI] [PubMed] [Google Scholar]
- 7.Witt ST, Laird AR, Meyerand ME. Functional neuroimaging correlates of finger-tapping task variations: an ALE meta-analysis. Neuroimage 2008; 42:343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hara D, Maki F, Tanaka S, Sasaki R, Hasegawa Y. MRI-based cerebellar volume measurements correlate with the International Cooperative Ataxia Rating Scale score in patients with spinocerebellar degeneration or multiple system atrophy. Cerebellum Ataxias 2016; 3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulz JB, Borkert J, Wolf S, Schmitz-Hubsch T, Rakowicz M, Mariotti C, et al. Visualization, quantification and correlation of brain atrophy with clinical symptoms in spinocerebellar ataxia types 1, 3 and 6. Neuroimage 2010; 49:158–168. [DOI] [PubMed] [Google Scholar]
- 10.Timmann D, Brandauer B, Hermsdörfer J, Ilg W, Konczak J, Gerwig M, et al. Lesion-symptom mapping of the human cerebellum. Cerebellum 2008; 7:602–606. [DOI] [PubMed] [Google Scholar]
- 11.Della Nave R, Ginestroni A, Tessa C, Cosottini M, Giannelli M, Salvatore E, et al. Brain structural damage in spinocerebellar ataxia type 2. A voxel-based morphometry study. Mov Disord 2008; 23:899–903. [DOI] [PubMed] [Google Scholar]
- 12.Schoch B, Dimitrova A, Gizewski ER, Timmann D. Functional localization in the human cerebellum based on voxelwise statistical analysis: a study of 90 patients. Neuroimage 2006; 30:36–51. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol 2003; 2:145–156. [DOI] [PubMed] [Google Scholar]
- 14.Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol 1999; 517 (Pt 2):591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamburin S, Fiaschi A, Andreoli A, Marani S, Manganotti P, Zanette G. Stimulus-response properties of motor system in patients with cerebellar ataxia. Clin Neurophysiol 2004; 115:348–355. [DOI] [PubMed] [Google Scholar]
- 16.Marquer A, Barbieri G, Pérennou D. The assessment and treatment of postural disorders in cerebellar ataxia: a systematic review. Ann Phys Rehabil Med 2014; 57:67–78. [DOI] [PubMed] [Google Scholar]
- 17.Fillmore PT, Phillips-Meek MC, Richards JE. Age-specific MRI brain and head templates for healthy adults from 20 through 89 years of age. Front Aging Neurosci 2015; 7:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsugi A, Iwata Y, Mori N, Horino H, Hiraoka K. Long latency electromyographic response induced by transcranial magnetic stimulation over the cerebellum preferentially appears during continuous visually guided manual tracking task. Cerebellum 2013; 12:147–154. [DOI] [PubMed] [Google Scholar]
- 19.Iwata NK, Ugawa Y. The effects of cerebellar stimulation on the motor cortical excitability in neurological disorders: a review. Cerebellum 2005; 4:218–223. [DOI] [PubMed] [Google Scholar]
- 20.Van Dun K, Bodranghien F, Manto M, Marien P. Targeting the cerebellum by noninvasive neurostimulation: a review. Cerebellum 2017; 16:695–741. [DOI] [PubMed] [Google Scholar]
- 21.Knikou M. The H-reflex as a probe: pathways and pitfalls. J Neurosci Methods 2008; 171:1–12. [DOI] [PubMed] [Google Scholar]
- 22.Kikuchi S, Mochizuki H, Moriya A, Nakatani-Enomoto S, Nakamura K, Hanajima R, et al. Ataxic hemiparesis: neurophysiological analysis by cerebellar transcranial magnetic stimulation. Cerebellum 2012; 11:259–263. [DOI] [PubMed] [Google Scholar]
- 23.McCall AA, Miller DM, Yates BJ. Descending influences on vestibulospinal and vestibulosympathetic reflexes. Front Neurol 2017; 8:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teune TM, van der Burg J, van der Moer J, Voogd J, Ruigrok TJ. Topography of cerebellar nuclear projections to the brain stem in the rat. Prog Brain Res 2000; 124:141–172. [DOI] [PubMed] [Google Scholar]
- 25.Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation of corticospinal pathways at the foramen magnum level in humans. Ann Neurol 1994; 36:618–624. [DOI] [PubMed] [Google Scholar]


