Abstract
Charcot–Marie–Tooth disease (CMT) is the most common hereditary neuropathy, and more than 80 CMT-causing genes have been identified to date. CMT4J is caused by a loss-of-function mutation in the Factor-Induced-Gene 4 (FIG4) gene, the product of which plays important roles in endosome–lysosome homeostasis. We hypothesized that Mammalian sterile 20-like kinase (MST) 1 and 2, tumor-suppressor genes, are candidate modifiers of CMT4J. We therefore examined the interaction between dFIG4 and Hippo (hpo), Drosophila counterparts of FIG4 and MSTs, respectively, using the Drosophila CMT4J model with the knockdown of dFIG4. The loss-of-function allele of hpo improved the rough eye morphology, locomotive dysfunction accompanied by structural defects in the presynaptic terminals of motoneurons, and the enlargement of lysosomes caused by the knockdown of dFIG4. Therefore, we identified hpo as a modifier of phenotypes induced by the knockdown of dFIG4. These results in Drosophila may provide an insight into the pathogenesis of CMT4J and contribute toward the development of disease-modifying therapy for CMT. We also identified the regulation of endosome–lysosome homeostasis as a novel probable function of Hippo/MST.
Keywords: CMT, CMT4J, Drosophila, endosome–lysosome system, FIG4, Hippo pathway, Hippo, lysosome, MST1, neurodegeneration
Introduction
Charcot–Marie–Tooth disease (CMT) is the most common hereditary neuropathy characterized by progressive motor and sensory polyneuropathies. CMT may be inherited in an autosomal dominant, an autosomal recessive, or an X-linked manner and more than 80 CMT-causing genes have been identified to date 1. CMT4J (OMIM #611228) is a severe, autosomal recessive inherited CMT caused by loss-of-function mutations in the Factor-Induced-Gene 4 (FIG4) gene 2. There is currently no specific treatment for CMT4J.
The endosome–lysosome system and autophagy are considered to play important roles in the pathogenesis of neurodegenerative diseases 3,4. FIG4 has been suggested to be crucially involved in endosome–lysosome membrane homeostasis because loss-of-function mutations in FIG4 counterparts induced enlarged vacuoles or lysosomes in yeast 5,6, nematodes 6, and mice 2. As endosomes and lysosomes are required for autophagy, the loss of FIG4 is expected to cause impaired autophagy, which is supported by previous findings obtained from FIG4 null mice 7. These findings indicated that molecules related to endosome–lysosome membrane homeostasis and/or autophagy modify the function of FIG4. The endosome–lysosome homeostasis is a potential target in the treatment of CMT4J; however, limited information is currently available.
Genetic interaction assays using Drosophila are a strong tool for identifying genetic modifiers for disease-related genes. We reported previously that the knockdown of Drosophila FIG4 (dFIG4) induced an aberrant eye morphology, shortened branches of motoneurons, and mobility defects in the Drosophila CMT4J model 8. We also observed the enlargement of lysosomes in dFIG4 knockdown Drosophila 8, which was consistent with another group’s findings using the null mutation of dFIG4 9.
In the present study, as an initial step to identify modifier genes for the dFIG4 knockdown phenotype, we focused on the cancer-related gene Hippo (hpo). Some causative genes of familial amyotrophic lateral sclerosis (ALS), which is characterized by the progressive loss of motoneurons, appear to be involved in cancer 10. This finding suggests that cancer-related genes modify the neurodegeneration process. The tumor-suppressor gene, Hippo, which is the Drosophila ortholog of Mammalian sterile 20-like kinase (MST) 1 and 2, encodes one of the core proteins of the Hippo pathway 11. A hyperactivated Hippo pathway has been observed in neurodegenerative diseases, including Alzheimer’s disease and ALS 12. Although the relationship between hpo and endosome–lysosome homeostasis currently remains unknown, hpo has been reported to regulate autophagy 13–16. On the basis of these findings, we hypothesized that hpo could be a candidate modifier of FIG4.
To test our hypothesis that hpo is a modifier of FIG4, we analyzed the interaction between the knockdown of dFIG4 and the loss-of-function allele of hpo using a Drosophila model.
Materials and methods
Fly stocks
Fly stocks were maintained at 25°C on standard food containing 0.65% agar, 10% glucose, 4% dry yeast, 5% corn flour, and 3% rice bran. The following fly strains were obtained from the Bloomington Drosophila Stock Center (BDSC): w; P[w+mC=GAL4-elav.L]3 (BDSC 8760)(elav-GAL4), P[ry+t7.2=hsFLP]12, y1w*;P[ry+t7.2=neoFRT]42DhpoKS240/CyO (BDSC 25085)(hpoKS240), yd2w1118P[ry+t7.2=ey-FLP.N]2;P[ry+t7.2=neoFRT]42DhpoKC202/CyO, P[w+mC=GAL4-Kr.C]DC3, P[w+mC=UAS-GFP.S65T]DC7 (BDSC 25090)(hpoKC202), and w1118; and P[w+mC=UAS-GFP.dsRNA.R]143 (BDSC 9331)(UAS-GFP-IR). y1w1118. P[w+mC=Lsp2-GAL4.H]3 (BDSC 108752)(FB-GAL4) was obtained from the Kyoto Stock Center. The RNAi line targeting the region corresponding to amino acid residues 605–714 of dFIG4 (UAS-dFIG4-IR605–714) was obtained from the Vienna Drosophila Resource Center. The establishment of the lines carrying GMR-GAL4 has been described previously 17. Fly lines bearing UAS-dFIG4-IR have been described previously 8. The phenotypes of these lines were not because of a possible insertional mutation or off-target effect as described previously 8.
Scanning electron microscopy
For compound eye observations, adult flies were anesthetized with diethyl ether, mounted on stages, and inspected under the scanning electron microscope V-7800 (Keyence Inc., Osaka, Japan) 18. In each experiment, at least six adult flies were selected randomly from each line for scanning electron microscopy to inspect eye morphologies. In each experiment, no significant variations were observed in eye phenotypes among six individuals from the same strain.
Immunohistochemistry
In the visualization of neuromuscular junctions (NMJs), third instar larvae were dissected in HL3 saline 19 and then fixed in 4% paraformaldehyde/PBS for 30 min. The blocking solution contained 2% bovine serum albumin and 0.1% Triton X-100 in PBS. FITC-conjugated goat anti-horseradish peroxidase (HRP) (1 : 1000; MP Biochemicals, Tokyo, Japan) was used as the detection antibody. Samples were mounted in Vectashield (Vector Laboratories; Burlingame, California, USA) and inspected under a confocal laser scanning microscope (Olympus FluoView FV10i; Olympus, Tokyo, Japan). Motoneurons in muscle 4 in abdominal segment 2 were inspected. The Meta Morph Imaging System 7.7 (Molecular Devices Inc., San Jose, California, USA) was used to measure nerve terminal branch lengths and Ib bouton sizes.
In the visualization of lysosomes, fat bodies were collected by dissecting third instar larvae in PBS and stained with 100 nM of LysoTracker Blue DND22 (Invitrogen, Tokyo, Japan) for 1 min. Stained samples were washed twice with PBS, mounted in Vectashield (Vector Laboratories, Burlingame, California, USA), and observed under a confocal laser scanning microscope (Olympus FluoView FV10iOlympus, Tokyo, Japan). The Meta Morph Imaging System 7.7 (Molecular Devices Inc.San Jose, California, USA) was used to measure the diameters of lysosomes.
Crawling assay
Crawling assays were performed as described previously 20. Third instar male larvae were placed in a 14-cm Petri dish containing 2% agarose (previously poured and allowed to harden) over a black sheet at a density of five larvae per dish. Larvae moved for 30 s, a video was recorded for 1 min, and the path of each larva and its moving speed were analyzed using ImageJ (Image Processing and Analysis in Java) (NIH; Bethesda, Maryland, USA).
Data analysis
Graph Pad Prism version 6.0 (MDF, Tokyo, Japan) was used to carry out each statistical analysis. A one-way analysis of variance was used to assess the significance of comparisons between groups of data. When the one-way analysis of variance showed a significant variation among groups, Dunnett’s test was used for pairwise comparisons between groups. All data are shown as the mean±standard error.
Results
The loss-of-function mutation in hpo significantly suppressed the rough eye morphology caused by the eye-specific dFIG4 knockdown
Flies with the eye disc-specific dFIG4 knockdown showed the rough eye morphology in adults, as we reported previously (Fig. 1a) 8. Two loss-of-function alleles among hpo, hpoKS240, and hpoKC202 in the heterozygous state markedly attenuated the aberrant compound eye morphology induced by the eye disc-specific dFIG4 knockdown (GMR>UAS-dFIG4-IR/hpoKS240 and GMR>UAS-dFIG4-IR/hpoKC202) (Fig. 1b and c). The suppressive effect observed was not because of potential background mutations because two independent loss-of-function alleles of hpo showed similar suppressive effects. These results indicate that dFIG4 genetically interacts with hpo.
Fig. 1.
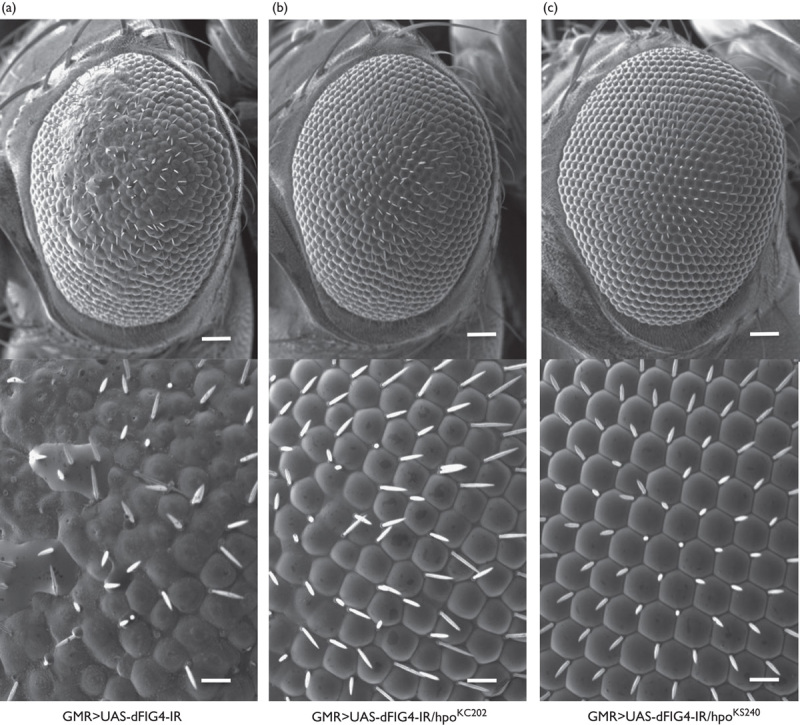
The rough eye morphology induced by the dFIG4 knockdown is suppressed by the loss-of-function mutation in hpo. Each panel shows a scanning electron micrograph of the adult compound eye. Each lower panel shows a higher magnification image of the corresponding upper panel. (a) GMR-GAL4/Y; UAS-dFIG4-IR/+; + (GMR>UAS-dFIG4-IR). (b) GMR-GAL4/Y; UAS-dFIG4-IR/hpoKC202;+(GMR>UAS-dFIG4-IR/hpoKC202). (c) GMR-GAL4/Y; UAS-dFIG4-IR/hpoKS240;+(GMR>UAS-dFIG4-IR/hpoKS240). Posterior is to the right and dorsal is to the top. Flies were developed at 28°C. Scale bars in each upper panel indicate 50 µm and scale bars in the bottom panel indicate 14.2 µm.
The loss-of-function mutation in hpo suppressed shortened motoneurons at presynaptic terminals in the NMJ caused by the dFIG4 knockdown
We analyzed the effects of the loss-of-function allele of hpo on the aberrant morphology of larval motoneuron terminals induced by the neuron-specific dFIG4 knockdown. Neuron-specific dFIG4 knockdown resulted in shorter presynaptic terminal branches of motoneurons in the NMJ (Fig. 2c) than those in the control (Fig. 2a), as reported previously 8. The total branch length of the motoneuron at the NMJ was significantly longer in larvae carrying the dFIG4 knockdown with the loss-of-function mutation in hpo (elav>UAS-dFIG4-IR/hpoKS240, Fig. 2d and e, black column) than in larvae carrying the dFIG4 knockdown only (elav>UAS-dFIG4-IR, Fig. 2c and e, gray column) (67.60±5.97 vs. 81.44±3.84 μm, P<0.05, Fig. 2e). The total branch length of the motoneuron at the NMJ of larvae carrying the loss-of-function mutation in hpo only (hpoKS240/+, Fig. 2b and e, hatched column) was similar to that of control larvae (elav>UAS-GFP-IR, Fig. 2a and e, white column) (121.62±13.19 vs. 115.35±9.49 μm, P=0.12, Fig. 2e). Thus, a half-dose reduction in hpo suppressed the morphological changes in presynaptic terminals induced by the neuron-specific dFIG4 knockdown. As elav-GAL4 is a pan neuron-specific driver, further analysis with a motor neuron-specific GAL4 driver would be necessary to further confirm these results.
Fig. 2.
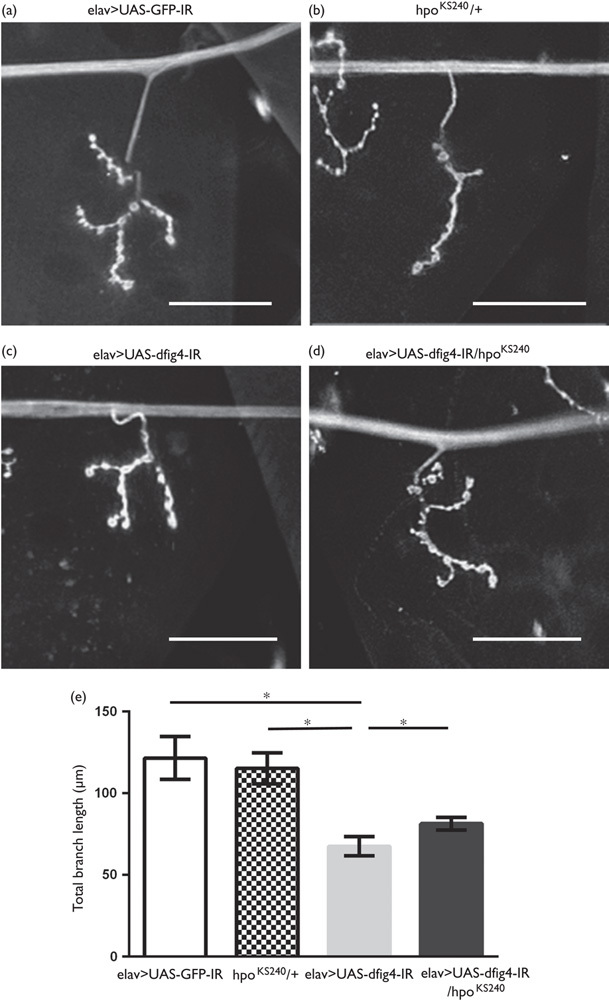
Shortened motoneurons in the neuromuscular junction caused by the dFIG4 knockdown are improved by the loss-of-function mutation in hpo. Each panel shows a representative image of anti-HRP staining of muscle 4 synapses in third instar larvae. (a) yw/Y; UAS-GFP-IR/+; elav-GAL4/+ (elav>UAS-GFP-IR, control), (b) yw/Y;hpoKS240/+; + (hpoKS240/+), (c) yw/Y; UAS-dFIG4-IR/+; elav-GAL4/+ (elav>UAS-dFIG4-IR), (d) yw/Y;UAS-dFIG4-IR/hpoKS240; elav-GAL4/+ (elav>UAS-dFIG4-IR/hpoKS240). (e) The quantified total branch length of the motoneuron at the NMJ. Columns and horizontal bars show the mean and SE of measurements, respectively. *P<0.05. The scale bars indicate 50 µm.
The loss-of-function mutation in hpo suppressed the reduced crawling ability induced by the dFIG4 knockdown
To examine the locomotive ability of larvae carrying the neuron-specific dFIG4 knockdown, we performed the well-established crawling assay 20. Larvae carrying the dFIG4 knockdown (elav>UAS-dFIG4-IR, Fig. 3, gray columns, n=21) showed significantly shorter crawling distances within 30 s and also slower average crawling speeds than control larvae (elav>UAS-GFP-IR, Fig. 3, white columns, n=28) (9.04±1.36 vs. 16.38±1.09 mm, P<0.001, Fig. 3a) (0.58±0.05 vs. 0.74±0.03 mm/s, Fig. 3b). Larvae carrying the dFIG4 knockdown with the loss-of-function mutation in hpo (elav>UAS-dFIG4-IR/hpoKS240, Fig. 3, black columns, n=20) showed significantly longer crawling distances (9.04±1.36 vs. 18.76±1.43 mm, P<0.001, Fig. 3a) (0.58±0.05 vs. 0.81±0.04 mm/s, P<0.001, Fig. 3b). Larvae carrying the loss-of-function mutation in hpo only (hpoKS240/+, Fig. 3, hatched columns, n=22) showed similar crawling ability to control larvae (17.50±1.51 vs. 16.38±1.09 mm, P=0.68, Fig. 3a) (0.79±0.05 vs. 0.74±0.03 mm/s, P=0.50, Fig. 3b). Thus, the half-dose reduction in hpo effectively suppressed the locomotive defect induced by the neuron-specific dFIG4 knockdown.
Fig. 3.
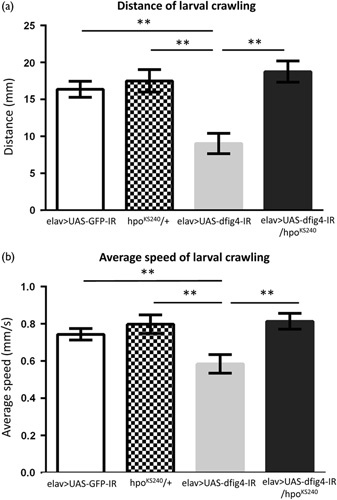
Reduced crawling ability induced by the dFIG4 knockdown is suppressed by the loss-of-function mutation in hpo. (a) Average larval crawling distance (mm) within 30 s. (b) Average crawling speed (mm/s). Columns and horizontal bars show the mean and SE of measurements, respectively. **P<0.001.
The loss-of-function mutation in hpo suppressed the enlarged lysosome diameter induced by the dFIG4 knockdown
As we reported previously, the dFIG4 knockdown induced the enlargement of lysosomes in Drosophila in the larval fat body (Fig. 4) 8. Larvae carrying the dFIG4 knockdown (FB>UAS-dFIG4-IR, Fig. 4b and d, light gray column, n=18) had lysosomes with significantly wider diameters than control larvae (FB>UAS-GFP-IR, Fig. 4a and d, white column, n=18) (2.77±0.57 vs. 6.43±1.93 mm, P<0.001, Fig. 4d). Larvae carrying the fat body-specific dFIG4 knockdown with the loss-of-function mutation in hpo (FB>UAS-dFIG4-IR/hpoKS240, Fig. 4c and d, gray column, n=18) showed significant suppression of the enlarged diameters of lysosomes (6.43±1.93 vs. 3.59±0.92 mm, P<0.001, Fig. 4d). Thus, the half-dose reduction in hpo effectively suppressed the abnormal enlargement of lysosomes induced by the fat body-specific dFIG4 knockdown.
Fig. 4.
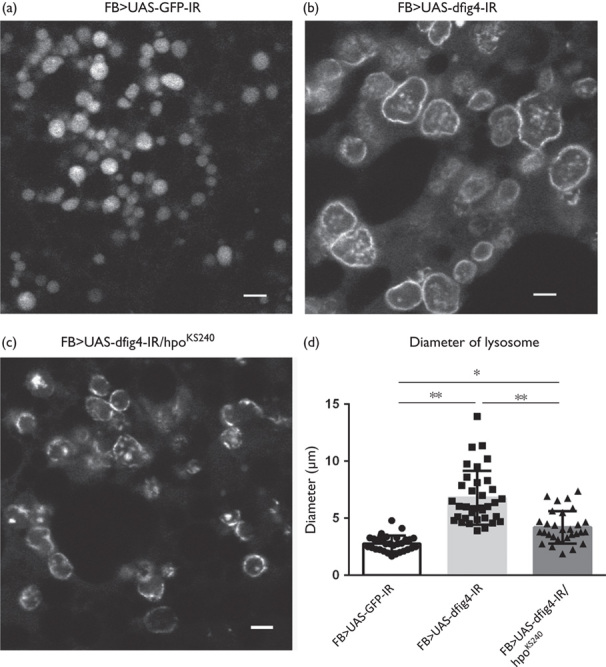
Enlarged lysosomes induced by the dFIG4 knockdown are recovered by the loss-of-function mutation in hpo. Larval fat bodies were stained with LysoTracker to visualize lysosomes. (a) yw/Y; UAS-GFP-IR/+; FB-GAL4/+ (FB>UAS-GFP-IR). (b) yw/Y; UAS-dFIG4-IR/+; FB-GAL4/+ (FB>UAS-dFIG4-IR). (c) yw/Y;UAS-dFIG4-IR/hpoKS240; FB-GAL4/+ (FB>UAS-dFIG4-IR/hpoKS240). (d) This graph shows the average diameter (μm) of lysosomes in larval fat bodies. The scale bars indicate 5 µm. *P<0.05, **P<0.001.
Discussion
A previous study reported that Hippo/MST is a genetic modifier of neurodegeneration in some ALS models. In superoxide dismutase 1 (sod1) mouse ALS models, the downregulation of MST delays the onset of disease and extends survival 13. Furthermore, reductions in the hpo gene dose suppress the eye degeneration phenotype and climbing defect in ALS Drosophila models targeted to VAPB 21. However, a relationship between CMT and Hippo/MST has not yet been reported. In addition to the suppressive effect of the rough eye phenotype by the dFIG4 knockdown, we found that the mutation in hpo effectively attenuated the reduced locomotive ability and abnormal formation of motoneurons induced by the neuron-specific dFIG4 knockdown. Therefore, the present study is the first to indicate a relationship between CMT and hpo in a Drosophila model. Furthermore, Hippo/MST has potential as a candidate therapeutic target for CMT4J. The present study also uncovered a novel probable function of hpo, namely, the downregulation of endosome–lysosome homeostasis, as suggested by the result that the dFIG4 knockdown-induced enlargement of lysosomes was ameliorated by the loss-of-function allele of hpo.
Limited information is currently available on the molecular mechanisms by which hpo influences dFIG4 knockdown-related phenotypes. FIG4 interacts with FAB1 kinase and the scaffolding protein VAC14. The FIG4-VAC14-FAB1 complex functions to increase the abundance of PI(3,5)P2, a signaling lipid located on the cytosolic surface of membranes of the late endosomal compartment 22. To date, there have been no studies on protein–protein interactions between Hippo and a certain component of the FIG4-VAC14-FAB complex, or on the influence of the Hippo pathway on the abundance of PI(3,5)P2. Therefore, further intensive studies are needed to clarify how Hippo regulates endosome–lysosome homeostasis.
The effects of the Hippo pathway on the regulation of autophagy remain controversial. Previous studies reported the upregulation of autophagy by Hippo, whereas others reported downregulation 13–16. Our results support the Hippo pathway downregulating autophagy. This is consistent with previous findings showing that MST1, the mammalian homologue of Hippo, mediated abnormal autophagy in the SOD1 ALS model mouse 13 and that an MST1 deficiency enhanced autophagy in post-traumatic spinal motoneurons 14. However, autophagy was shown to be upregulated by the Hippo pathway in a cell culture study or Drosophila model of Dantato-rubro-pallido-luysian atrophy 15,16. These controversial findings may be at least partially because of the differences in the diseases studied.
Conclusion
Here, we identified hpo as a modifier of dFIG4, a CMT4J-causing gene. This discovery will contribute toward clarifying the pathogenesis of CMT as well as the development of disease-modifying therapy.
Acknowledgements
The authors acknowledge our lab members for their technical assistance and discussions.
The authors thank all members of the Chromosome engineering laboratory for their helpful discussions, technical support, and suggestions. We thank the Bloomington Drosophila stock center, the Vienna Drosophila RNAi center, and the Kyoto Drosophila Genetic Resource Center for providing us with fly lines.
This study was supported by the Japan Agency for Medical Research and Development (AMED) (17dk0207030h0002 and 17ek0109222h0001; to T.T.) and JSPS KAKENHI Grant Numbers JP26893227 and JP16K19519; by a Grant for Joint Research Project of The Center for Advanced Insect Research Promotion, Kyoto Institute of Technology; and by a Grant-in-Aid by the Nakabayashi Trust for ALS Research, Tokyo, Japan (Y.A.) and by a Grant for the JSPS Core-to-Core Program, Asia-Africa Science Platforms, and the JSPS Program for Advancing Strategic International Networks to Accelerate the Circulation of Talented Researchers (grant no. S2802 to M.Y.).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Timmerman V, Strickland AV, Züchner S. Genetics of Charcot–Marie–Tooth (CMT) Disease within the Frame of the Human Genome Project Success. Genes 2014; 5:13–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chow CY, Zhang Y, Dowling JJ, Jin N, Adamska M, Shiga K, et al. Mutation of FIG4 causes neurodegeneration in the pale tremor mouse and patients with CMT4J. Nature 2007; 448:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denton D, Xu T, Kumar S. Autophagy as a pro-death pathway. Immunol Cell Biol 2015; 93:35–42. [DOI] [PubMed] [Google Scholar]
- 4.Hu YB, Dammer EB, Ren RJ, Wang G. The endosomal–lysosomal system: from acidification and cargo sorting to neurodegeneration. Transl Neurodegener 2015; 4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonangelino CJ, Nau JJ, Deux JE, Brinkman M, Wurmser AE, Gary JD, et al. Osmotic stress-induced increase of phosphatidylinositol 3,5-bisphosphate requires Vac14p, an activator of the lipid kinase Fab1p. J Cell Biol 2002; 156:1015–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicot AS, Fares H, Payrastre B, Chisholm AD, Labouesse M, Laporte J. The phosphoinositide kinase PIKfyve/Fab1p regulates terminal lysosome mutation in Caenorhabditis elegans. Mol Biol Cell 2006; 17:3062–3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson CJ, Lenk GM, Meisler MH. Defective autophagy in neurons and astrocytes from mice deficient in PI(3,5)P2. Hum Mol Genet 2009; 18:4868–4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyotani A, Azuma Y, Yamamoto I, Yoshida H, Mizuta I, Mizuno T, et al. Knockdown of the Drosophila FIG4 induces deficient locomotive behavior, shortening of motor neuron, axonal targeting aberration, reduction of life span and defects in eye development. Exp Neurol 2016; 277:86–95. [DOI] [PubMed] [Google Scholar]
- 9.Bharadwaj R, Cunningham KM, Zhang K, Lloyd TE. FIG4 regulates lysosome membrane homeostasis independent of phosphatase function. Hum Mol Genet 2016; 25:681–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaguchi M, Azuma Y, Yoshida H. ALS and cancer. J Carcinog Mutagen 2016; 7:e122. [Google Scholar]
- 11.Staley BK, Irvine KD. Hippo signaling in Drosophila: recent advances and insights. Dev Dyn 2012; 241:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang SP, Wang LH. Disease implication of hyper-Hippo signaling. Open Biol 2016; 6:160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JK, Shin JH, Hwang SG, Gwag BJ, McKee AC, Lee J, et al. MST1 functions as a key modulator of neurodegeneration in a mouse model of ALS. Proc Natl Acad Sci USA 2013; 110:12066–12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang M, Tao W, Yuan Z, Liu Y. Mst-1 deficiency promotes post-traumatic spinal motor neuron survival via enhancement of autophagy flux. J Neurochem 2017; 143:244–256. [DOI] [PubMed] [Google Scholar]
- 15.Wilkinson DS, Jariwala JS, Anderson E, Mitra K, Meisenhelder J, Chang JT, et al. Phosphorylation of LC3 by the Hippo kinases STK3/STK4 is essential for autophagy. Mol Cell 2015; 57:55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calamita P, Fanto M. Slimming down fat makes neuropathic hippo: The Fat/Hippo tumor suppressor pathway protects adult neurons through regulation of autophagy. Autophagy 2011; 7:907–909. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi Y, Hirose F, Matsukage A, Yamaguchi M. Identification of three conserved regions in the DREF transcription factors from Drosophila melanogaster and Drosophila virilis. Nucleic Acids Res 1999; 27:510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ly LL, Suyari O, Yoshioka Y, Tue NT, Yoshida H, Yamaguchi M. dNF-YB plays dual roles in cell death and cell differentiation during Drosophila eye development. Gene 2013; 520:106–118. [DOI] [PubMed] [Google Scholar]
- 19.Stewart BA, Atwood HL, Renger JJ, Wang J, Wu CF. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J Comp Physiol A 1994; 175:179–191. [DOI] [PubMed] [Google Scholar]
- 20.Nichols CD, Becnel J, Pandey UB. Methods to assay Drosophila behavior. J Vis Exp 2012; 61:e3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanhueza M, Chai A, Smith C, McCray BA, Simpson TI, Taylor JP, et al. Network analyses reveal novel aspects of ALS pathogenesis. PLoS Genet 2015; 11:e1005107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudge SA, Anderson DM, Emr SD. Vacuole size control: regulation of PtdIns(3,5)P2 levels by the vacuole-associated Vac14-Fig4 complex, a PtdIns(3,5)P2-specific phosphatase. Mol Biol Cell 2004; 15:24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]


