Abstract
Metaplastic carcinoma is a rare subtype of breast cancer that comprises histologically diverse malignancies. Metaplastic carcinoma tends to have an aggressive clinical presentation, high metastatic potential, and more chances of local recurrence, as compared with intraductal carcinoma. Although many clinical and pathologic finding have been reported, to our knowledge, there are few reports of imaging findings for this type of tumor.
In this study, we reviewed data of 5 patients with histopathologically proven metaplastic breast carcinoma. Multimodality imaging findings including those from mammography, ultrasonography, magnetic resonance imaging, and positron emission tomography–computed tomography were recorded.
The results indicated that metaplastic carcinomas tend to show more benign imaging features such as round or oval shape with circumscribed margins and less axillary lymph node metastasis compared with invasive ductal carcinoma. High signal intensity on T2-weighted magnetic resonance imaging due to its cystic or necrotic component may be useful for diagnosis of metaplastic carcinoma.
Key Words: metaplastic breast carcinoma, MRI, CT, sonography, mammography, PET-CT
Metaplastic breast carcinoma is a very rare breast cancer, characterized by mixed epithelial and mesenchymal differentiation. It accounts for less than 5% of all breast cancers.1–6 Previously histologic classification by Wargotz et al,7 including matrix-producing carcinoma, squamous cell carcinoma, spindle cell carcinoma, carcinosarcoma, and metaplastic carcinoma with osteoclastic giant cells, has been used to describe the metaplastic breast carcinoma. However, the World Health Organization officially recognized metaplastic breast carcinoma as a distinct pathologic entity in 2000. Updated histologic World Health Organization suggested 6 types of metaplastic carcinomas, including squamous cell carcinoma, spindle cell carcinoma, low-grade adenosquamous carcinoma, metaplastic carcinoma with mesenchymal differentiation, fibromatosis-like metaplastic carcinoma, low-grade adenosquamous carcinoma, and mixed metaplastic carcinoma.8,9 In our study, histopathologic examination revealed 2 cases of low-grade adenosquamous carcinoma and mixed metaplastic carcinoma, 2 cases of low-grade adenosquamous carcinoma, and 1 case of squamous cell carcinoma. The differential diagnosis between invasive ductal carcinoma and metaplastic breast carcinoma is important for management and prognosis.3 Metaplastic carcinoma tends to have a high metastatic potential, more chances of local recurrence, and poor prognosis, as compared with intraductal carcinoma. It can be explained by its greater size, pathologic heterogeneity, higher proliferation index, poorer differentiation, and suboptimal response to systemic chemotherapy or hormonal therapy. A recent study reported that most metaplastic breast carcinomas are estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (Her2) negative and tend to have a poor prognosis with fewer treatment options.1 The worse prognosis of metaplastic breast carcinoma can ;be explained by greater size, histopathologic heterogeneity, higher proliferation index, and poorer differentiation.10 Although clinical and pathologic findings have been described,1,10 to our knowledge, imaging findings related to metaplastic carcinoma have rarely been described. The purpose of this study was to describe multimodality imaging findings of metaplastic breast carcinoma, including mammography, sonography, magnetic resonance imaging (MRI), positron emission tomography–computed tomography (PET-CT), and histopathologic features.
CASE REPORT
Case 1
A 49-year-old woman presented to her surgeon with a palpable lump in right breast. Mammography showed an indistinct round isodense mass with nipple retraction and skin thickening in the right breast, mid-lower portion (Fig. 1A). Ultrasonography showed a microlobulated round hypoechoic mass (Fig. 1B), which showed remarkable fluorodeoxyglucose (FDG) uptake on PET-CT (maximum standardized uptake value [maxSUV], 12.8; Fig. 1C). Subsequent diagnostic core biopsy indicated an invasive ductal carcinoma. The patient underwent modified radical mastectomy. Histopathologic examination revealed a low-grade adenosquamous carcinoma measuring 3.1 × 2.5 × 2.0 cm. Immunohistochemistry of the tumor was negative for ER, PR, and HER2 expression (Table 1). The patient developed left and right upper lobe lung mass 7 and 24 months later, respectively. Biopsy of the lung mass showed squamous cell carcinoma, suggestive of metastasis from breast. The patient was treated with radiation therapy, and at final follow-up, the patient was alive with stable disease for 4 years.
FIGURE 1.
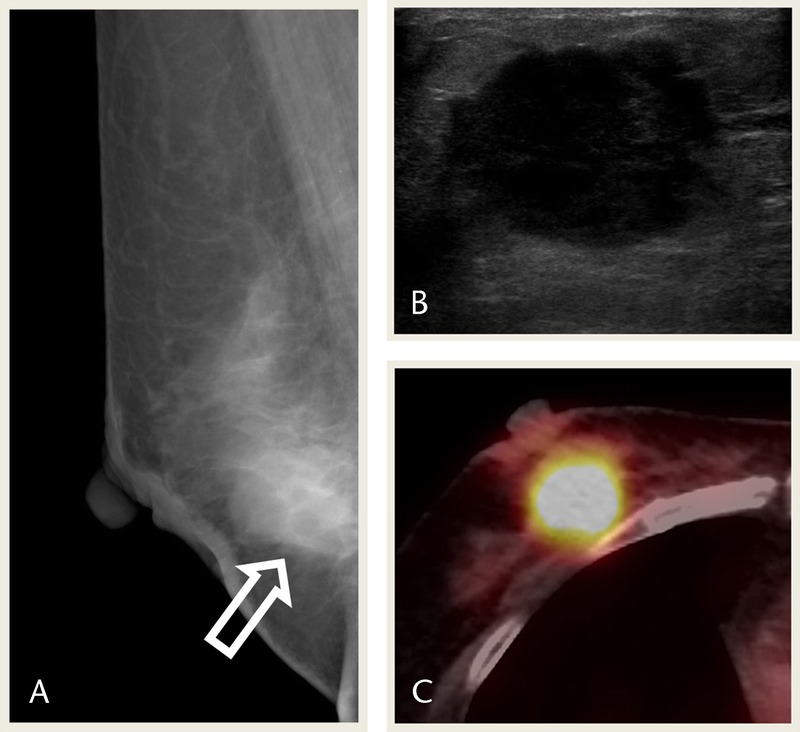
A 49-year-old woman presented with a palpable right breast lump. A, Mammography MLO view shows an indistinct round isodense mass in the right breast, mid lower portion (arrow). B, USG image shows a microlobulated round hypoechoic mass. C, PET-CT image shows remarkable FDG uptake in the mass (maxSUV 12.8). MLO, mediolateral oblique; USG, ultrasonography.
TABLE 1.
Summary of Pathology and Imaging Findings in 5 Metaplastic Breast Carcinomas
Case 2
A 55-year-old woman presented with a large palpable mass in her left breast, which she had discovered 1 year earlier and increased in size. The large heterogeneous hypoechoic mass in the left breast found on ultrasonography (Fig. 2A) showed remarkable FDG uptake on PET-CT (maxSUV, 12.39; Fig. 2D). Breast MRI showed a T1 iso-SI mass with early heterogeneous enhancement and early washout (Fig. 2B). Results of a subsequent diagnostic core biopsy revealed an adenosquamous carcinoma. Modified radical mastectomy was performed. Histopathologic examination of the resected specimen showed low-grade adenosquamous carcinoma. Skin invasion were observed. Both ER and PR were negative, and HER2 was positive (Table 1). The patient underwent preoperative adjuvant chemotherapy, followed by postoperative radiotherapy. Seven months later, the patient developed a right axilla recurrence and transferred to an outside hospital.
FIGURE 2.
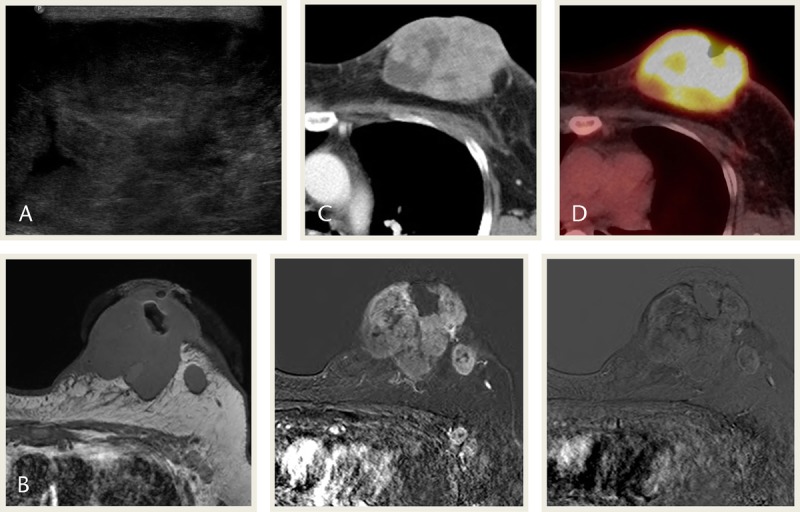
A 55-year-old woman presented with a huge palpable mass in the left breast. A, USG image shows a heterogeneous hypoechoic mass in the left breast. B, MRI images show an iso-signal mass in T1WI. The mass shows early heterogeneous enhancement in subtraction image and early washout in reverse subtraction image. C, Contrast-enhanced CT image shows heterogeneous enhancement. D, PET-CT image shows remarkable FDG uptake in the mass (maxSUV, 12.39). MLO, mediolateral oblique; T1WI, T1-weighted image.
Case 3
A 53-year-old woman presented with a palpable lump in the right breast, subareolar area. Mammography showed an indistinct oval hyperdense mass in the right breast, subareolar area (Fig. 3A). Ultrasonography revealed an indistinct angular hypoechoic mass and suspicious axillary lymph node (Figs. 3B, C). Subsequent diagnostic core biopsy of the tumor indicated squamous cell carcinoma. Immunohistochemistry of the tumor was negative for ER and PR and positive for HER2 (Table 1). She was transferred to an outside hospital.
FIGURE 3.
A 53-year-old woman presented with a palpable lump in the right breast. A, Mammography CC view shows an indistinct oval hyperdense mass in the right breast, subareolar area (arrow). B, USG images show an indistinct angular hypoechoic mass in the right breast, subareolar area. C, Suspicious lymph node is seen in right axilla, but fine needle aspiration was negative. CC, craniocaudal view; USG, ultrasonography.
Case 4
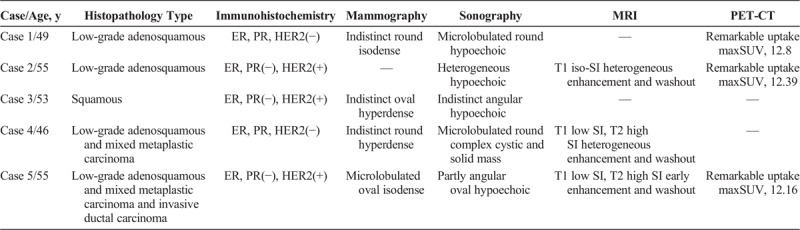
A 46-year-old woman presented with a mass in the right breast, which was discovered during self-examination and stable for several months. She had an operation history of bilateral mammoplasty. Mammography showed an indistinct round hyperdense mass in the right breast, upper outer quadrant. Metastatic lymph node was also noted in right axilla (Fig. 4A). Ultrasonography showed a microlobulated, round, complex cystic and solid mass with metastatic lymph node (Figs. 4B, C), which showed remarkable FDG uptake on PET-CT (maxSUV, 11.4 and 10.5, respectively; Fig. 4E). Magnetic resonance imaging revealed a heterogeneous T2 high-SI mass with peripheral enhancement in the right breast, upper outer quadrant, which showed early enhancement in subtraction image and washout in reverse subtraction image, with metastatic lymph node of similar appearance (Figs. 4D). Subsequent diagnostic core biopsy revealed low-grade adenosquamous carcinoma and mixed metaplastic carcinoma. The tumor was negative for ER, PR, and HER2 (Table 1). The patient underwent adjuvant chemotherapy followed by lumpectomy and postoperative radiotherapy. Histopathologic examination of the resected specimen revealed no residual tumor and no metastatic axillary lymph nodes. Four months after operation, brain angio-CT showed 2 enhancing nodules that were suspicious for metastatic disease. The patient underwent palliative radiotherapy and has had stable disease for the past 8 months.
FIGURE 4.
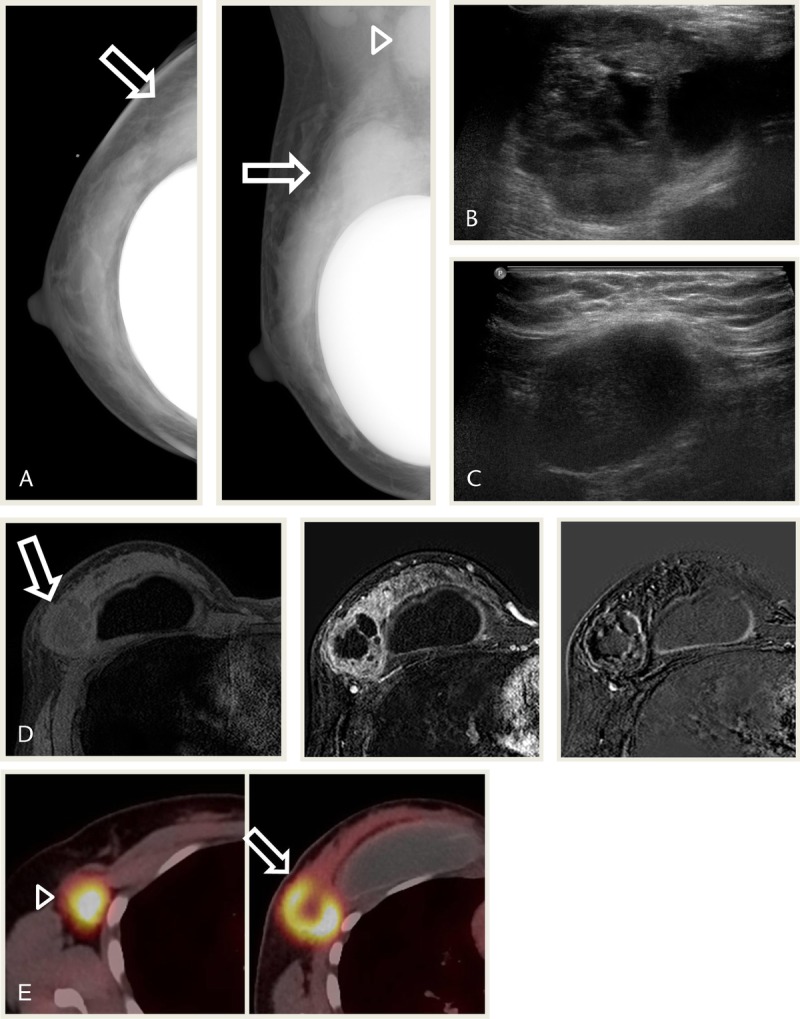
A 46-year-old woman presented with a palpable mass in the right breast after mammoplasty. A, Mammography CC and MLO views show an indistinct round hyperdense mass in the right UOQ (arrows). Metastatic lymph node is noted in right axilla (arrow head). B and C, USG images show a microlobulated round complex cystic and solid mass with metastatic lymph node. D, MRI images show a mass in the right breast UOQ with heterogeneous high SI in T2WI, low SI in T1WI, heterogeneous peripheral enhancement. The mass shows early enhancement in subtraction image and washout in reverse subtraction image (arrows). E, PET-CT images show remarkable FDG uptake in the mass of the breast (arrow; maxSUV, 11.4) and metastatic lymph node in right axilla (arrowhead; maxSUV, 10.5). CC, craniocaudal view; MLO, mediolateral oblique; T1WI, T1-weighted image; T2WI, T2-weighted image; UOQ, upper outer quadrant; USG, ultrasonography.
Case 5
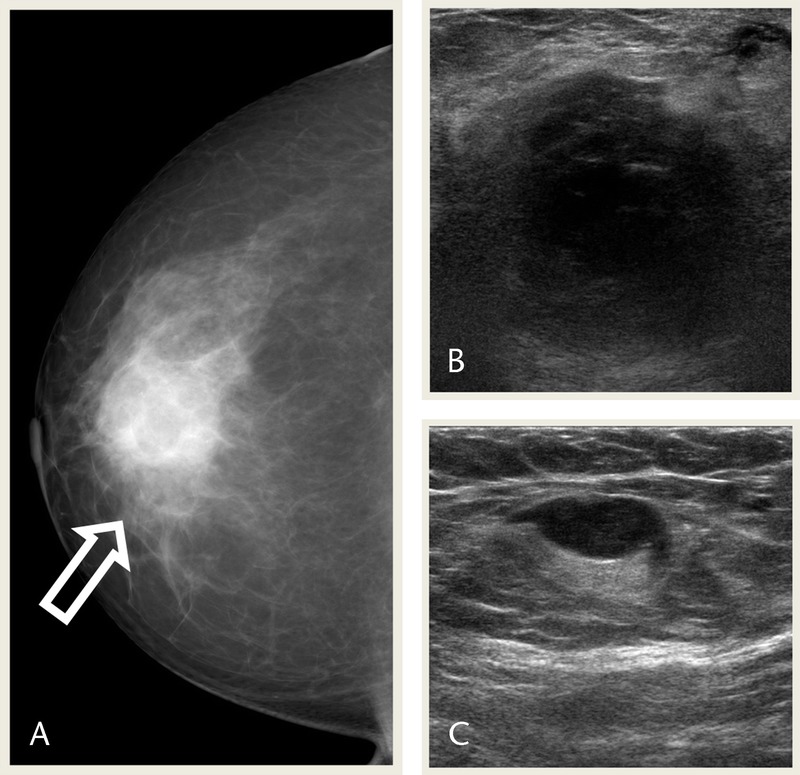
A 55-year-old woman presented with a palpable mass in the right breast. Mammography showed a microlobulated oval isodense mass in the right breast upper, outer quadrant (Fig. 5A). Ultrasonography show a partly angular oval hypoechoic mass with several satellite nodules in the right breast, upper outer quadrant, with suspicious lymph node in right axilla (Figs. 5B, C). Magnetic resonance imaging showed an irregular spiculated heterogeneous T2 high-, T1 low-SI mass with early enhancement and delayed washout (Fig. 5D). Positron emission tomography–CT showed remarkable FDG uptake in the main mass and satellite nodules in the right breast, upper outer quadrant (maxSUV, 12.16 and 5.25, respectively; Fig. 5E).
FIGURE 5.
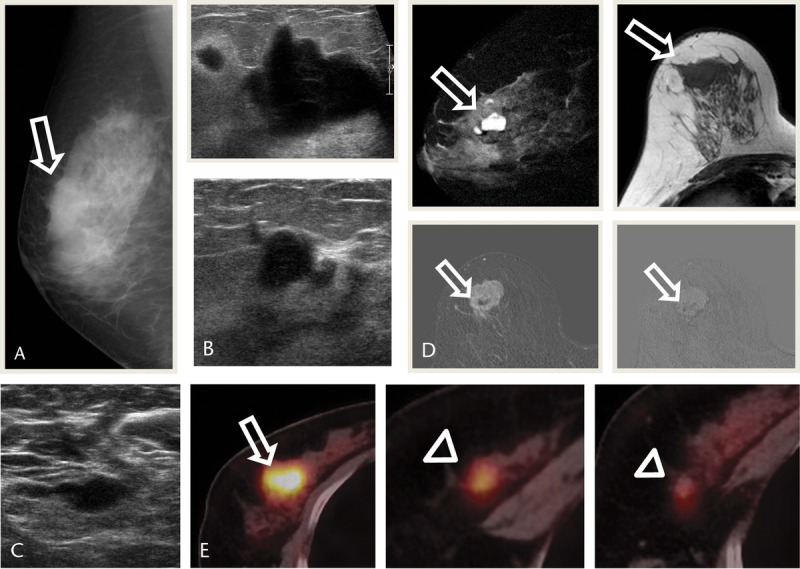
A 55-year-old woman presented with a palpable mass in the right breast. A, Right mammography MLO view shows a microlobulated oval isodense mass in the right UOQ (arrow). B, USG images show a partly angular oval hypoechoic mass with several satellite nodules in the right breast UOQ. C, USG image shows suspicious lymph node in right axilla, but FNA was negative. D, MRI images show an irregular spiculated heterogeneous mass with T2 high, T1 low SI, early enhancement and delayed washout in subtraction and reverse subtraction images (arrows). E, images show FDG uptake in the main mass (arrow) and satellite nodules (arrowheads; maxSUV, 12.16 and 5.25, respectively). MLO, mediolateral oblique; UOQ, upper outer quadrant; USG, ultrasonography.
Subsequent diagnostic core biopsy revealed an invasive ductal carcinoma.
Modified radical mastectomy was performed. Histopathologic examination of the resected specimen showed low-grade adenosquamous carcinoma and mixed metaplastic carcinoma and invasive ductal carcinoma measuring 2.5 × 2.5 × 2.4 cm. Metastatic lymph nodes were absent, and the tumor was negative for ER and PR and positive for HER2 expression (Table 1). The patient subsequently received adjuvant chemotherapy. She has been free of recurrence during 7 months of follow-up.
DISCUSSION
Most benign and malignant tumors originate from glandular epithelium. In a small percentage of malignancies, glandular epithelium undergoes a process called metaplasia, presenting differentiation to nonglandular mesenchymal tissue.4 Metaplastic breast carcinoma is a rare subtype of ductal carcinoma undergoing metaplasia including squamous differentiation, a spindle-cell pattern of growth, and/or heterogeneous mesenchymal morphological elements.2,4,6 The most common metaplastic components include squamous metaplasia.4 Metaplastic breast carcinoma accounts for less than 5% of all breast cancers.1–6 It occurs more commonly in black and Hispanic women.11 Metaplastic breast carcinomas usually present in approximately 50-year-old women as a fast-growing palpable large mass, with lower rates of axillary nodal involvement, higher rates of both local recurrence and metastasis, and higher rates of ER, PR, and Her2 negativity, as well as a suboptimal response to systemic therapies when compared with other invasive breast cancers.4,6,9,10 It is often associated with poorer survival, despite the survival advantage of histologic-specific novel chemotherapeutic strategies and thus a potential area for further research.1 In the current study, we described 5 metaplastic breast carcinoma patients. The most common type of metaplastic carcinoma is known as squamous, followed by spindle cell and matrix producing. Osseous or sarcomatoid metaplasia is a rare type.4 In our study, low-grade adenosquamous carcinoma was noted in 2 cases, and low-grade adenosquamous carcinoma with mixed metaplastic carcinoma in 2 cases squamous cell carcinoma in 1 case. Moreover, most cases are not associated with hormone receptors. ER and PR were negative in 5 of our patients, whereas 3 of the tumors were positive for Her2. In addition, surgical specimen axillary lymph node metastases were noted in 1 case. Despite the poor prognosis of metaplastic breast carcinoma, reduced axillary node metastasis is observed due to its hematogenous over lymphatic spread. This may also result in higher rates of local or distant metastases during the follow-up.4 The results of our study are similar to those of previous reports.
On mammography, metaplastic breast carcinomas show a predominantly circumscribed, dense, noncalcified mass.2,4,6,11,12
On sonography, metaplastic breast carcinoma appears as gently lobulated complex echogenicity with solid components as well as cystic components that are related to necrosis and cystic degeneration in the histologic analysis.2,4,6,11 Posterior acoustic enhancement is common.4,10
Breast MRI could be used as a problem-solving tool with higher sensitivity and specificity in cases of equivocal findings with conventional breast imaging modalities.2 On magnetic resonance images, metaplastic breast carcinomas present as a round to lobular mass, frequently smooth margins, and generally isointense or hypointense, similar to other histologic types of invasive breast carcinoma. Nevertheless, high signal intensity on T2-weighted images due to necrotic component and cystic degeneration is a frequent finding.2,4,6,11 The T2 high signal is also observed in mucinous carcinoma, invasive ductal carcinoma with infarction or necrosis, intracystic papillary carcinoma, and invasive papillary carcinoma, in addition to metaplastic breast carcinoma.4,6 The reported enhancement characteristics of these lesions include heterogeneous, rim-like, or containing nonenhancing internal components. The frequently reported kinetic pattern is early enhancement and a delayed washout corresponding to the enhancing peripheral portion and nonenhancing internal components.2,4,12
The mammographic, sonographic, and MRI imaging characteristics of metaplastic breast carcinomas can be similar to invasive ductal carcinoma as well as benign lesion, and so subject to misinterpretation.4,12 Overlapping findings prevent complete differentiation of such tumors on imaging.2,4 Awareness of the overlapping imaging findings and clinical features can assist in diagnosing these malignancies.12
Metaplastic breast carcinomas should be differentiated from invasive ductal carcinoma and primary breast sarcoma, to determine appropriate treatment options and management.2,12,13 Preoperative diagnosis of metaplastic breast carcinoma by core needle biopsy might be possible, but in most cases, transition foci of metaplastic element were noted after excision biopsy. Therefore, extensive sampling such as excision biopsy should be performed to avoid misleading from small sample.4
In conclusion, metaplastic breast carcinoma is a rare subtype of breast cancer accounting for less than 5% of all breast cancers. It is characterized by a larger size at presentation, lower rates of axillary nodal involvement, higher rates of both local and distant recurrence, and higher rates of ER, PR, and Her2 negativity. Most metaplastic breast carcinomas have a poor prognosis due to a suboptimal response to systemic therapy. On mammography and sonography, metaplastic breast carcinoma shows benign features including circumscribed margin, round shape, and posterior acoustic enhancement. T2 high signal intensity, early enhancement, and a delayed washout in a peripheral rim and nonenhancing internal components on magnetic resonance image would be helpful in differentiating from other breast malignancies. Therefore, multimodality imaging studies with immunohistochemical and pathologic studies are necessary in the diagnosis of metaplastic breast carcinoma. Further research studies may be required to develop a targeted therapeutic regimen for improving clinical outcomes.
Footnotes
The authors declare no conflict of interest.
This work was supported in part by the Soonchunhyang University Research Fund.
Ethical statement: This study was approved by an institutional review board of the Soonchunhyang University Cheonan Hospital and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The institutional review board of our university waived the need to obtain informed consent.
The manuscript has not been published before or is not under consideration for publication anywhere else and has been approved by all co-authors in the title page.
REFERENCES
- 1.Schwartz TL, Mogal H, Papageorgiou C, et al. Metaplastic breast cancer: histologic characteristics, prognostic factors and systemic treatment strategies. Exp Hematol Oncol. 2013;2:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang YW, Lee MH, Kwon KH, et al. Magnetic resonance imaging of metaplastic carcinoma of the breast: sonographic and pathologic correlation. Acta Radiol. 2004;45:18–22. [DOI] [PubMed] [Google Scholar]
- 3.Yang WT, Hennessy B, Broglio K, et al. Imaging differences in metaplastic and invasive ductal carcinomas of the breast. AJR Am J Roentgenol. 2007;189:1288–1293. [DOI] [PubMed] [Google Scholar]
- 4.Choi BB, Shu KS. Metaplastic carcinoma of the breast: multimodality imaging and histopathologic assessment. Acta Radiol. 2012;53:5–11. [DOI] [PubMed] [Google Scholar]
- 5.Lai YC, Hsu CY, Chou YH, et al. Sonographic presentations of metaplastic breast cancers. J Chin Med Assoc. 2012;75:589–594. [DOI] [PubMed] [Google Scholar]
- 6.Velasco M, Santamaría G, Ganau S, et al. MRI of metaplastic carcinoma of the breast. AJR Am J Roentgenol. 2005;184:1274–1278. [DOI] [PubMed] [Google Scholar]
- 7.Wargotz ES, Does PH, Norris HJ. Metaplastic carcinomas of the breast. II. Spindle cell carcinoma. Hum Pathol. 1989;20:732–740. [DOI] [PubMed] [Google Scholar]
- 8.Frank GA, Danilova NV, Andreeva IuIu, et al. WHO classification of tumors of the breast, 2012. Arkh Patol. 2013;75:53–63. [PubMed] [Google Scholar]
- 9.Langlands F, Cornford E, Rakha E, et al. Imaging overview of metaplastic carcinomas of the breast: a large study of 71 cases. Br J Radiol. 2016;20140644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wani FA. Metaplastic breast cancer: pathological subtypes, clinical presentation, imaging characteristics, immunohistochemistry, treatment and prognosis. Int J Med Sci Public Health. 2014;3:1029–1033. [Google Scholar]
- 11.Ryckman EM, Murphy TJ, Meschter SC, et al. AIRP best cases in radiologic-pathologic correlation: metaplastic squamous cell carcinoma of the breast. Radiographics. 2013;33:2019–2024. [DOI] [PubMed] [Google Scholar]
- 12.Leddy R, Irshad A, Rumboldt T, et al. Review of metaplastic carcinoma of the breast: imaging findings and pathologic features. J Clin Imaging Sci. 2012;2:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashimoto BE, Morgan GN, Kramer DJ, et al. Systematic approach to difficult problems in breast sonography. Ultrasound Q. 2008;24:31–38. [DOI] [PubMed] [Google Scholar]


