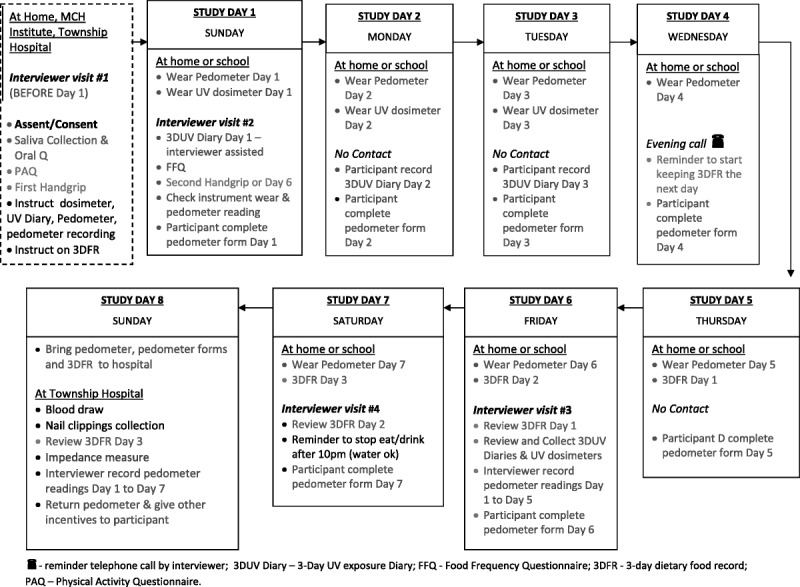
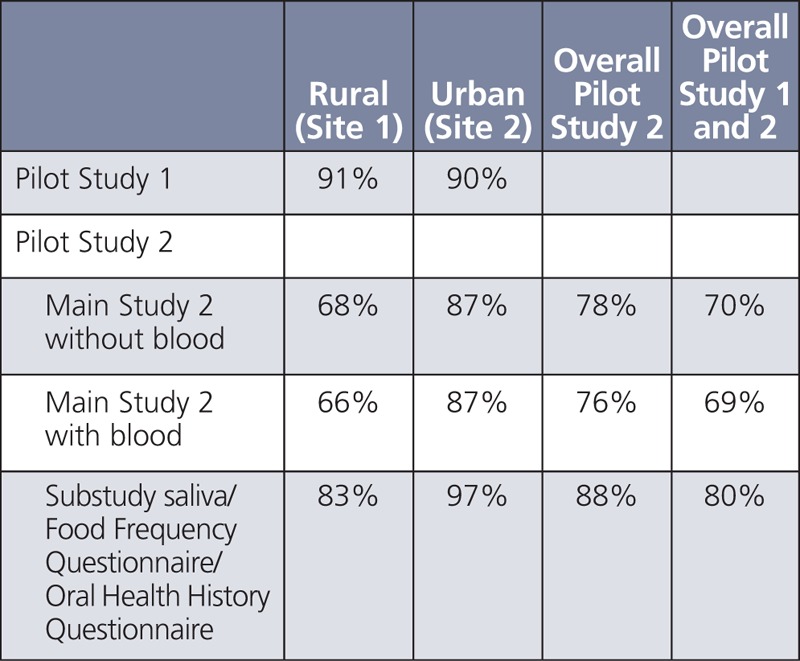
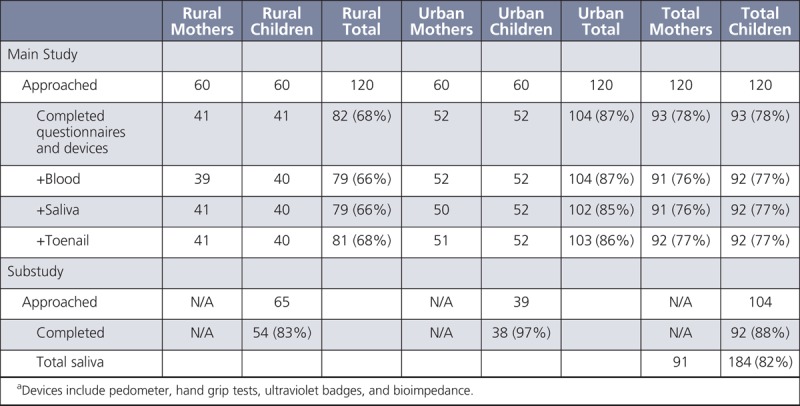
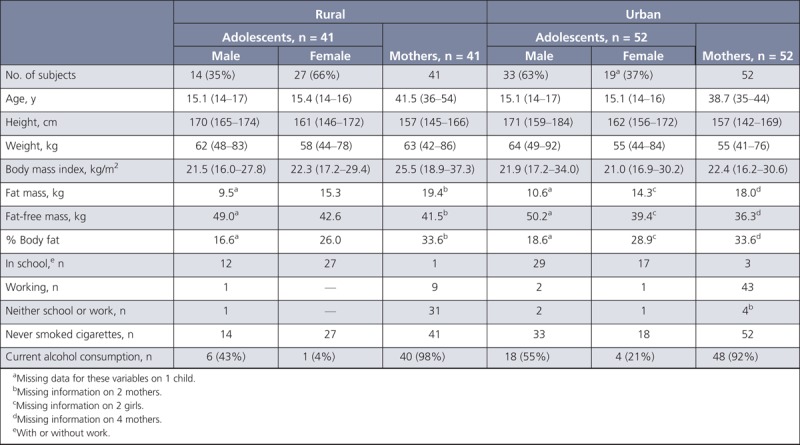
Nancy Potischman
Nancy Potischman, PhD
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,
Liwen Fang
Liwen Fang, MD, PhD
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,
Ling Hao
Ling Hao, MD, PhD
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,
Regan R Bailey
Regan R Bailey, PhD, MPH
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,
David Berrigan
David Berrigan, PhD, MPH
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,
Robert John Berry
Robert John Berry, MD, MPHTM
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,
Alison Brodie
Alison Brodie, PhD
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,
Ann Chao
Ann Chao, PhD
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,
Jing Chen
Jing Chen, MSc
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,
Kevin Dodd
Kevin Dodd, PhD
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,
Yajing Feng
Yajing Feng, MSc
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,
Guansheng Ma
Guansheng Ma, PhD
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,
Yuna He
Yuna He, PhD
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,
Jing Fan
Jing Fan, MSc
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,
Michael Kimlin
Michael Kimlin, PhD
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,
Cari Kitahara
Cari Kitahara, PhD
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,
Martha Linet
Martha Linet, MD, MPH
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,
Zhu Li
Zhu Li, MD, MPH
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,
Ailing Liu
Ailing Liu, PhD
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,
Yashan Liu
Yashan Liu, MD
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,
Joshua Sampson
Joshua Sampson, PhD
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,
Joseph Su
Joseph Su, PhD, MPH
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,
Jiandong Sun
Jiandong Sun, PhD
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,
Natasha Tasevska
Natasha Tasevska, MD, PhD, MSc
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,
Lichen Yang
Lichen Yang, MD, PhD
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,
Ruilan Yang
Ruilan Yang, MD
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,
Qian Zhang
Qian Zhang, PhD
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,
Ning Wang
Ning Wang, MSc
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,
Linhong Wang
Linhong Wang, MD, MS
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,
Wang Yu
Wang Yu, MD, PhD
1Nancy Potischman, PhD, is the Director of Population Studies, in the Office of Disease Prevention in the Office of the Director of the National Institutes of Health and the principal investigator of the Chinese Children and Families Cohort Study (CFCS) Pilot Study 2.
2Liwen Fang, MD, PhD, is Director of Cancer Division, National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
3Ling Hao, MD, PhD, is a Project Officer, US Centers for Disease Control and Prevention China Office, Beijing, China.
4Regan R. Bailey, PhD, MPH, formerly at the Office of Dietary Supplements, National Institutes of Health, Bethesda, Maryland.
5David Berrigan, PhD, MPH, is a Program Director, Division of Cancer Control and Population Studies, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
6Robert John Berry, MD, MPHTM, is a medical epidemiologist, US Centers for Disease Control and Prevention, Atlanta, Georgia, and the US Principal Investigator of the Community Intervention Program in China (retired).
7Alison Brodie, PhD, Queensland University of Technology, Brisbane, Australia.
8Ann Chao, PhD, is a Senior Advisor for Cancer and Noncommunicable Diseases, Center for Global Health, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
9Jing Chen, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
10Kevin Dodd, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
11Yajing Feng, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
12Guansheng Ma, PhD, is Chair of Department of Nutrition and Food Safety, School of Public Health, Peking University, Beijing, China.
13Yuna He, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
14Jing Fan, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
15Michael Kimlin, PhD, is professor of Cancer Prevention, University of the Sunshine Coast & Cancer Council Queensland, Brisbane, Australia.
16Cari Kitahara, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
17Martha Linet, MD, MPH, served as Chief of the Radiation Epidemiology Branch from 2002 to 2014 in the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and principal investigator of the larger CFCS Pilot Study 1.
18Zhu Li, MD, MPH, is a consultant and Principal Investigator of the Community Intervention Program in China, Peking University Health Science Center, Beijing, China (retired).
19Ailing Liu, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
20Yashan Liu, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
21Joshua Sampson, PhD, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
22Joseph Su, PhD, MPH, is a professor in the Department of Epidemiology and codirector of the Cancer Prevention and Population Sciences Program at the University of Arkansas, Little Rock.
23Jiandong Sun, PhD, Queensland University of Technology, Brisbane, Australia.
24Natasha Tasevska, MD, PhD, MSc formerly at the National Cancer Institute, National Institutes of Health.
25Lichen Yang, MD, PhD, is the nutrition team lead for CFCS and Director of Trace-Elements Division, National Center for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
26Ruilan Yang, MD, Department of Maternal and Child Health in Rural and Urban Centers in China.
27Qian Zhang, PhD, Chinese Center for Disease Control and Prevention, Beijing, China.
28Ning Wang, MSc, Chinese Center for Disease Control and Prevention, Beijing, China.
29Linhong Wang, MD, MS, is the Director of the National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
30Wang Yu, MD, PhD, Director General (former), Chinese Center for Disease Control and Prevention, Beijing, China.
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30