Abstract
The melanocortin receptor system consists of five closely related G-protein coupled receptors (MC1R, MC2R, MC3R, MC4R and MC5R). These receptors are involved in many of the key biological functions for multicellular animals, including human beings. The natural agonist ligands for these receptors are derived by processing of a primordial animal gene product, proopiomelanocortin (POMC). The ligand for the MC2R is ACTH (Adrenal Corticotropic Hormone), a larger processed peptide from POMC. The natural ligands for the other 4 melanocortin receptors are smaller peptides including α-melanocyte stimulating hormone (α-MSH) and related peptides from POMC (β-MSH and γ-MSH). They all contain the sequence His-Phe-Arg-Trp that is conserved throughout evolution. Thus, there has been considerable difficulty in developing highly selective ligands for the MC1R, MC3R, MC4R and MC5R. In this brief review, we discuss the various approaches that have been taken to design agonist and antagonist analogues and derivatives of the POMC peptides that are selective for the MC1R, MC3R, MC4R and MC5R receptors, via peptide, nonpeptide and peptidomimetic derivatives and analogues and their differential interactions with receptors that may help account for these selectivities.
Keywords: Melanocortin Receptors (MCRs: MC1R, MC2R, MC3R, MC4R, MC5R); α-MSH: α-melanocyte stimulate hormone; POMC: Proopiomelanocortin; ACTH: adrenal corticotropic hormone; GPCRs: G-protein coupled receptors; ASIP: agouti signaling protein; AGRP: agouti related protein; MTI: Ac-Ser-Tyr-Ser-Met-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2; MT-II: Ac-Nle4-c[Asp5,D-Phe7,Lys10]α-MSH(4-10)-NH2,(Ac-Nle-c[Asp-His-DPhe-Arg-Trp-Lys]-NH2). SHU9119: Ac-Nle4-c[Asp5,D-Nal(2′)7,Lys10]α-MSH(4-10)-NH2,(Ac-Nle-c[Asp-His- D-Nal(2′)-Arg-Trp-Lys]-NH2)
INTRODUCTION
The melanocortin receptor system consists of five 7-transmembrane G-protein coupled receptors generally referred to as MC1R, MC2R, MC3R, MC4R and MC5R. The melanocortin 1 receptor (MC1R) is found primarily in the periphery, especially in the skin; the MC2R in the adrenal gland: the MC3R in the brain and periphery; the MC4R in the brain and periphery; and the MC5R throughout the body [1]. They are involved in many critical functions including feeding behavior, energy homeostasis, response to stress, response to UV radiation, sexual function and behavior, pain response, fear flight, and many others [1]. In this regard they are involved in many of our serious degenerative diseases including obesity, and other feeding disorders; sexual dysfunction; cancer; skin disorders; diabetes; and many others [2–4]. Yet thus far, despite many years of effort, there are only a few medications on the market for this class of receptors and their natural ligands, the melanotropins. These included ACTH and MT-I. In this brief review/overview, we will discuss efforts to obtain ligands that might address some of the diseases associated with this class of receptors. Since the literature is vast in this area, we will have to concentrate on only a limited number of issues associated with these receptors and their ligands, concentrating on conformational and topographical considerations. Nonetheless, we hope that this review will provide the impetus for further studies in drug development in this area.
EMERGING BIOLOGICAL ROLES OF THE MELA-NOCORTIN SYSTEM
Historically, the primary functions of the melanocortin system were its involvement in pigmentation in what is now referred to as the melanocortin 1 receptor (MC1R), and it was also found to be present in skin cancer including melanoma cancer. The primary ligand for this receptor was thought to be α-melanocyte stimulating hormone (α-MSH), which is found in the pituitary gland and is a product of the proopiomelanocortin (POMC) gene. The other established hormone, which was a product of the POMC gene, was adrenal corticotropic hormone (ACTH). Its primary function was its involvement in the response to stress including fear-flight and adrenal function. In the early 1990s it became possible to clone the melanocortin receptors, and the field exploded when it was discovered that were additional genes and functions that could be attributed to the melanocortin system and that responded to the products of POMC including α-MSH. Within a few years the MC3R, MC4R and MC5R were cloned and sequenced and the examination of their biological functions were begun that continue to this day [5–9]. Antibodies and other biological tools were utilized to examine the distribution of these receptors in various tissues and cells in animals including humans [10]. At the same time numerous studies were initiated to determine the function(s) of these receptors. Details will not be discussed here, but it was found that the melanocortin receptors were clearly involved in many of the key physiological functions necessary for animal survival and reproduction. Among others, these functions have included: 1) feeding behavior [11, 12]; 2) energy balance [13]; 3) sexual function and behavior [3, 14, 15]; 4) cardiovascular function [16, 17]; 5) kidney function [18, 19]; 6) immune response [20–23]; 7) pain [24, 25]; 8) sebaceous gland secretion [26, 27] and many others e.g., [28–30] Indeed, new functions are still being discovered at this time [22]. Not surprisingly, therefore, these receptors and their natural hormone and neurotransmitter ligands are involved in many of the primary degenerative diseases in humans, including cancer, heart disease, kidney disease, diabetes, etc. Interestingly, despite considerable effort, at the time the receptors were being cloned though there were stable α-MSH analogues such as [Nle4, D-Phe7]-α-MSH (NDP-α-MSH) [31], but no potent antagonist analogues of α-MSH and ACTH so that it could be proven unequivocally what the biological receptors were for particular biological functions.
GENERAL FEATURES OF THE MELANOTROPIN PEPTIDES
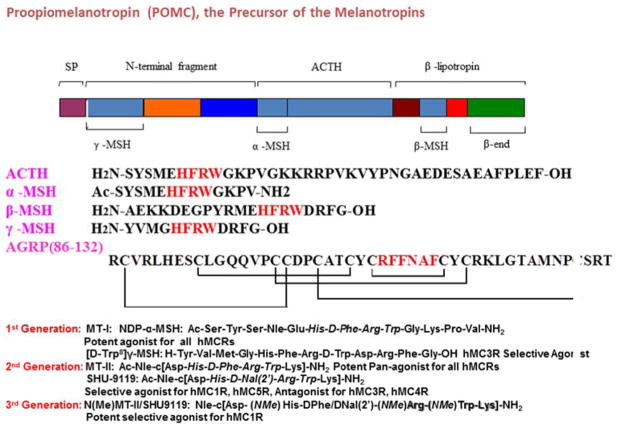
The melanocortin receptors (MCRs) belong to the family of seven transmembrane (TM) spanning G-protein coupled receptors (GPCRs) that stimulate the cAMP signal transduction pathway. The endogenous melanotropin agonists peptides, α, β, and γ-melanocyte stimulating hormones (MSH) and adrenocorticotropic hormone (ACTH) are derived by the posttranslational processing of the proopiomelanocortin (POMC) gene transcript (Fig. 1) [1, 32]. All MSH ligands contain a His-Phe-Arg-Trp core sequence (Fig. 1), which is important for melanocortin receptor stimulation [33]. The melanocortin receptor system is unique among GPCRs in terms of having both naturally occurring agonists and antagonists. The melanocortin antagonists, agouti-related protein (AGRP) and agouti signaling protein (ASIP), are the only two endogenous antagonists of GPCRs identified to date [34–36].
Fig. 1.
The Melanocortin System and 3 generations of melanotropin ligands.
DESIGN OF BIOAVAILABLE MELANOTROPIN PEPTIDES AND PEPTIDOMIMETICS
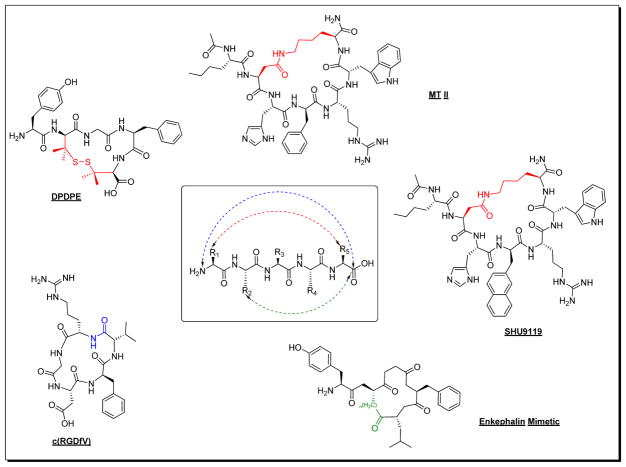
The melanocortin system [1] remains a challenging target for rational peptide and peptidomimetic design as the 3D-topographical requirements for specific melanocortin receptor subtype recognition have not been fully elucidated. Nevertheless, the numerous multifaceted physiological functions of the five known subtypes of human melanocortin receptors (hMCRs), continue to provide a strong stimulus for further development of potent and selective melanocortin agonists and antagonists. On the other hand, development of selective ligands for the melanocortin system bears intrinsic challenges due to conserved amino acid sequences and their structural similarity in the 7 transmembrane GPCR fold [37] and the limited structural variations of the endogenous melanotropin ligands. Unlike other protein targets, hMCRs, have separate natural agonist and antagonist molecules. This imposes a second dimension of design of melanocortin receptor ligands for achieving selectivity not only for receptor subtype but also for the agonists and antagonist properties. Designing such molecules that possess both functional selectivity and hMCR subtype selectivity from the melanotropin core sequence His-Phe-Arg-Trp has been difficult, but great progress has been made in recent years as outlined in Fig. (1). Nevertheless, melanotropin peptide drug discovery still faces several substantial challenges, including metabolic instability of peptides owing to rapid degradation by endogenous proteolytic enzymes and other pharmacokinetic properties of peptides that have required sophisticated drug delivery technologies. Consequently, it is generally necessary to employ strategies to develop modified peptides and peptidomimetics [31, 38–41] (Fig. 2). For example, modifications of amide bonds (e.g., N-alkylation or replacement with non-hydrolyzable surrogates [42, 43]; D-amino acid scan/unnatural amino acid substitutions in linear α-, β- and γ-melanocortin stimulating hormone (MSH)-derived sequences [44]; hybridization of the native MSH sequences with each other and with sequences of other bioactive peptides [45]; implementation of various global and local conformational constraints via peptide cyclization and employment of constrained amino acids [46, 47]; manipulation of steric factors that influence receptor-ligand interaction [48, 49]; construction of small molecules based on β-turn peptidomimetics and “privileged structure” scaffolds [50], and multiple N-Methylation [51]. These strategies have successfully enhanced the metabolic stability of peptide analogues [51]. Successfully developed peptide drugs exemplify this, and many also demonstrate the significance of drug delivery technologies in achieving desirable in vivo efficacy through non-oral routes of administration. Maintaining stability in vivo and oral availability have also been a challenge, and several strategies have been developed in order to achieve this, such as application of natural cycloid template and conjugation (e.g., to polyethylene glycol, antibodies or other synthetic/recombinant proteins, and to serum albumin) as well as sustained release formulations applicable to parental routes of administration. This review will focus on the 3-D structural properties of peptides and the chemical biology strategies to investigate peptide secondary structures (i.e., β–turns, γ–turns and 310-helices) as key pharmacophore elements for peptide molecular recognition at their targets.
Fig. 2.
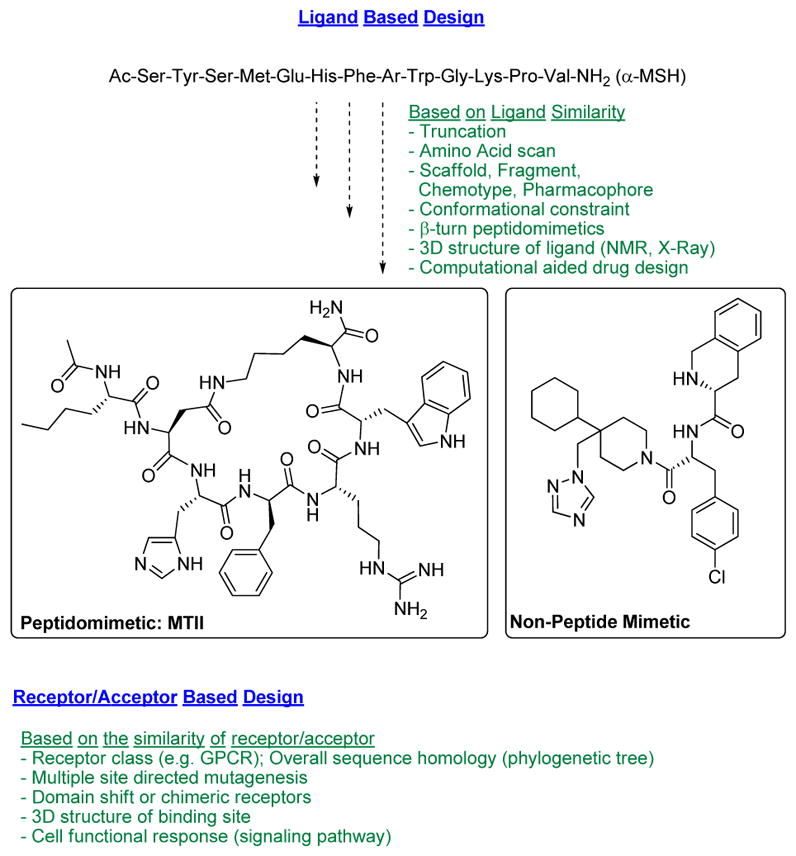
Design of biologically active peptides and peptidomimetics.
Peptide Conformation and Drug Design
Many peptides are conformationally flexible in aqueous solution, but upon interacting with their biologically relevant molecule they assume a preferred conformation. Thus the reduction of conformational freedom can lead to insights regarding the receptor-bound conformation. The reduction of conformational freedom may eventually lead to the receptor-bound conformation, which results in the selective interaction of a ligand with a receptor.
Peptide conformation has been structurally exploited in varying ways, including amino acid substitutions, amide bond replacements, scaffold modification (e.g., natural peptide analog to first-generation peptidomimetic), macrocyclization, secondary structure mimicry, and non-peptidic templates (e.g., designed small-molecule) [52]. Such drug design and synthetic chemistry efforts have expanded our understanding of β–turns, γ–turns, β–strands, α–helices and 310-helices in terms of both their 3D structural properties and molecular recognition at their targets. Finally, a multidisciplinary understanding of peptide conformation and drug design is essential to overcome the challenges traditionally associated with peptide drugs and enable the creation of next-generation agents.
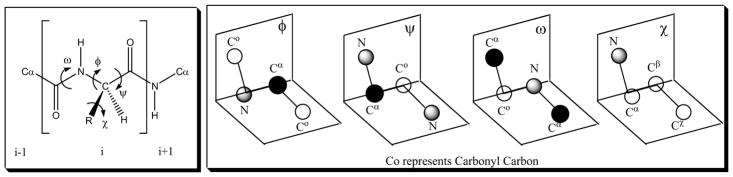
Conformationally constrained peptides can provide crucial information about biologically active conformations. A major goal of using conformational constraints is to determine which peptide conformation is required for binding to the receptor in an agonist or antagonist state. The backbone and side chain conformational features of a peptide can be defined by the torsional angles φ, ψ, ω and χ (Fig. 2). A Ramachandran plot ( ψ versus φ) may be used to analyze the preferred combinations of torsion angles for ordered secondary structures (i.e., conformations of peptides, such as α–helix, β-turn, γ-turn, or β-sheet). With respect to the amide bond torsion angle (ω), the trans geometry is more energetically-favored for most natural dipeptide substructures; however, when the C-terminal partner is Pro or another N-alkylated amino acid, the cis geometry is probable and may also contribute to β-turn or γ-turn stabilization. Molecular flexibility is directly related to covalent and/or noncovalent bonding interactions within a particular peptide. In this regard, a single replacement of hydrogen by a methyl moiety within amino acids (i.e., Nα-methyl, Cα-methyl or Cβ-methyl) may have significant consequences on the local conformational properties of a peptide. The peptide including the preferred conformation in Chi space (χ) (Fig. 3) [31] and/or Cα–Cβ scaffold may also be transformed to create novel amino acid building blocks such as β-methyl amino acids. Furthermore, the Cβ-carbon may be substituted to create “chimeric” amino acids.
Fig. 3.
Definitions of peptide backbone (φ, ψ, ω) conformations and peptide side chain torsional angle χ1, χ2.
Conformational constraint of flexible bioactive peptides can significantly improve potency, selectivity, stability, and bioavailability compared with endogenous peptides. The determination of a biologically active conformation of peptide is a difficult process. General strategies have been developed and tested in many laboratories [52].
Modification of the Peptide Side Chain Conformation
At the time the receptors were being cloned there were virtually no potent selective agonist or antagonist ligands for the melanocortin receptors, even for the MC1R and the MC2R. However, it was shown that α-MSH did not interact with the MC2R, but that ACTH did interact with the other melanocortin receptors. An important breakthrough was the discovery in our laboratory that replacement of the D-Phe7 residue with D-Nal(2′) but NOT D-Nal(1′) in MT-II: Ac-Nle4-c[Asp5, D-Phe7, Lys10]α-MSH(4-10)-NH2, (Ac-Nle-c[Asp-His-DPhe-Arg-Trp-Lys]-NH2) [53] led to an analogue of α-MSH, SHU9119: Ac-Nle4-c[Asp5, D-Nal(2′)7, Lys10]α-MSH(4-10)-NH2, (Ac-Nle-c[Asp-His-D-Nal(2′)-Arg-Trp-Lys]-NH2) that was a nanomolar binding antagonist at the MC3R and MC4R, but an agonist at the MC1R and MC5R [54]. This important discovery in combination with biophysical studies led to the realization that topographical differences in the structures of melanotropin analogues and derivatives are critical for parsing out different receptor selectivities for both agonists and antagonists at the MCRs, though all have essentially the same pharmacophore, -His-Phe-Arg-Trp-, for agonist activity [55]. Among many other discoveries related to biological function, the discovery that MC3R and/or MC4R are involved in feeding behavior [12, 56] and erectile function [14, 57] were demonstrated using the SHU-9119: Ac-Nle4-c[Asp5, D-Nal (2′)7, Lys10]α-MSH(4-10)-NH2, (Ac-Nle-c[Asp-His-DNal(2′)-Arg-Trp-Lys]-NH2) antagonist analogue.
Several amino acid substitution studies have been carried out on MT-II and SHU9119 to modify the structure [58, 59]. Replacement of His6 in SHU9119 with conformationally constrained amino acids resulted in selective agonists for hMC1R and hMC5R, and antagonists for hMC3R and hMC4R [60, 61]. Introduction of conformational constrained β-methyltryptophan into MTII, results in differential potencies, dissociation rates, and prolonged activities at the melanocortin receptors. MTII analogs modified at the Trp9 position with β-methyltryptophan have been evaluated using the classical frog and lizard skin bioassays, and at the cloned human MC1R receptors [62]. The 2S,3S analog did not have the highest affinity and potency out of the four MTII derivatives at the cloned human MC1R, but exhibited the slowest dissociation rate from the MC1R (25% slower than MTII). The 2S,3S-MTII analog presumably has a topographical conformation that allows for “enhanced” binding to the MC1R than the other three analogs, and thus this receptor–ligand interactions results in slower dissociation of the ligand from the receptor. Alternatively the ligand-receptor complex may lead to a conformation that enhances prolonged activity. The above studies indicate that topographical constraints, such as β-methyl amino acids, can provide a useful tool to determine the preferred side chain orientation, and this data may be used to design of ligands with potent and selective activity. Data from NMR studies suggest that the preferred side chain populations of the Trp9 residue modifications of MTII were gauche (-) in the 2S,3S, trans in the 2S,3R, and gauche(+) in the 2R,3R and 2R,3S analogs [63]. This led to the understanding that the conformational restriction at His6 and around DNal(2′)7 may help in receptor selectivities. Among the other studies carried out, it also has been shown that the activity of melanocortin ligands is sensitive to the side chain χ conformation [41, 52] (Fig. 3).
The availability of selective antagonist such as SHU-9119 and the selective MC1R antagonist was essential for demonstrating that the melanocortin system also was involved in pain, and that pain perception for females and males involve different biological mechanisms involving the melanocortin system [24, 25].
Modification of the Peptide Backbone
Another strategy in the design of peptide drugs is peptide backbone modifications that generally refers to isosteric or isoelectronic exchange of NHCO units in the peptide backbone or introduction of additional groups. Some of the most frequent modifications to the peptide backbone are shown in Fig. (4).
Modification of the peptide backbone can also serve to introduce local backbone constraints. For example, N-alkylation restricts the ϕ torsional angle, but eliminates the hydrogen bonding capability of the amide bond (e.g., 51). N-Methyl amino acids have been incorporated into bioactive derivatives of melanotropins, opioid peptides, bradykinin, thyrotropin releasing hormone (THR), angiotensin II, and cholecystokinin (CCK), and many other bioactive peptides. α-Methyl (and α-alkyl or –aryl) substituted amino acids often can induce or stabilize particular turn and helical structures (Fig. 4).
Fig. 4.
Frequent modifications employed to the peptide backbone.
Peptide Secondary Structure Mimetics
An understanding of 3-D structural and conformational properties of peptides that correlates with their binding and bioactivity at therapeutic targets has inspired creativity in peptide drug design. For example, many of the native peptides for GPCRs have a turn moiety as a part of their pharmacophore structure. In this regard, chemistry approaches aimed at creating versatile synthetic templates to mimic β-turns, γ–turns and a 310-helix secondary structures have been of great interests. Historically, substitution of D-amino acids, Nα-Me-amino acids, Cα-Me-amino acids, dehydro-amino acids and cyclic amino acids within a peptide lead compound have been fruitful (Fig. 5). Among the early approaches to enforce β– and γ–turns was the conversion of linear to cyclic peptides that employed side chain to side chain or other constraints [64]. In the melanocortin system, application of several of these strategies has resulted in a successful drug candidates such as MTII: Ac-Nle4-c[Asp5, D-Phe7, Lys10]α-MSH(4-10)-NH2, a universal agonist for all the hMCRs [54]; SHU9119: Ac-Nle4-c[Asp5, D-Nal (2′)7, Lys10]α-MSH(4-10)-NH2, a potent agonist for the hMC1R and hMC5R and a potent antagonist for the hMC3R and hMC4R [54]. The two ligands, although lacking exclusive receptor subtype selectivity, have been extensively used in understanding the biological properties of hMCRs.
Fig. 5.
Examples of converting biologically active linear peptides to potent cyclic peptides.
3D Peptide Conformation
In the melanocortin system, the primary sequence of the endogenous agonists (MSHs) and antagonists (AGRP, ASIP) are different, and they interact differently with the melanocortin receptors to produce the active and inactive conformations of the ligand-receptor complex. A critical approach is to understand receptor structure is to evaluate its conformation in three-dimensional (3D) space. X-ray crystal structures provide 3D conformation, but may be misleading in terms of function. Incorporation of these X-ray co-ordinates in to a computer-aided examination of function in 3D space is being pursued. This allows one to further explore the region/site or the surrounding 3D space occupied by the key amino acids of the protein (where the potent ligand has an affinity) so as to better understand biological actions.
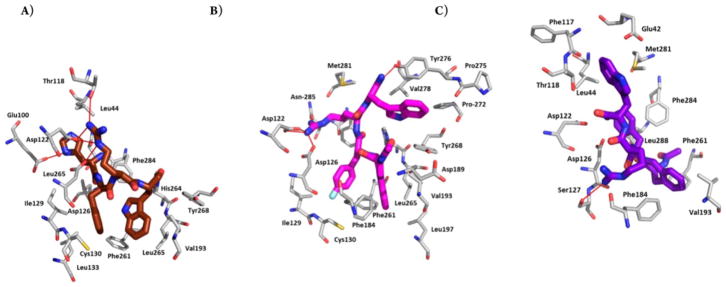
Peptide analogues which are sufficiently constrained, whose conformational and dynamic properties are known from multidimensional nuclear magnetic resonance (NMR) and other spectroscopic methods, in combination with modern molecular mechanics methods and docking experiments can greatly aid peptide ligand development. Exploring the accessible conformational space on the docking site of the receptor/acceptor can be served as the starting point for further design of analogues to develop conformation-biological activity relationships. Such molecular modeling studies provide a useful tool toward the generation of potent peptide or peptidomimetic ligand structures. Fig. (6) shows the molecular docking result of our novel designed local constrained tetrapeptides: Ac-Aia-pF-D-Phe-Arg-Trp-NH2, a selective hMC4R agonist, and Ac-Aba-D-Phe-Arg-Trp-NH2, an agonist of the hMC4R; antagonist of the hMC5R, interacted with the hMC4R receptor [65]. In these cases, homology modeling with the few known structures in these classes has been somewhat successful, but further developments are clearly needed.
Ligand-Receptor Interactions
Latest studies have demonstrated that the hMC3R plays a complementary role in weight control, α-MSH is equally potent at the MC3R and the MC4R, but γ-MSH has higher affinity at MC3R than MC4R. Agouti related protein (AGRP) is a potent antagonist at both MC3R and MC4R, but agouti is a potent antagonist only at the MC4R. These differences indicate that these two receptors, though members of the same system, are likely to possess functional and structural differences in response to ligands. Determination of the molecular basis of ligand binding and receptor signaling should, therefore, provide important insights into the mechanism of MC3R action. As is typical of GPCRs, the MC3R consists of the heptahelical transmembrane structure, with an extracellular NH2 terminus and intracellular COOH terminus. Modeling studies suggested that the hydrophobic pocket of the ligand binding sites are different between the MC3R and MC4R, whereas charge–charge interactions between the ligand and the receptor are similar in the two melanocortin receptors (MCRs). This prediction has been verified experimentally by Yang et al. [66, 67]. In addition to D154 and D158 in TM3 (corresponding to D122 and D126 in the MC4R), there are eight additional acidic residues that can potentially form a salt bridge with Arg in the pharmacophore (His-Phe-Arg-Trp) of the ligands. These include: E73, E80, E92, D121, E131, D178, E221, and D332 [68]. The results presented suggest that acidic residues in TMs 1 and 3 are important for ligand binding whereas those in TMs 2 and 7 are important for both binding and signaling [69].
MULTIVALENCY AND THE MELANOCORTIN SYSTEM
The realization that for many of our degenerative diseases there are multiple changes in the expressed genome and/or that treatment attempts lead to further changes in expressed genes leads one to consider the possibility of designing drugs that have 2 or more pharmacophores all within a single molecule [70]. For design there are several possible approaches including design of overlapping pharmacophores or adjacent pharmacophores or use of linkers to separate pharmacophores depending on whether the goal is to take advantage of proximity affects or to crosslink receptors/acceptors. In any case a primary consideration is that the two or more pharmacophores cannot interfere with the other(s), and vise versa, when each pharmacophore interacts with its receptor/acceptor. Peptides are ideal for this because they often have ancillary residues that are not critical for ligand-receptor/acceptor interaction.
In the case of the melanocortin system, POMC itself is a multivalent protein in that it produces via processing several biologically active peptides. There are 3 MSH related peptides, in its primary structure, plus ACTH, β-endorphin and other peptides that modulate many of the multiple functions needed for survival. To address multiple targets for the same purpose, we have designed two different kinds of multivalent ligands for different goals. In the first approach we designed homo- and hetero-bivalent ligands to crosslink membrane receptors in cancer cell lines that expressed melanocortin and cholecystokinin receptors [71] (Fig. 7). With appropriate spacing between pharmacophores we were able to demonstrate cross-linking of the different receptors. A most interesting and exciting observation in these studies was the observation that cross linking occurred very rapidly (within a few minutes) in cells that had both receptors, but very little binding occurred when only a single receptor was present. This kinetic advantage can be very useful in detecting cancer vs. non-cancer tissue. This kinetic advantage also should be applicable for in vivo imaging for detection of cancer following cancer treatments [72–75].
Fig. 7.

Example of a cross-linking novel designed homo multivalent MSH related ligand for receptor.
In a different application, ligands were designed to address the question of whether melanocortin and opioid receptors could have overlapping pharmacophores [76]. It was found that they could if carefully designed so as not to interfere with each other on receptor interaction.
SUMMARY AND CONCLUSIONS
At this time there are a couple of thousand references in the literature examining the biological functions of the melanocortin systems. In this short review we have only been able to discuss a relatively small number of such functions and of possible applications of melanocortin systems to drug design and development, and to the possible application of receptor selective or multiply selective ligands for the melanocortin receptor system. What we hope we have done in this short focused review is illustrate of the possible applications of carefully designed melanotropin ligands for treatment of disease. Our major conclusion is there is still much to learn, but it is evident that carefully designed ligands for melanocortin receptors can provide useful drugs for the treatment of our most prevalent degenerative diseases. For those interested in nonpeptide ligands for the melanocortin receptors, a few reviews are provided [77–79]. Our major conclusions are: 1) the melanocortin receptor system is of central importance for many of our major degenerative diseases; 2) properly designed, bioavailable ligands with unique biological activity profiles are needed to evaluate and develop drugs for these receptors in various diseases; 3) the use of novel conformational and topographical design features is of critical importance; 4) further insights into melanocortin receptor conformations and ligand receptor interactions are needed; 5) side chain and backbone conformations are critically important and their relationships to each other fundamental to further development of melanotropin ligands; and 6) the application of the multivalency concept to design of ligands for the melanocortin receptors may be essential to drug design in this area.
Fig. 6.
Best docking pose for (A) [Nle4, D-Phe7]-α-MSH (dark brown), (B) Aia peptide: Ac-Aia-pF-D-Phe-Arg-Trp-NH2 (magenta), and (C) Aba peptide: Ac-Aba-D-Phe-Arg-Trp-NH2 (purple) in hMC4R. Extracellular part of TMH4 (green helix) and -5 (yellow helix) are hidden for better visibility of the poses.
Acknowledgments
Supported in part by grants from the U.S. Public Health Service, NIH.
LIST OF ABBREVIATIONS
- Aba
α-aminobutyric acid
- MSH
melanocyte stimulating hormone
- ACTH
adrenal corticotropic hormone
- MT-I
melanotan-I, Ac-SerRyr-Ser-Nle-Asp-His-D-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2
- MT-II
melanotan-II, Ac-Nle-c[Asp-His-D-Phe-Arg-Trp-Lys]-NH2
- SHU-9119
Ac-Nle-c[Asp-His-D-Nal(2′)-Arg-Trp-Lys]-NH2
- POMC
proopiomelanocortin
- GPCR
G-protein coupled receptor
- AGRP
agouti-related protein
- MCR
melanocortin receptor
- D-Nal(2′)
D-2′-naphthylalanine
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
References
- 1.Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27(7):736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- 2.Roberts JL, Eberwine JH, Gee CE. Analysis of Pomc Gene-Expression by Transcription Assay and Insitu Hybridization Histochemistry. Cold Spring Harb Symp Quant Biol. 1983;48:385–391. doi: 10.1101/sqb.1983.048.01.042. [DOI] [PubMed] [Google Scholar]
- 3.Hadley ME. Discovery that a melanocortin regulates sexual functions in male and female humans. Peptides. 2005;26(10):1687–1689. doi: 10.1016/j.peptides.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 4.do Carmo JM, da Silva AA, Rushing JS, Pace B, Hall JE. Differential control of metabolic and cardiovascular functions by melanocortin-4 receptors in proopiomelanocortin neurons. Am J Physiol-Reg, I. 2013;305(4):R359–R368. doi: 10.1152/ajpregu.00518.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gantz I, Konda Y, Tashiro T, Shimoto Y, Miwa H, Munzert G, Watson S, Delvalle J, Yamada T. Molecular-Cloning of a Novel Melanocortin Receptor. J Biol Chem. 1993;268(11):8246–8250. [PubMed] [Google Scholar]
- 6.Chhajlani V, Muceniece R, Wikberg JES. Molecular-Cloning of a Novel Human Melanocortin Receptor. Biochem Biophys Res Commun. 1993;195(2):866–873. doi: 10.1006/bbrc.1993.2125. [DOI] [PubMed] [Google Scholar]
- 7.Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, Delvalle J, Yamada T. Molecular-Cloning, Expression, and Gene Localization of a 4th Melanocortin Receptor. J Biol Chem. 1993;268(20):15174–15179. [PubMed] [Google Scholar]
- 8.Klovins J, Haitina T, Ringholm A, Lowgren M, Fridmanis D, Slaidina M, Stier S, Schioth HB. Cloning of two melanocortin (MC) receptors in spiny dogfish - MC3 receptor in cartilaginous fish shows high affinity to ACTH-derived peptides while it has lower preference to gamma-MSH. Eur J Biochem. 2004;271(21):4320–4331. doi: 10.1111/j.1432-1033.2004.04374.x. [DOI] [PubMed] [Google Scholar]
- 9.Gantz I, Shimoto Y, Konda Y, Miwa H, Dickinson CJ, Yamada T. Molecular-Cloning, Expression, and Characterization of a 5th Melanocortin Receptor. Biochem Biophys Res Commun. 1994;200(3):1214–1220. doi: 10.1006/bbrc.1994.1580. [DOI] [PubMed] [Google Scholar]
- 10.Thornwall M, Dimitriou A, Xu XL, Larsson E, Chhajlani V. Immunohistochemical detection of the melanocortin 1 receptor in human testis, ovary and placenta using specific monoclonal antibody. Horm Res. 1997;48(5):215–218. doi: 10.1159/000185518. [DOI] [PubMed] [Google Scholar]
- 11.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of malanocortinergic neurons in feeding and the Agouti obesity syndrome. FASEB J. 1997;11(3):1011–1011. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 12.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385(6612):165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 13.Hagan MM, Rushing PA, Schwartz MW, Yagaloff KA, Burn P, Woods SC, Seeley RJ. Role of the CNS melanocortin system in the response to overfeeding. J Neurosci. 1999;19(6):2362–2367. doi: 10.1523/JNEUROSCI.19-06-02362.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wessells H, Gralnek D, Dorr R, Hruby VJ, Hadley ME, Levine N. Effect of an alpha-melanocyte stimulating hormone analog on penile erection and sexual desire in men with organic erectile dysfunction. Urology. 2000;56(4):641–646. doi: 10.1016/s0090-4295(00)00680-4. [DOI] [PubMed] [Google Scholar]
- 15.Wessells H, Levine N, Hadley ME, Dorr R, Hruby V. Melanocortin receptor agonists, penile erection, and sexual motivation: human studies with Melanotan II. Int J Impot Res. 2000;12:S74–S79. doi: 10.1038/sj.ijir.3900582. [DOI] [PubMed] [Google Scholar]
- 16.Ni XP, Pearce D, Butler AA, Cone RD, Humphreys MH. Genetic disruption of gamma-melanocyte-stimulating hormone signaling leads to salt-sensitive hypertension in the mouse. J Clin Invest. 2003;111(8):1251–1258. doi: 10.1172/JCI16993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ni XP, Butler AA, Cone RD, Humphreys MH. Central receptors mediating the cardiovascular actions of melanocyte stimulating hormones. J Hypertens. 2006;24(11):2239–2246. doi: 10.1097/01.hjh.0000249702.49854.fa. [DOI] [PubMed] [Google Scholar]
- 18.Ni XP, Kesterson R, Hruby VJ, Cone RD, Humphreys MH. Functional Evidence for Melanocortin-3 Receptors (Mc3-R) in Rat-Kidney. Hypertension. 1995;26(3):565–565. [Google Scholar]
- 19.Mayan H, Ling KT, Lee EY, Wiedemann E, Kalinyak JE, Humphreys MH. Dietary sodium intake modulates pituitary proopiomelanocortin mRNA abundance. Hypertension. 1996;28(2):244–249. doi: 10.1161/01.hyp.28.2.244. [DOI] [PubMed] [Google Scholar]
- 20.DeBold CR, Nicholson WE, Orth DN. Immunoreactive proopiomelanocortin (POMC) peptides and POMC-like messenger ribonucleic acid are present in many rat nonpituitary tissues. Endocrinology. 1988;122(6):2648–2657. doi: 10.1210/endo-122-6-2648. [DOI] [PubMed] [Google Scholar]
- 21.Lunec J, Pieron C, Sherbet GV, Thody AJ. Alpha-melanocyte-stimulating hormone immunoreactivity in melanoma cells. Pathobiology. 1990;58(4):193–197. doi: 10.1159/000163583. [DOI] [PubMed] [Google Scholar]
- 22.Tatro JB. Receptor biology of the melanocortins, a family of neuroimmunomodulatory peptides. Neuroimmunomodulation. 1996;3(5):259–284. doi: 10.1159/000097281. [DOI] [PubMed] [Google Scholar]
- 23.Nagahama M, Funasaka Y, Fernandez-Frez ML, Ohashi A, Chakraborty AK, Ueda M, Ichihashi M. Immunoreactivity of alpha-melanocyte-stimulating hormone, adrenocorticotrophic hormone and beta-endorphin in cutaneous malignant melanoma and benign melanocytic naevi. Brit J Dermatol. 1998;138(6):981–985. doi: 10.1046/j.1365-2133.1998.02263.x. [DOI] [PubMed] [Google Scholar]
- 24.Juni A, Cai M, Stankova M, Waxman AR, Arout C, Klein G, Dahan A, Hruby VJ, Mogil JS, Kest B. Sex-specific Mediation of Opioid-induced Hyperalgesia by the Melanocortin-1 Receptor. Anesthesiology. 2010;112(1):181–188. doi: 10.1097/ALN.0b013e3181c53849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mogil JS, Wilson SG, Chesler EJ, Rankin AL, Nemmani KVS, Lariviere WR, Groce MK, Wallace MR, Kaplan L, Staud R, et al. The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proc Natl Acad Sci U S A. 2003;100(8):4867–4872. doi: 10.1073/pnas.0730053100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thiboutot D, Sivarajah A, Gilliland K, Cong ZY, Clawson G. The melanocortin 5 receptor is expressed in human sebaceous glands and rat preputial cells. J Invest Dermatol. 2000;115(4):614–619. doi: 10.1046/j.1523-1747.2000.00094.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen WB, Kelly MA, OpitzAraya X, Thomas RE, Low MJ, Cone RD. Exocrine gland dysfunction in MC5-R-deficient mice: Evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell. 1997;91(6):789–798. doi: 10.1016/s0092-8674(00)80467-5. [DOI] [PubMed] [Google Scholar]
- 28.Newton J, Cai M, Xu H, Reed K. Melanocortin binding activity in term and preterm human placenta. Am J Obstet Gynecol. 2012;206(1):S104–S104. [Google Scholar]
- 29.Andersen M, Meyer MK, Nagaev I, Nagaeva O, Wikberg JES, Mincheva-Nilsson L, Andersen GN. TNF-Alpha Inhibitors Normalizes Melanocortin Receptor Subtype 2, 3 and 4 Expression in CD8+, CD14+and CD19+Leukocyte Subsets in Rheumatoid Arthritis. Arthritis Rheumatol. 2014;66:S666–S667. [Google Scholar]
- 30.Montero-Melendez T, Madeira MFM, Norling LV, Alsam A, Curtis MA, da Silva TA, Perretti M. Association between Periodontal Disease and Inflammatory Arthritis Reveals Modulatory Functions by Melanocortin Receptor Type 3. Am J Pathol. 2014;184(8):2333–2341. doi: 10.1016/j.ajpath.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sawyer TK, Sanfilippo PJ, Hruby VJ, Engel MH, Heward CB, Burnett JB, Hadley ME. 4-Norleucine, 7-D-Phenylalanine-Alpha-Melanocyte-Stimulating Hormone - a Highly Potent Alpha-Melanotropin with Ultralong Biological-Activity. Proc Natl Acad Sci Biol. 1980;77(10):5754–5758. doi: 10.1073/pnas.77.10.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cone RD. The central melanocortin system and its role in energy homeostasis. Annales d’endocrinologie. 1999;60(1):3–9. [PubMed] [Google Scholar]
- 33.Hruby VJ, Wilkes BC, Hadley ME, Alobeidi F, Sawyer TK, Staples DJ, Devaux AE, Dym O, Castruccin AMD, Hintz MF, et al. Alpha-Melanotropin - the Minimal Active Sequence in the Frog-Skin Bioassay. J Med Chem. 1987;30(11):2126–2130. doi: 10.1021/jm00394a033. [DOI] [PubMed] [Google Scholar]
- 34.Nijenhuis WAJ, Oosterom J, Adan RAH. AgRP(83–132) acts as an inverse agonist on the human-melanocortin-4 receptor. Mol Endocrinol. 2001;15(1):164–171. doi: 10.1210/mend.15.1.0578. [DOI] [PubMed] [Google Scholar]
- 35.McNulty JC, Jackson PJ, Thompson DA, Chai BX, Gantz I, Barsh GS, Dawson PE, Millhauser GL. Structures of the agouti signaling protein. J Mol Biol. 2005;346(4):1059–1070. doi: 10.1016/j.jmb.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 36.Yang YK, Dickinson C, Lai YM, Li JY, Gantz I. Functional properties of an agouti signaling protein variant and characteristics of its cognate radioligand. Am J Physiol Reg I. 2001;281(6):R1877–R1886. doi: 10.1152/ajpregu.2001.281.6.R1877. [DOI] [PubMed] [Google Scholar]
- 37.Barrett P, Macdonald A, Helliwell R, Davidson G, Morgan P. Cloning and Expression of a New Member of the Melanocyte-Stimulating Hormone-Receptor Family. J Mol Endocrinol. 1994;12(2):203–213. doi: 10.1677/jme.0.0120203. [DOI] [PubMed] [Google Scholar]
- 38.Zhang JY, Xiong CY, Hruby VJ. Stereoselective synthesis of 4,8-disubstituted azabicyclo[4.3.0]nonane amino acid esters: Dipeptide beta-turn mimetics for melanocortin peptide receptors. Pept Revolution. 2004;2004:200–201. [Google Scholar]
- 39.Zhang JY, Xiong CY, Ying JF, Wang W, Hruby VJ. Stereoselective synthesis of novel dipeptide beta-turn mimetics targeting melanocortin peptide receptors. Org Lett. 2003;5(17):3115–3118. doi: 10.1021/ol0351347. [DOI] [PubMed] [Google Scholar]
- 40.Bondebjerg J, Xiang ZM, Bauzo RM, Haskell-Luevano C, Meldal M. A solid-phase approach to mouse melanocortin receptor agonists derived from a novel thioether cyclized peptidomimetic scaffold. J Am Chem Soc. 2002;124(37):11046–11055. doi: 10.1021/ja0123913. [DOI] [PubMed] [Google Scholar]
- 41.Hruby VJ, Li GG, HaskellLuevano C, Shenderovich M. Design of peptides, proteins, and peptidomimetics in chi space. Biopolymers. 1997;43(3):219–266. doi: 10.1002/(SICI)1097-0282(1997)43:3<219::AID-BIP3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 42.Foret J, de Courcy B, Gresh N, Piquemal JP, Salmon L. Synthesis and evaluation of non-hydrolyzable D-mannose 6-phosphate surrogates reveal 6-deoxy-6-dicarboxymethyl-D-mannose as a new strong inhibitor of phosphomannose isomerases. Bioorgan Med Chem. 2009;17(20):7100–7107. doi: 10.1016/j.bmc.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 43.Spatola AF, Darlak K. Amide Bond Surrogates - Pseudopeptides and Macrocycles. Tetrahedron. 1988;44(3):821–833. [Google Scholar]
- 44.Grieco P, Balse PM, Weinberg D, MacNeil T, Hruby VJ. D-amino acid scan of gamma-melanocyte-stimulating hormone: Importance of Trp(8) on human MC3 receptor selectivity. J Med Chem. 2000;43(26):4998–5002. doi: 10.1021/jm000211e. [DOI] [PubMed] [Google Scholar]
- 45.Cai M, Stankova M, Cabello C, Decot B, Mayorov A, Trivedi D, Hruby VJ. Novel alpha-MSH/gamma-MSH hybrid analogues that lead to selective ligands for the human MC1R and human MC3R. Pept Revolution. 2004:651–652. [Google Scholar]
- 46.Mayorov AV, Cai M, Palmer ES, Tanaka DK, Cain JP, Dedek MM, Tan B, Trivedi D, Hruby VJ. Cyclic lactam hybrid alpha-MSH/Agouti-related protein (AGRP) analogues with nanomolar range binding affinities at the human melanocortin receptors. Bioorg Med Chem Lett. 2011;21(10):3099–3102. doi: 10.1016/j.bmcl.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayorov AV, Cai M, Chandler KB, Petrov RR, Van Scoy AR, Yu ZR, Tanaka DK, Trivedi D, Hruby VJ. Development of cyclic gamma-MSH analogues with selective hMC3R agonist and hMC3R/hMC5R antagonist activities. J Med Chem. 2006;49(6):1946–1952. doi: 10.1021/jm0510326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schreyer A, Blundell T. CREDO: A Protein-Ligand Interaction Database for Drug Discovery. Chem Biol Drug Des. 2009;73(2):157–167. doi: 10.1111/j.1747-0285.2008.00762.x. [DOI] [PubMed] [Google Scholar]
- 49.Baltz RH, Miao V, Wrigley SK. Natural products to drugs: daptomycin and related lipopeptide antibiotics. Nat Prod Rep. 2005;22(6):717–741. doi: 10.1039/b416648p. [DOI] [PubMed] [Google Scholar]
- 50.Driggers EM, Hale SP, Lee J, Terrett NK. The exploration of macrocycles for drug discovery - an underexploited structural class. Nat Rev Drug Discov. 2008;7(7):608–624. doi: 10.1038/nrd2590. [DOI] [PubMed] [Google Scholar]
- 51.Schafmeister CE, Po J, Verdine GL. An all-hydrocarbon cross-linking system for enhancing the helicity and metabolic stability of peptides. J Am Chem Soc. 2000;122(24):5891–5892. [Google Scholar]
- 52.Hruby VJ. Designing peptide receptor agonists and antagonists. Nat Rev Drug Discov. 2002;1(11):847–858. doi: 10.1038/nrd939. [DOI] [PubMed] [Google Scholar]
- 53.Alobeidi F, Castrucci AMD, Hadley ME, Hruby VJ. Potent and Prolonged Acting Cyclic Lactam Analogs of Alpha-Melanotropin - Design Based on Molecular-Dynamics. J Med Chem. 1989;32(12):2555–2561. doi: 10.1021/jm00132a010. [DOI] [PubMed] [Google Scholar]
- 54.Hruby VJ, Sharma SD. Designing peptide and protein ligands for biological receptors. Curr Opin Biotechnol. 1991;2(4):599–605. doi: 10.1016/0958-1669(91)90086-k. [DOI] [PubMed] [Google Scholar]
- 55.Hruby VJ, Wilkes BC, Hadley ME, Al-Obeidi F, Sawyer TK, Staples DJ, de Vaux AE, Dym O, Castrucci AM, Hintz MF, et al. alpha-Melanotropin: the minimal active sequence in the frog skin bioassay. J Med Chem. 1987;30(11):2126–2130. doi: 10.1021/jm00394a033. [DOI] [PubMed] [Google Scholar]
- 56.Chance WT, Sheriff S, Dayal R, Balasubramaniam A. Refractory hypothalamic alpha-MSH satiety and AGRP feeding systems in rats bearing MCA sarcomas. Peptides. 2003;24(12):1909–1919. doi: 10.1016/j.peptides.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 57.Wessells H, Fuciarelli K, Hansen J, Hadley ME, Hruby VJ, Dorr R, Levine N. Synthetic melanotropic peptide initiates erections in men with psychogenic erectile dysfunction: Double-blind, placebo controlled crossover study. J Urology. 1998;160(2):389–393. [PubMed] [Google Scholar]
- 58.Grieco P, Lavecchia A, Cai M, Trivedi D, Weinberg D, MacNeil T, Van der Ploeg LHT, Hruby VJ. Structure-activity studies of the melanocortin peptides: Discovery of potent and selective affinity antagonists for the hMC3 and hMC4 receptors. J Med Chem. 2002;45(24):5287–5294. doi: 10.1021/jm0202526. [DOI] [PubMed] [Google Scholar]
- 59.Grieco P, Balse-Srinivasan P, Han G, Weinberg D, MacNeil T, Van der Ploeg LHT, Hruby VJ. Extensive structure-activity studies of lactam derivatives of MT-II and SHU-(9119): their activity and selectivity at human melanocortin receptors 3, 4, and 5. J Pept Res. 2003;62(5):199–206. doi: 10.1034/j.1399-3011.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 60.Doedens L, Opperer F, Cai M, Beck JG, Dedek M, Palmer E, Hruby VJ, Kessler H. Multiple N-Methylation of MT-II Backbone Amide Bonds Leads to Melanocortin Receptor Subtype hMC1R Selectivity: Pharmacological and Conformational Studies. J Am Chem Soc. 2010;132(23):8115–8128. doi: 10.1021/ja101428m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grieco P, Han GX, Weinberg D, MacNeil T, Van der Ploeg LHT, Hruby VJ. Design and synthesis of highly potent and selective melanotropin analogues of SHU9119 modified at position 6. Biochem Biophys Res Commun. 2002;292(4):1075–1080. doi: 10.1006/bbrc.2002.6739. [DOI] [PubMed] [Google Scholar]
- 62.Haskell-Luevano C, Nikiforovich G, Sharma SD, Yang YK, Dickinson C, Hruby VJ, Gantz I. Biological and conformational examination of stereochemical modifications using the template melanotropin peptide, Ac-Nle-c[Asp-His-Phe-Arg-Trp-Ala-Lys]-NH2, on human melanocortin receptors. J Med Chem. 1997;40(11):1738–1748. doi: 10.1021/jm960845e. [DOI] [PubMed] [Google Scholar]
- 63.Ying JF, Kover KE, Gu XY, Han G, Trivedi D, Kavarana MJ, Hruby VJ. Solution structures of cyclic melanocortin agonists and antagonists by NMR. Biopolymers. 2003;71(6):696–716. doi: 10.1002/bip.10596. [DOI] [PubMed] [Google Scholar]
- 64.Hruby VJ. Conformational Restrictions of Biologically-Active Peptides Via Amino-Acid Side-Chain Groups. Life Sci. 1982;31(3):189–199. doi: 10.1016/0024-3205(82)90578-1. [DOI] [PubMed] [Google Scholar]
- 65.Van der Poorten O, Feher K, Buysse K, Feytens D, Zoi I, Schwartz SD, Martins JC, Tourwei D, Cai M, Hruby VJ, et al. Azepinone-Containing Tetrapeptide Analogues of Melanotropin Lead to Selective hMC4R Agonists and hMC5R Antagonist. ACS Med Chem Lett. 2015;6(2):192–197. doi: 10.1021/ml500436s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen M, Cai M, Aprahamian CJ, Georgeson KE, Hruby VJ, Harmon CM, Yang Y. Contribution of the conserved amino acids of the melanocortin-4 receptor in [corrected] [Nle4,D-Phe7]-alpha-melanocyte-stimulating [corrected] hormone binding and signaling. J Biol Chem. 2007;282(30):21712–21719. doi: 10.1074/jbc.M702285200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen M, Aprahamian CJ, Celik A, Georgeson KE, Garvey WT, Harmon CM, Yang Y. Molecular characterization of human melanocortin-3 receptor ligand-receptor interaction. Biochemistry-US. 2006;45(4):1128–1137. doi: 10.1021/bi0521792. [DOI] [PubMed] [Google Scholar]
- 68.Tao YX, Segaloff DL. Functional characterization of melanocortin-3 receptor variants identify a loss-of-function mutation involving an amino acid critical for G protein-coupled receptor activation. J Clin Endocrinol Metab. 2004;89(8):3936–3942. doi: 10.1210/jc.2004-0367. [DOI] [PubMed] [Google Scholar]
- 69.Wang SX, Fan ZC, Tao YX. Functions of acidic transmembrane residues in human melanocortin-3 receptor binding and activation. Biochem Pharmacol. 2008;76(4):520–530. doi: 10.1016/j.bcp.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hruby VJ, Agnes RS, Davis P, Ma SW, Lee YS, Vanderah TW, Lai J, Porreca F. Design of novel peptide ligands which have opioid agonist activity and CCK antagonist activity for the treatment of pain. Life Sci. 2003;73(6):699–704. doi: 10.1016/s0024-3205(03)00390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vagner J, Xu LP, Handl HL, Josan JS, Morse DL, Mash EA, Gillies RJ, Hruby VJ. Heterobivalent Ligands crosslink multiple cell-surface receptors: The human melanocortin-4 and delta-opioid receptors. Angew Chem Int Edit. 2008;47(9):1685–1688. doi: 10.1002/anie.200702770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cai M, Liu Z, Qu H, Fan H, Zheng Z, Hruby VJ. Utilize conjugated melanotropins for the earlier diagnosis and treatment of melanoma. Eur J Pharmacol. 2011;660(1):188–193. doi: 10.1016/j.ejphar.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brabez N, Lynch RM, Xu L, Gillies RJ, Chassaing G, Lavielle S, Hruby VJ. Design, Synthesis, and Biological Studies of Efficient Multivalent Melanotropin Ligands: Tools toward Melanoma Diagnosis and Treatment. J Med Chem. 2011;54(20):7375–7384. doi: 10.1021/jm2009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barkey NM, Tafreshi NK, Josan JS, De Silva CR, Sill KN, Hruby VJ, Gillies RJ, Morse DL, Vagner J. Development of Melanoma-Targeted Polymer Micelles by Conjugation of a Melanocortin 1 Receptor (MC1R) Specific Ligand. J Med Chem. 2011;54(23):8078–8084. doi: 10.1021/jm201226w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brabez N, Xu L, Gillies R, Chassaing G, Lavielle S, Hruby VJ. Design, synthesis and study of new multimeric ligands: Application as vectors for cancer diagnosis and therapy. Biopolymers. 2011;96(4):513–513. [Google Scholar]
- 76.Lee YS, Agnes RS, Cain JP, Kulkarni V, Cai MY, Salibay C, Ciano K, Petrov R, Mayorov A, Vagner J, et al. Opioid and melanocortin receptors: Do they have overlapping pharmacophores? Biopolymers. 2008;90(3):433–438. doi: 10.1002/bip.20814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen C, Jinghua Yu, Fleck BA, Hoare SRJ, Saunders J, Foster AC. Phenylguanidines as selective nonpeptide melano-cortin-5 receptor antagonists. J Med Chem. 2004;47:4083–4088. doi: 10.1021/jm0400496. [DOI] [PubMed] [Google Scholar]
- 78.Kang L, McIntyre KW, Gillooly Kathleen M, Yang Y, Haycock J, Roberts S, Khanna A, Herpin TF, Yu G, Wu X, Morton GC, Tuerdi H, Koplowitz B, Walker SB, Wardwell-Swanson J, Macor JE, Michael Lawrence R, Carlson KE. A selective small molecule agonist of the melanocortin-1 receptor inhibits lipopolysaccharide-induced cytokine accumulation and leukocyte infiltration in mice. J Leukocyte Biol. 2006;80:897–904. doi: 10.1189/jlb.1204748. [DOI] [PubMed] [Google Scholar]
- 79.Herpin TF, Yu G, Carlson KE, Morton GC, Wu X, Kang L, Tuerdi H, Khanna A, Tokarski JS, Lawrence ML, Macor JE. Discovery of tyrosine-based potent and selective melanocortin-1 receptor small-molecule agonists with anti-inflammatory properties. J Med Chem. 2003;46:1123–1126. doi: 10.1021/jm025600i. [DOI] [PubMed] [Google Scholar]