Abstract
Clinical studies have demonstrated that a single sub-anesthetic dose of the dissociative anesthetic ketamine induces rapid and sustained antidepressant actions in treatment-resistant patients. Although this finding has been met with enthusiasm, ketamine’s widespread use is limited by its abuse potential and dissociative properties. Recent preclinical research has focused on unraveling the molecular mechanisms underlying the unique antidepressant actions of ketamine in an effort to develop novel pharmacotherapies, which will mimic ketamine’s antidepressant actions but lack its undesirable effects. Here, we review hypotheses for the mechanism of action of ketamine as an antidepressant, including direct synaptic or extra-synaptic (GluN2B-selective) NMDAR inhibition, selective inhibition of NMDARs localized on GABAergic interneurons, and the role of α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor (AMPAR) activation. We also discuss links between ketamine’s antidepressant actions and downstream mechanisms regulating synaptic plasticity, including brain-derived neurotrophic factor (BDNF), eukaryotic elongation factor 2 (eEF2), mechanistic target of rapamycin (mTOR), and glycogen synthase kinase-3 (GSK-3). Mechanisms that do not involve direct inhibition of the NMDAR, including a role for ketamine’s (R)-ketamine enantiomer and hydroxynorketamine (HNK) metabolites, specifically (2R,6R)-HNK, are also discussed. Proposed mechanisms of ketamine’s action are not mutually exclusive and may act in a complementary fashion to exert the acute changes in synaptic plasticity, leading to sustained strengthening of excitatory synapses, which are necessary for antidepressant behavioral actions. Understanding the molecular mechanisms underpinning ketamine’s antidepressant actions will be invaluable for the identification of targets, which will drive the development of novel, effective, next-generation pharmacotherapies for the treatment of depression.
Introduction
Major depressive disorder (MDD) is a devastating mental disorder affecting approximately 16 percent of the world population, causing serious health and socio-economic consequences 1. Although interventions such as pharmacotherapies and cognitive behavioral psychotherapies are available, a high proportion of patients remain treatment-resistant 2. Moreover, even when effective, existing monoaminergic-based pharmacotherapies often take several weeks or months to exert their full therapeutic effects 3. Placebo-controlled trials have provided strong evidence for the rapid-acting (within hours) and sustained (lasting up to 7 days) antidepressant effects of a single administration of a sub-anesthetic dose of the non-competitive N-methyl-D-aspartate receptor (NMDAR) antagonist ketamine in treatment-resistant depressed patients 4–8. Moreover, antidepressant effects of ketamine have been demonstrated in many antidepressant-relevant tests in experimental animals (e.g. 9, 10–14); also see 15. However, ketamine’s routine clinical use for the treatment of depression is restricted due to its dissociative effects, changes in sensory perception, intravenous route of administration, as well as its abuse liability 16. These limitations have led investigators to explore the exact mechanisms of action underlying ketamine’s antidepressant clinical responses in an effort to understand its primary targets that will lead to the development of novel treatment interventions for depression. These treatments are intended to mimic the unique antidepressant actions of ketamine but lack its undesirable side effects.
The first clinical trial reporting antidepressant actions of ketamine was published in 2000, where ketamine was administered intravenously (40-min infusion) at the sub-anesthetic dose of 0.5 mg/kg 4. This contrasts with the typical dose of ketamine used in anesthesia of up to 2 mg/kg 17. A robust antidepressant effect of ketamine was achieved within four hours post-infusion compared with depressed subjects who received placebo 4. A subsequent double-blind randomized clinical trial demonstrated the efficacy of ketamine in treatment-resistant major depressed patients, who failed at least two conventional antidepressant treatments 5. The antidepressant effects of ketamine manifested within 2 hours post-infusion and 35% of patients maintained response for at least 7 days 5. Following these initial reports, several other clinical trials demonstrated rapid antidepressant actions of ketamine in treatment-refractory patients (e.g. 8, 18). Importantly, in an effort to address the functional un-blinding of treatment status (ketamine versus placebo) due to the acute dissociative effects of ketamine, Murrough et al., (2013) using a psychoactive placebo, midazolam, demonstrated a 64% response rate for the patients administered ketamine compared to 28% for those who received midazolam 19. In addition to the therapeutic effects of ketamine in major depressed patients, ketamine exerts antidepressant actions in patients suffering with bipolar depression, with a similar response rate 20, 21.
The actions of ketamine to induce rapid antidepressant effects are in sharp contrast with the delayed effect onset of currently approved antidepressant treatments, which is particularly important in cases of patients with suicidal ideation, where a lag in the onset of antidepressant action has been associated with increased risk for suicidal behavior 22. Ketamine has been also shown to induce a rapid amelioration of suicidal ideation in major depressed patients 23, 24 and to rapidly reduce anhedonia 25–27.
Here, we review hypotheses for the mechanism of action of ketamine as a rapid-acting antidepressant drug, including direct NMDAR inhibition (extra-synaptic NMDAR inhibition; inhibition of spontaneous NMDAR-mediated neurotransmission; inhibition of NMDAR-dependent burst firing of lateral habenula neurons), inhibition of GABAergic interneuron NMDARs (resultant pyramidal neuron disinhibition), and the role of the ketamine metabolite (2R,6R)-HNK. These pre-clinically demonstrated mechanisms of ketamine action are not mutually exclusive and may act in concert to exert the antidepressant actions of the drug.
NMDAR inhibition-mediated mechanisms
NMDARs are glutamatergic, ligand-gated, ion channel receptors which exist as heterotetramers. Seven different NMDAR subunits have been identified to date: GluN1, GluN2A, GluN2B, GluN2C, GluN2D, GluN3A and GluN3B 28. NMDARs typically contain two GluN1 subunits and either two GluN2 subunits or a mixture of GluN2/GluN3 subunits 28, 29. NMDAR activation requires concurrent binding of L-glutamate and glycine/D-serine at the GluN2 and GluN1 subunits respectively, as well as voltage-dependent repulsion of magnesium (Mg2+) block at the ion channel pore via membrane depolarization, resulting in calcium influx 28. Trullas and Skolnick, 1990, were the first to show that the NMDAR non-competitive channel blocker MK-801 and the competitive NMDAR inhibitor AP-7 decrease immobility time in the forced-swim test in mice, a measure of antidepressant efficacy 30. It was also reported that chronic, but not acute, administration of 17 different classical antidepressants in mice decreases radioligand binding to NMDARs, indicative of adaptive changes to the receptor 31, 32. Therefore, Skolnick et al. (1996) 33 hypothesized that direct NMDAR inhibition might represent a target for faster-acting antidepressant actions.
Inhibition of NMDARs expressed on GABAergic interneurons (Disinhibition hypothesis)
Although ketamine is expected to block excitatory glutamatergic neurotransmission via NMDAR inhibition, it was shown to increase overall activity in the prefrontal cortex in healthy volunteers 34, which was hypothesized to be due a preferential inhibition of NMDARs expressed on GABAergic interneurons 35–37. This preferential action of ketamine at inhibitory interneurons is supported by early findings showing that the NMDAR antagonist MK-801 initially inhibits firing of fast-spiking interneurons and subsequently increases firing of pyramidal neurons in freely-moving rats 36. This is postulated to be due to the higher frequency of interneuron firing compared with the pyramidal neurons 38, which allows for increased depolarization-dependent relief of Mg2+ block, thus permitting ketamine access to bind at the NMDAR channel pore selectively on interneurons 39. In addition, ketamine is reported to have higher affinity for GluN2D NMDAR subunits 40, 41, which are highly expressed in forebrain inhibitory interneurons 42, 43. Inhibition of NMDARs specifically on GABAergic interneurons is predicted to induce a decrease in overall inhibition, leading to pyramidal cell disinhibition and an enhancement of excitatory glutamatergic neurotransmission in the medial prefrontal cortex (mPFC), and potentially other mood-relevant cortico-limbic brain regions 35 (see Figure 1). In rats, ketamine administration at sub-anesthetic doses results in a significant increase in extracellular glutamate levels 35 and an increase in glutamate cycling 44 in the prefrontal cortex. Further supporting this hypothesis, administration of partial inverse agonists at the benzodiazepine binding site of alpha5-containing GABAA receptors, which are selectively expressed in the forebrain, including prefrontal cortex and hippocampus, promote coherent network activity via disinhibition of excitatory neurotransmission 45 and exert rapid antidepressant actions in several animal tests 46–48. Notably, ketamine 13, similar to negative allosteric modulators of alpha5-containing GABAA receptors 47, enhance gamma band electroencephalography power, which is hypothesized to be directly related to cortical disinhibition 49–52, further supporting the role of cortical disinhibition in the rapid antidepressant actions of these drugs.
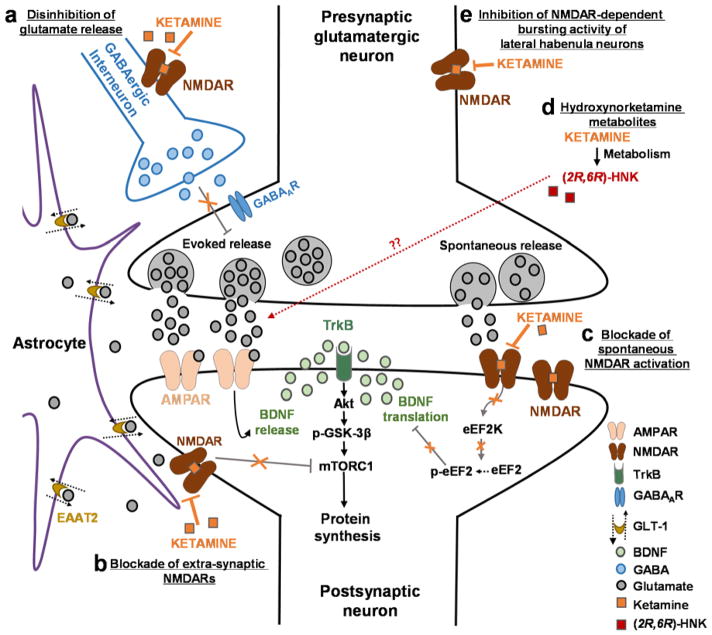
Figure 1. Proposed mechanisms of ketamine action as an antidepressant.
(A) Disinhibition hypothesis: Based on the disinhibition hypothesis, ketamine is proposed to selectively block N-methyl-D-aspartate receptors (NMDARs) expressed on GABAergic inhibitory interneurons, which leads to a disinhibition of pyramidal neurons and enhanced glutamatergic firing. Evoked released glutamate binds to and activates post-synaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) resulting in enhanced brain-derived neurotrophic factor (BDNF) release, activation of the tropomyosin receptor kinase B (TrkB) receptor and subsequently promotion of protein synthesis via the activation of the mechanistic target of rapamycin complex 1 (mTORC1). (B) Inhibition of extra-synaptic NMDARs: Ketamine is proposed to selectively block extra-synaptic GluN2B-containing NMDARs, which are tonically activated by low levels of ambient glutamate regulated by the glutamate transporter 1 located on astrocytes. Inhibition of the extra-synaptic GluN2B-NMDARs is hypothesized to de-suppress mTORC1 function, which in turn will induce protein synthesis. (C) Blockade of spontaneous NMDAR activation: This hypothesis proposes that ketamine blocks NMDAR-mediated spontaneous neurotransmission, which results in the inhibition of the eukaryotic elongation factor 2 kinase (eEF2K) activity, thus preventing phosphorylation of its eEF2 substrate. This effect subsequently leads to an enhancement of BDNF translation. (D) Ketamine hydroxynorketamine (HNK) metabolites: This hypothesis posits that ketamine exerts NMDAR inhibition-independent antidepressant actions via the action of its metabolites, (2R,6R)-HNK and (2S,6S)-HNK. Ketamine is metabolized to HNKs following administration, and these HNK metabolites act to promote AMPAR-mediated synaptic potentiation. These mechanisms of ketamine action are not mutually exclusive and may act complementary in exerting the antidepressant actions of the drug as all hypotheses propose acute changes in synaptic plasticity, leading to sustained strengthening of excitatory synapses, being necessary for antidepressant responses.
Abbreviations: EAAT2, excitatory amino acid transporter 2; GABA, gamma aminobutyric acid; GSK, glycogen synthase kinase
However, there is also evidence arguing against a primary role of suppression of the inhibitory GABAergic interneuron activity in ketamine’s action as an antidepressant. In particular, ketamine administration to mice with a global reduction of GABAA receptor function, reversed behavioral despair and novelty-induced hyper-anxiety and selectively potentiated GABAergic synaptic inhibition within the mPFC 53. Similarly, potentiation of GABAergic inhibitory input to pyramidal cells via a disinhibition of somatostatin-positive GABAergic interneurons induced sustained antidepressant-like effects in mice 54. Enhancement of inhibition with pharmacological activation of GABAA or GABAB receptors also resulted in an antidepressant effect in rats 55, 56. Moreover, pharmacological-induced disinhibition of synaptic neurotransmission via administration of the ionotropic GABAA receptor antagonist picrotoxin did not reduce behavioral despair in mice 10. In addition, mice lacking NMDAR (GluN1) in parvalbumin-expressing interneurons, designed to mimic disinhibition of pyramidal cell activity, retained ketamine-induced antidepressant activity 57.
Inhibition of spontaneous NMDAR-mediated transmission
Spontaneous synaptic vesicular glutamate release “at rest”, occurring via a spontaneous fusion of presynaptic vesicles of the pre-synaptic terminal 58, 59, results in miniature excitatory postsynaptic currents (mEPSCs) that have a role in regulating synaptic strength and protein synthesis 60–62. In particular, mEPSCs tonically suppress protein synthesis 62, whereas folimycin-induced selective depletion of spontaneously releasable vesicular pools induces synaptic potentiation in rat hippocampal slices 63. Ketamine and other NMDAR antagonists, including AP-5 and MK-801, were shown to block NMDAR-mediated neurotransmission at rest (NMDAR-mEPSCs), thus inducing a de-suppression of protein synthesis leading to synaptic potentiation in the CA1 region of the hippocampus and behavioral antidepressant actions 10, 63 (see Figure 1). Importantly, ketamine’s inhibition of NMDAR-mEPSCs occurs at physiological levels of Mg2+, an effect that was associated with the rapid antidepressant behavioral actions of ketamine 64. In contrast, memantine, a non-competitive NMDAR channel blocker, failed to exert antidepressant actions in animal tests 64 and in humans 65–67, and which was suggested to be because memantine does not block NMDAR-mEPSCs under physiological Mg2+ levels 64. Spontaneous NMDAR-mediated neurotransmission is hypothesized to contribute to ketamine’s antidepressant actions by enhancing synaptic neurotransmission through a protein synthesis-dependent mechanism involving eukaryotic elongation factor 2 kinase (eEF2K) and BDNF (see Figure 1), as described later 10.
Direct inhibition of extra-synaptic NMDARs
Both immunohistochemical and electrophysiological studies have confirmed the existence of extra-synaptic NMDARs, which are not located in the post-synaptic density 68, and are primarily comprised of GluN2B-containing heterotetramers 28, 29. The extra-synaptic GluN2B-containing NMDARs, and in particular those that are localized on dendrites adjacent to glial cells, are not activated by the typical transient synaptic glutamate release, but are chronically activated by low-levels of ambient glutamate within the extracellular space 69, 70. These tonic ambient glutamate levels are directly regulated by the glutamate transporter EAAT2 (GLT-1), which is expressed on glial cells (see Figure 1) 69, 70. Ketamine is hypothesized to specifically inhibit extra-synaptic GluN2B-NMDARs, thus preventing ambient glutamate-induced tonic activation of these receptors, an effect that is expected to induce an excitation of pyramidal neurons 71. Under basal conditions, activation of cortical extra-synaptic GluN2B-selective NMDARs acts through the mTOR signaling pathway to suppress protein synthesis, which maintains synaptic homeostasis 71–74; therefore, blockade of extra-synaptic GluN2B-containing NMDARs would de-suppress protein synthesis and induce antidepressant actions via an mTOR-dependent mechanism (see Figure 1), as described later.
In support of a role of GluN2B-NMDARs in ketamine’s antidepressant actions is the findings that ketamine administration does not further decrease behavioral despair in mice lacking GluN2B-specific NMDARs localized to pyramidal neurons 71, suggesting that ketamine might act via inhibition of GluN2B-specific NMDARs on pyramidal neurons to exert its antidepressant effects. However, developmental homeostatic effects in genetically modified mice cannot be ruled out. In fact, mice lacking GluN2B-specific NMDARs localized to pyramidal neurons are characterized by low baseline levels of behavioral despair 71, possibly precluding any further effects of ketamine on this outcome. Furthermore, it is unclear how ketamine, with no selectivity for GluN2B subunit inhibition, specifically acts at this site to induce its antidepressant actions. In fact, it has been reported that at physiological magnesium concentrations ketamine has greater selectivity for inhibiting GluN2C- and GluN2D-containing NMDARs compared with GluN2B- and GluN2A-containing receptors 40, 41.
Independent of the mechanism of ketamine action, GluN2B selective antagonists exert rapid antidepressant actions in rodent models 9, 75–78. Moreover, deletion of GluN2B-containing NMDARs from pyramidal cortical neurons in the brain of mice induced an enhancement of protein synthesis and increased the number of excitatory inputs measured in the prefrontal cortex, concomitant with decreased behavioral despair in the forced-swim test and tail-suspension test, and reduced corticosterone-induced behavioral deficits 71. The value of targeting GluN2B selectively is further supported by the finding that GluN2B-selective NMDAR blockers may exert antidepressant actions in humans; however, these antidepressant effects do not appear as rapidly as the effects of ketamine. In particular, intravenous administration of the GluN2B-NMDAR antagonist CP-101,606 (traxoprodil) did not induce a rapid antidepressant response at the first time point measured (2 days following treatment), but induced a significant antidepressant action 5 and 8 days following a single administration 79. Although this study provided evidence for a beneficial action of this drug, it had a small sample size (n=15 subjects/group). CP-101,606 is not currently in development for the treatment of depression and there have been no further studies confirming this initial finding. Moreover, there is a controversy regarding whether the effects of this compound are solely due to block of GluN2B-NMDARs, since it also possesses high affinity at sigma-1 receptors 80, 81, which have been suggested as a target for antidepressant actions 82–84. Another GluN2B-preferring NMDAR antagonist, MK-0657 (CERC-301), induced modest improvement in mood scores in depressed patients (Hamilton Depression Rating Scale, but not the Montgomery-Åsberg Depression Rating Scale), 5 days, but not 1–4 days, following a single infusion 85. A larger phase II clinical trial failed to identify significant antidepressant actions of MK-0657 (as reported in 86).
Inhibition of NMDAR-dependent bursting activity of lateral habenula neurons
The lateral habenula (LHb) is a highly conserved region of the epithalamus that acts as an intermediary between the forebrain, and midbrain monoaminergic systems 87, 88. Glutamatergic LHb neurons are transiently activated by aversive stimuli including acute stressors 89, 90 and exert a feedforward inhibitory influence on the activity of midbrain dopamine neurons by virtue of their connections with GABAergic cells in the rostromedial tegmental area 91–93. Activation of LHb neurons is also associated with depression-related phenotypes in animal models 94–96 and in patients with MDD 88, 97. It has been recently demonstrated that LHb neurons show enhanced burst activity in rats characterized by congenital helpless behavior 98. The same authors showed that direct application of ketamine to LHb slice preparations decreases abnormally high NMDAR-dependent burst firing 98. Importantly, in vivo, ketamine-induced reduction in bursting activity was associated with an acute antidepressant effect in congenially helpless rats measured in the forced-swim and sucrose preference tests 98. Although these findings are exciting and promising, the role of the LHb in regulating the antidepressant actions of ketamine was only assessed acutely (i.e, 1 h following drug infusion) and thus it is critical to investigate whether reducing NMDAR-dependent burst firing in the LHb can elicit long-lasting (e.g., 24 hours post-treatment) antidepressant behavioral actions, similar to the effects of ketamine when administered peripherally to rodents. Moreover, future studies should aim to determine whether different classes of putative rapid acting antidepressants also act via this mechanism and to determine whether these effects converge with the other known antidepressant-relevant actions of ketamine.
NMDAR inhibition-independent mechanisms
Following the finding that ketamine exerts rapid and sustained antidepressant actions in treatment-resistant depressed patients 4, 5, several human trials have been initiated to investigate the antidepressant potential of alternative NMDAR antagonists that, similar to ketamine, inhibit the NMDAR in a voltage-dependent manner. However, clinical trials indicate that these alternative NMDAR antagonists, lack the rapid, robust and/or long-lasting antidepressant actions of ketamine in humans 99. In particular, memantine, repeatedly failed to exert antidepressant actions in major depressed patients 65–67. In addition, a single intravenous administration of AZD6765 (i.e., lanicemine), a low-trapping non-selective NMADR channel blocker, exerted transient (~110 min) antidepressant responses in major depressed patients, which were not maintained 100. Although a follow-up study, where patients received 3 intravenous infusions of AZD6765 per week (total of 3 weeks), reported significant improvement in depressed mood and symptom remission at the end of treatment 101, this is in contrast with the sustained antidepressant actions of ketamine following a single infusion. Additionally, a four-country, 49 site placebo-controlled study comparing AZD6765 to placebo as an adjunctive treatment for depression in 302 patients, failed to show separation from placebo 102. This literature leads to the conclusion that while alternative NMDAR channel-blocking antagonists may exert clinical antidepressant actions, such actions are not of the same time frame or magnitude as those exerted by ketamine. Similar to these human data, animal studies show that the NMDAR channel-blocking antagonist MK-801 does not exert sustained antidepressant actions, though it does have acute actions in some studies 9, 10, 13, 103.
Another finding challenging the NMDAR inhibition hypothesis of ketamine’s antidepressant mechanism of action is the fact that partial agonists at the NMDAR glycineB binding site, including GLYX-13 (i.e., rapastinel) and D-cycloserine manifest antidepressant effects in clinical trials 104, 105 and in animal tests 106–109, without sharing ketamine’s NMDAR inhibition-mediated side effects 109. Furthermore, in vivo evidence shows that GLYX-13 is able to reduce ketamine-induced memory deficits in mice 110, which are NMDAR inhibition-mediated.
(R)-ketamine
Ketamine is an enantiomeric mixture of (R)-ketamine and (S)-ketamine. (S)-ketamine has ~4-fold greater affinity/potency at inhibiting the NMDAR compared to its (R)-ketamine enantiomer 13, 111–115. Hashimoto and colleagues were the first to report superior and longer-lasting antidepressant actions of (R)-ketamine compared with (S)-ketamine in rodent models 116–118. These findings were subsequently replicated by Zanos et al. (2016), who showed that (S)-ketamine’s antidepressant behavioral actions require higher doses compared to those of (R)-ketamine 13. The superiority of (R)-ketamine does not seem to be related to a U-shaped dose response of the drugs, as it has been shown superior to (S)-ketamine with up to a 30-fold range of doses in multiple mouse tests of antidepressant efficacy 13, 118. Importantly, administration of equal, antidepressant-relevant, doses of (R)- and (S)-ketamine in mice did not yield different levels of these enantiomers in the brain of mice 13, indicating that the antidepressant superiority of (R)-ketamine in rodent models is not due to greater brain exposure. These data indicate that it is unlikely that ketamine exerts its full antidepressant actions solely via inhibition of the NMDAR, at least in rodents. Nevertheless, we note that pre-clinical rodent studies have also indicated rapid-acting antidepressant behavioral actions of (S)-ketamine in mice 13, 116–118. In addition, in patients with depression, intravenous, 40-min infusion of (S)-ketamine (0.2 and 0.4 mg/kg) has been reported to exert antidepressant responses within 2 hours following administration, an effect that was sustained for at least 3 days, with some patients reporting beneficial effects for up to a period of two weeks following a single administration 18. In addition, intranasal administration of 28–84 mg (S)-ketamine twice a week for a total period of two weeks induced antidepressant actions in treatment-resistant depressed patients as an adjunct treatment 119. To date, there is no human clinical trial directly comparing the antidepressant efficacy of (S)- and (R)-ketamine enantiomers, or assessing antidepressant actions of (R)-ketamine in depressed patients.
(2S,6S;2R,6R)-hydroxynorketamine (HNK) metabolite
Following ketamine administration, (2S,6S;2R,6R)-HNK is the major HNK metabolite found in the plasma and brain of mice 13, as well as the plasma of humans 120. While maximal concentrations of (2S,6S;2R,6R)-HNK in the plasma of patients receiving ketamine are lower than ketamine levels (0.16 vs 0.78 μM, respectively), total exposure of (2S,6S;2R,6R)-HNK is higher than that of the parent drug (5.72 vs 4.36 μM) 120. In addition, (2S,6S;2R,6R)-HNK exposure is ~1.8-fold higher in humans compared to mice (121 and unpublished analyses) when given at antidepressant doses. These data indicate that there may be sufficient total exposure of (2S,6S;2R,6R)-HNK to exert biologically meaningful effects, but also suggest the possibility that other ketamine metabolites may be additive in humans to exert the full antidepressant actions of ketamine.
Early pharmacodynamic studies assessing the anesthetic properties of ketamine and its principle metabolite norketamine, and (2S,6S;2R,6R)-HNK demonstrated that ketamine and norketamine manifested anesthetic effects and induced hyper-locomotor activity during the post-anesthetic recovery period in rats, whereas (2S,6S;2R,6R)-HNK had no effect on these outcomes 122; for review see 123. (2S,6S;2R,6R)-HNK was thus described as an “inactive” metabolite in regards to anesthetic action.
There is evidence that metabolism of ketamine to (2S,6S;2R,6R)-HNK is necessary for its antidepressant action in rodent tests 13. This was shown by chemically altering ketamine via deuteration at the C6 position, which did not change its binding affinity for the NMDAR, but dramatically decreased its in vivo metabolism to (2S,6S;2R,6R)-HNK. This manipulation prevented ketamine’s antidepressant actions in mice 13, indicating that metabolism of ketamine to (2S,6S;2R,6R)-HNK is required for ketamine’s antidepressant responses. In addition, greater antidepressant behavioral responses of a single administration of ketamine have been observed in female compared to male rats and mice 13, 124, 125. In mice, this behavioral effect was associated with higher brain levels of (2S,6S;2R,6R)-HNK, but not ketamine or norketamine levels 13, further supporting a role of this metabolite in the antidepressant actions of ketamine.
Both the (2S,6S)- and/or (2R,6R)-HNK enantiomers are sufficient on their own to exert dose-dependent antidepressant actions in several rodent tests including the 1-hour 13 and 24-hour 13, 126 forced-swim test, learned helplessness paradigm 13, as well as reversal of social interaction deficits following chronic social defeat, and anhedonia deficits following chronic corticosterone administration 13. In accordance with the findings that (R)-ketamine is a more potent antidepressant compared to the (S)-ketamine 13, 116, 117, 127, the (2R,6R)-HNK metabolite, which is solely produced via the metabolism of (R)-ketamine, exerts more potent and longer-lasting antidepressant actions compared with the (2S,6S)-HNK enantiomer, which is produced via the metabolism of (S)-ketamine. Nevertheless, Yang et al. (2017) 128 failed to identify antidepressant-relevant actions of a single dose of (2R,6R)-HNK (10 mg/kg) following chronic social defeat stress in mice, indicating that further studies are required to establish the effective doses of this metabolite in different animal tests predictive of antidepressant efficacy. Indeed, a dose of 20 mg/kg was capable of reversing anhedonia following chronic social defeat stress in mice 13.
Important for the mechanism of action of (2R,6R)-HNK as an antidepressant (and thus ketamine’s action) is the fact that at relevant concentrations (i.e., 10 mg/kg or brain Cmax = ~10 μmol/kg in mice), (2R,6R)-HNK does not appear to inhibit the NMDAR. [3H]-MK-801 binding displacement studies showed that the affinity of (2R,6R)-HNK to displace MK-801 from the NMDAR is >100 μM, and that of (2S,6S)-HNK is 7–20 μM 111, 112. In addition, at 10 μM concentration, (2R,6R)-HNK does not functionally inhibit the NMDARs localized at stratum radiatum interneurons in hippocampal slices, compared to ~50% inhibition by ketamine at this concentration 13. Suzuki et al. (2017) 129 recently confirmed that (2R,6R)-HNK does not functionally inhibit NMDAR-mEPSCs at 10 μM 129. These authors also reported that at a higher concentration (50 μM), (2R,6R)-HNK induces a modest (~40%) inhibition of NMDAR-mEPSCs 129, which could result in off-target effects of this metabolite at high doses. However, (2R,6R)-HNK did not induce any NMDAR inhibition-mediated side effects in mice in the open-field test (locomotor activity; doses up to 125 mg/kg), the rota-rod test (motor incoordination; doses up to 125 mg/kg) and the pre-pulse inhibition test (sensory dissociation; doses up to 375 mg/kg) 13, 130, in contrast to the antidepressant dose of 10 mg/kg.
Downstream mechanisms involved in ketamine’s antidepressant actions
α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor (AMPAR)
α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptors (AMPARs) are ionotropic transmembrane glutamatergic receptors and the principal receptors responsible for the transduction of fast synaptic neurotransmission in the brain, and are targets for multiple signaling pathways that regulate synaptic plasticity 131. The disinhibition hypothesis of ketamine action proposes that an increase in synaptic glutamatergic neurotransmission causes an acute activation of the post-synaptic AMPARs 132. We note that ketamine-induced enhancement of synaptic excitatory neurotransmission would be predicted to not only activate post-synaptic AMPARs, but also NMDARs. Although synaptic NMDAR activation has not yet been reported/studied to underlie ketamine’s antidepressant actions, it is likely to contribute to the antidepressant effects of the drug. Indeed, activation of both AMPARs and NMDARs is required for synaptic potentiation and synaptic plasticity 133, which both are thought to be involved in the antidepressant actions of ketamine 134.
Quantitative EEG measurements in humans 101, as well as rats e.g. 135 and mice e.g. 13 revealed ketamine-induced increases in gamma-band power, which, in addition to a putative measure of cortical disinhibition, also indicates activation of fast ionotropic excitatory receptors, including AMPARs 136–138. Pre-treatment with a subthreshold dose of an AMPAR agonist (C×546) enhanced the antidepressant effects of ketamine in the forced-swim test in rats 139, indicating that AMPAR activation might be involved in ketamine’s antidepressant effects. Indeed, pre-treatment with the AMPAR antagonist NBQX prevents the antidepressant-like actions of ketamine in the forced-swim test 9, 13, 117, 139, 140, tail-suspension test 117, 141, novelty-suppressed feeding test 142, learned helplessness test 141 and stress-induced sucrose preference deficits 117, 143. Importantly, NBQX does not prevent the antidepressant actions of monoamine-acting antidepressant drugs 9, 144, highlighting AMPAR activation as a unique mechanism underlying ketamine’s antidepressant actions. Notably, as AMPAR activation typically leads to membrane depolarization and voltage-dependent release of NMDAR Mg2+ blockade 28, inhibition of AMPARs could also be mechanistically linked to preventing NMDAR activation, thus inactivation of both AMPARs and NMDARs could be responsible for the lack of ketamine’s antidepressant actions following administration of NBQX.
Ketamine administration also results in an upregulation of the membrane AMPAR subunits, GluA1 and GluA2, in the hippocampus three hours post-injection 63. AMPAR containing GluA1 and/or GluA2 subunit upregulation was also observed in mPFC 75 and hippocampal 13 synaptoneurosome fractions at 24 hours post-injection, indicating a rapid-triggered and sustained recruitment of AMPAR in the synapse, consistent with synaptic strengthening. In fact, low doses of ketamine were shown to induce an enhancement of AMPAR-mediated synaptic transmission in the mPFC 145 and hippocampus 146 of rats, as measured by AMPAR currents in pyramidal neurons and extracellular in vivo electrophysiological recordings in CA3 pyramidal neurons respectively. Additionally, application of ketamine to hippocampal slices (non-stimulated 10, 63 and stimulated 147) enhanced AMPAR-mediated synaptic potentiation in the CA1 region. The AMPAR subunit GluA2 was shown to be required for ketamine’s induction of synaptic potentiation, since ketamine did not induce AMPAR-mediated synaptic potentiation of Schaffer collateral-CA1 synapses in hippocampal slices of mice lacking the GluA2 gene 63. In addition, GluA2 knockout mice did not manifest ketamine-induced antidepressant responses 63. Similar to ketamine, other NMDAR antagonists, including MK-801 63 and AP-5 148 mimicked ketamine’s effect in inducing AMPAR-mediated synaptic potentiation. This finding was hypothesized to indicate that ketamine, via blocking the NMDAR at rest, drives synaptic potentiation, leading to synaptic plasticity changes that might be relevant to the antidepressant actions of NMDAR antagonists 10. However, MK-801 failed to induce long-lasting antidepressant actions in several animal tests 9, 10, 13, 103.
In line with an AMPAR activation-dependent mechanism of ketamine’s antidepressant action, (2R,6R)-HNK induces an increase in AMPAR-mediated excitatory post-synaptic potentials recorded from the CA1 region of hippocampal slices following stimulation of Schaffer collateral axons, suggesting an enhancement of excitatory synaptic transmission 13 (see Figure 1). This effect appears independent of any possible NMDAR inhibition by (2R,6R)-HNK, since the NMDAR antagonist AP-5 was present in the vehicle wash solution 13. In support of this, (2R,6R)-HNK, at the same concentration which did not alter NMDAR EPSCs (i.e., 10 μM), increases the frequency of AMPAR-mediated excitatory post-synaptic currents recorded from CA1 stratum radiatum interneurons, which receives glutamatergic inputs from the Schaffer collaterals 13. Similar to ketamine, (2R,6R)-HNK treatment in mice induces an acute and transient increase in high frequency gamma power 13. Importantly, administration of the AMPAR antagonist NBQX prior to (2R,6R)-HNK abolished the gamma power oscillation increases, as well as the acute and sustained antidepressant effects of this metabolite in mice, indicating that acute AMPAR activation is required for gamma power increase and its rapid and sustained antidepressant actions 13. In addition, (2R,6R)-HNK administration in mice, while not altering the levels of GluA1 or GluA2 AMPAR subunits 1 hour post-injection in hippocampal synaptoneurosomes, increases these AMPAR subunits 24 hours post-injection 13, indicating that maintenance of the antidepressant actions of this metabolite requires sustained activation of the AMPARs. Consistent with a mechanistic model where the sustained activity of synaptic AMPARs is required for the long-lasting antidepressant actions of (2R,6R)-HNK, it was shown that similar to ketamine 140 blockade of the AMPAR (with NBQX) 23.5 hours after (2R,6R)-HNK administration abolished its antidepressant actions at 24 hours post-injection 13.
Brain-derived neurotrophic factor (BDNF)
Brain-derived neurotrophic factor (BDNF) is a growth factor that regulates neurite outgrowth, functional neuronal connections, synapse formation and synaptic plasticity in the central nervous system 149–152. With regards to depression, systemic or intra-hippocampal administration of BDNF exerts antidepressant-like effects 153–155, and over-expression of BDNF in the hippocampus leads to resilience to chronic stress 156. Activation of the high-affinity BDNF receptor, tropomyosin receptor kinase B (TrkB), was shown to be necessary for these antidepressant-related behavioral actions 157, 158. Moreover, classical antidepressants induce BDNF-related changes following several weeks of administration 159. In contrast, ketamine administration rapidly (within 30 min of administration) increases the phosphorylation (activation) of hippocampal TrkB 10 and induces a rapid increase in total BDNF protein levels 10, 160. In addition, ketamine and (2R,6R)-HNK administration increases synaptoneurosomal BDNF protein levels 24 hours post-injection in the hippocampus of mice 13.
BNDF signaling was shown to be necessary for ketamine’s antidepressant actions. In particular, ketamine failed to exert antidepressant actions in mice with Bdnf gene knockdown specifically in the forebrain 10 and intra-mPFC infusion of a BDNF-neutralizing antibody prevented ketamine’s antidepressant behavioral responses 161, showing that BDNF release is essential for the actions of ketamine. In support of this, mice expressing the human BDNFVal66met (rs6265) single nucleotide polymorphism (SNP) – especially Met/Met carriers –, which induces deficits in BDNF processing and activity-dependent secretion 162, do not manifest ketamine-induced antidepressant effects 163. Similar to these findings in mice, Laje et al., (2012) 164 demonstrated that major depressed patients carrying the Met rs6265 allele did not respond to ketamine, further suggesting that increase in BDNF synthesis is required for the antidepressant actions of ketamine.
Eukaryotic elongation factor 2 kinase (eEF2K)
Eukaryotic elongation factor 2 kinase (eEF2K, also known as calmodulin-dependent protein kinase III), belongs to the atypical alpha-kinase family, and its activity is dependent on calcium and calmodulin cellular levels. Its primary downstream substrate (eEF2) is associated with the regulation of protein synthesis and synaptic plasticity 165. Under physiological conditions, NMDAR-dependent activation of eEF2K results in inactivation (phosphorylation) of eEF2 leading to the blockade of the elongation phase of protein synthesis and thus inhibition of protein translation 166, 167. Administration of eEF2K inhibitors reduced behavioral despair in the forced-swim test 30 min post-injection in mice 10. Autry et al., (2011) proposed that a single sub-anesthetic dose of ketamine, via inhibition of spontaneous synaptic NMDAR-mediated glutamatergic neurotransmission, decreases activation of eEF2K 10 resulting in eEF2 de-phosphorylation and a subsequent disinhibition of protein translation in vitro 10, 62. In vivo eEF2 dephosphorylation was shown to de-suppress BDNF protein translation, which was hypothesized to mediate the long-term effects of ketamine via the induction of synaptic plasticity 10. Mice lacking the eEF2K gene do not manifest ketamine-induced increases in hippocampal BDNF protein expression and lack ketamine antidepressant-like responses in the 30-min forced-swim test 63.
(2R,6R)-HNK administration also induced a decrease in hippocampal eEF2 phosphorylation 1 and 24 hours post-treatment, concomitant with increased BDNF levels at 24 hours 13, suggesting that protein synthesis through the eEF2 kinase/BDNF translation pathway might be involved in the antidepressant actions of this metabolite. This finding is of particular importance, since the concentrations achieved in the brain following peripheral administration of this metabolite are not associated with NMDAR inhibition 129, 130, thus synaptic plasticity changes and downstream signaling alterations occur independent of NMDAR inhibition. Indeed, several NMDAR inhibition-independent mechanisms have been proposed, which may explain eEF2 dephosphorylation caused by (2R,6R)-HNK administration 168–170. These findings might suggest that ketamine acts to inhibit eEF2K activity via an NMDAR inhibition-independent mechanism, which converges with the mechanism of antidepressant action of its HNK metabolite as well.
Mechanistic target of rapamycin (mTOR)
Enhanced BDNF translation and/or release, as well as activation of the BDNF receptor target TrkB can further activate downstream pathways important in synaptic plasticity. BDNF-mediated activation of TrkB receptors induces an activation of the phosphatidylinositol 3-kinase (PI3K), which, by changing the inner plasma membrane composition of inositol phospholipids, causes a translocation of Akt (protein kinase B) to the plasma membrane 171. Alternatively, TrkB activation induces a downstream activation of MEK-MAPK/Erk signaling pathway. These two pathways drive protein translation via the activation of the mechanistic target of rapamycin complex 1 (mTORC1) 172. Mechanistic target of rapamycin (mTOR) is a serine/threonine kinase that regulates neurogenesis, dendritic spine growth, protein translation initiation, and protein synthesis via a phosphorylation of p70S6 kinase and repression of 4E binding proteins (4EBP 173–175). mTOR signaling has been implicated in the antidepressant responses of several classical antidepressant drugs 176.
A single antidepressant-dose ketamine administration induced a fast-onset (within 30 min of administration) induction of phospho-mTOR 71, 75, 124, 139, 177–179, phospho-p70S6 kinase and phospho-4EBP1 75, 177 in the prefrontal cortex and hippocampus of mice and rats, suggesting a mechanism whereby ketamine-induced protein translation occurs in an mTOR activation-dependent manner. These changes are transient and the levels of mTOR-signaling molecules return to baseline levels 2 hrs following ketamine administration 75, indicating that acute activation of mTOR and thus protein translation may induce sustained synaptic plasticity changes responsible for the prolonged effects of ketamine. Additional evidence for the involvement of mTOR signaling in the antidepressant actions of ketamine is the finding that ketamine administration induced a rapid increase in phospho-Akt and phospho-ERK levels (activation), which are upstream of mTOR signaling activation 75. Intracerebroventricular administration of both PI3K-Akt and MEK-ERK inhibitors abolished ketamine-induced effects on mTOR pathway phosphoproteins 75. Although some studies failed to replicate an effect of ketamine administration on mTOR-related signaling 180, this might be due to the different doses of ketamine or the experimental procedures used 181. Indeed, mTOR activation was shown to be required for ketamine’s behavioral antidepressant actions 182. Specifically, intracerebroventricular pre-treatment with the selective mTOR inhibitor rapamycin blocks ketamine-induced synaptic molecular changes in mice 75, as well as the antidepressant actions of the drug in rats 183 and mice 75. These findings implicate mTOR as a key downstream point of convergence for explaining ketamine’s rapid-acting antidepressant actions. Importantly, and in accordance with mTOR being involved in the antidepressant actions of ketamine, both the antidepressant behavioral effects of ketamine, as well as its actions on the mTORC1 signaling were blocked by pre-treatment with the AMPAR antagonist NBQX 75. Additionally, (2S,6S)-HNK metabolite administration was shown to rapidly induce mTOR phosphorylation in rats 177.
Activation of mTOR signaling is linked to deactivation of the serine/threonine kinase glycogen synthase kinase-3 (GSK-3). In particular, upstream phosphorylation (deactivation) of GSK-3 induces mTOR activation (see Figure 1) 184, 185. Mice harboring a knock-in mutation at both GSK-3α and GSK-3β genes, which prevents the phosphorylation-dependent inactivation of the kinase, do not manifest ketamine-induced antidepressant behavioral responses 186. Administration of combined subthreshold doses of ketamine and lithium (a non-selective GSK-3 inhibitor), or a selective GSK-3 inhibitor, induced an activation of the mTORC1 signaling pathway, phosphorylation of GSK-3, synaptoneurogenesis and enhanced antidepressant actions 187, suggesting that mTORC1 activation and phosphorylation of GSK-3 might be a convergent mechanism involved in ketamine’s antidepressant actions. Phosphorylation of GSK-3 might be caused by ketamine-induced activation of the mTOR upstream kinase Akt, which regulates the activity of GSK-3 188. This is supported by the finding that PI3K/Akt antagonism prevented ketamine-induced phosphorylation of GSK-3β and mTORC1, and abolished ketamine’s antidepressant actions 189.
Conclusions
Most hypotheses regarding ketamine’s mechanism of action as an antidepressant have presumed an essential role of inhibition of the NMDAR. These hypotheses include direct effects on spontaneous NMDAR-mediated transmission, preferential inhibition of the NMDAR on GABAergic interneurons, and a role for extra-synaptic (plausibly GluN2B-specific) NMDAR inhibition. However, a growing body of evidence indicates that additional mechanisms are likely involved in mediating the unique properties of ketamine as an antidepressant, which may include ketamine metabolites. Indeed, it was shown that ketamine exerts NMDAR inhibition-independent antidepressant actions, and that these effects require the metabolism of ketamine to the (2S,6S;2R,6R)-HNK metabolite 13. Moreover, the (2R,6R)-HNK metabolite is sufficient to induce antidepressant actions, similar to those observed following ketamine administration, in animal tests. This metabolite also exerts electrophysiological, electroencephalographic and molecular actions that might explain ketamine’s unique antidepressant actions 13. These data highlight the need to consider alternative mechanisms, in addition to NMDAR inhibition, to unravel ketamine’s mechanism of action as an antidepressant.
There is a consensus from most pre-clinical research that AMPAR activity is required for the antidepressant actions of ketamine (see Figure 1). Increased probability of glutamate release, either by interneuron-mediated disinhibition or direct action of (2R,6R)-HNK on pyramidal neurons may result in activation of AMPARs, and a subsequent activation of downstream neuroplasticity-related signaling pathways, including those regulated by BDNF and mTORC1, to promote protein synthesis and synaptic plasticity that are involved in ketamine’s behavioral antidepressant actions. Alternatively, eEF2 inactivation as a result of NMDAR inhibition at rest, may regulate production of BDNF, resulting in an upregulation of AMPARs. Importantly, we note that all the proposed mechanisms of ketamine’s antidepressant actions are not mutually exclusive and may in fact complement each other to result in the unique antidepressant effects of the drug. Indeed, a net result of all these processes is a sustained potentiation of excitatory synapses in cortico-mesolimbic brain circuits involved in the maintenance of mood and stress-reactivity 190. Additional mechanisms, not discussed in the present review, include ketamine’s effects on the monoaminergic systems 191–194, as well as its anti-inflammatory actions, which are postulated to be involved in the mechanisms underlying its antidepressant actions; the reader is directed to reviews by Sleigh et al. (2014) 195 and Loix et al. (2011) 196.
Understanding the mechanisms underpinning ketamine’s antidepressant actions not only provides invaluable information on the neurobiology of major depression but it also drives the identification of novel therapeutic targets for the development of the next generation rapid-acting antidepressants, which will be effective and lack undesirable side effects. NMDAR inhibition produces serious side effects, even at low, sub-anesthetic and antidepressant-relevant doses, which makes long-term use of agents fully blocking this receptor impractical for the treatment of depression 197–201. The NMDAR inhibition-independent hypothesis of the antidepressant actions of ketamine reviewed here provides a framework to guide future studies on the identification of novel targets for the long-term treatment of depression lacking such side effects.
Acknowledgments
This is supported by an NIH grant MH107615 and a Harrington Discovery Institute Scholar-Innovator grant to T.D.G. We thank Dr. Paul Shepard for reviewing the LHb text section and Ms. Jaclyn Highland for proof-reading the manuscript.
Footnotes
Conflicts of interest
P.Z. and T.D.G. are listed as co-authors in a patent applications related to the pharmacology and use of (2S,6S)- and (2R,6R)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation and post-traumatic stress disorders.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA : the journal of the American Medical Association. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. The American journal of psychiatry. 2006;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 3.Insel TR, Wang PS. The STAR*D trial: revealing the need for better treatments. Psychiatric services. 2009;60(11):1466–1467. doi: 10.1176/ps.2009.60.11.1466. [DOI] [PubMed] [Google Scholar]
- 4.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 5.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 6.Price RB, Iosifescu DV, Murrough JW, Chang LC, Al Jurdi RK, Iqbal SZ, et al. Effects of ketamine on explicit and implicit suicidal cognition: a randomized controlled trial in treatment-resistant depression. Depress Anxiety. 2014;31(4):335–343. doi: 10.1002/da.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010;71(12):1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lapidus KA, Levitch CF, Perez AM, Brallier JW, Parides MK, Soleimani L, et al. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry. 2014;76(12):970–976. doi: 10.1016/j.biopsych.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63(4):349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 10.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475(7354):91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li N, Liu R-J, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate Receptor Antagonists Rapidly Reverse Behavioral and Synaptic Deficits Caused by Chronic Stress Exposure. Biological psychiatry. 2011;69(8):754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poleszak E, Wlaz P, Szewczyk B, Wlaz A, Kasperek R, Wrobel A, et al. A complex interaction between glycine/NMDA receptors and serotonergic/noradrenergic antidepressants in the forced swim test in mice. J Neural Transm. 2011;118(11):1535–1546. doi: 10.1007/s00702-011-0630-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zanos P, Piantadosi SC, Wu HQ, Pribut HJ, Dell MJ, Can A, et al. The Prodrug 4-Chlorokynurenine Causes Ketamine-Like Antidepressant Effects, but Not Side Effects, by NMDA/GlycineB-Site Inhibition. J Pharmacol Exp Ther. 2015;355(1):76–85. doi: 10.1124/jpet.115.225664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zanos P, Gould TD. Convergent mechanisms underlying rapid antidepressant action. CNS Drugs. 2018 doi: 10.1007/s40263-018-0492-x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51(3):199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 17.Marland S, Ellerton J, Andolfatto G, Strapazzon G, Thomassen O, Brandner B, et al. Ketamine: use in anesthesia. CNS Neurosci Ther. 2013;19(6):381–389. doi: 10.1111/cns.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh JB, Fedgchin M, Daly E, Xi L, Melman C, De Bruecker G, et al. Intravenous Esketamine in Adult Treatment-Resistant Depression: A Double-Blind, Double-Randomization, Placebo-Controlled Study. Biol Psychiatry. 2016;80(6):424–431. doi: 10.1016/j.biopsych.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170(10):1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67(8):793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zarate CA, Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71(11):939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. JAMA. 2004;292(3):338–343. doi: 10.1001/jama.292.3.338. [DOI] [PubMed] [Google Scholar]
- 23.Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66(5):522–526. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ballard ED, Ionescu DF, Vande Voort JL, Niciu MJ, Richards EM, Luckenbaugh DA, et al. Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res. 2014;58:161–166. doi: 10.1016/j.jpsychires.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ballard ED, Wills K, Lally N, Richards EM, Luckenbaugh DA, Walls T, et al. Anhedonia as a clinical correlate of suicidal thoughts in clinical ketamine trials. J Affect Disord. 2017;218:195–200. doi: 10.1016/j.jad.2017.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lally N, Nugent AC, Luckenbaugh DA, Niciu MJ, Roiser JP, Zarate CA., Jr Neural correlates of change in major depressive disorder anhedonia following open-label ketamine. J Psychopharmacol. 2015;29(5):596–607. doi: 10.1177/0269881114568041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lally N, Nugent AC, Luckenbaugh DA, Ameli R, Roiser JP, Zarate CA. Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry. 2014;4:e469. doi: 10.1038/tp.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vyklicky V, Korinek M, Smejkalova T, Balik A, Krausova B, Kaniakova M, et al. Structure, function, and pharmacology of NMDA receptor channels. Physiol Res. 2014;63(Suppl 1):S191–203. doi: 10.33549/physiolres.932678. [DOI] [PubMed] [Google Scholar]
- 29.Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, et al. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62(3):405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trullas R, Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur J Pharmacol. 1990;185(1):1–10. doi: 10.1016/0014-2999(90)90204-j. [DOI] [PubMed] [Google Scholar]
- 31.Paul IA, Nowak G, Layer RT, Popik P, Skolnick P. Adaptation of the N-methyl-D-aspartate receptor complex following chronic antidepressant treatments. J Pharmacol Exp Ther. 1994;269(1):95–102. [PubMed] [Google Scholar]
- 32.Nowak G, Li Y, Paul IA. Adaptation of cortical but not hippocampal NMDA receptors after chronic citalopram treatment. Eur J Pharmacol. 1996;295(1):75–85. doi: 10.1016/0014-2999(95)00585-4. [DOI] [PubMed] [Google Scholar]
- 33.Skolnick P, Layer RT, Popik P, Nowak G, Paul IA, Trullas R. Adaptation of N-methyl-D-aspartate (NMDA) receptors following antidepressant treatment: implications for the pharmacotherapy of depression. Pharmacopsychiatry. 1996;29(1):23–26. doi: 10.1055/s-2007-979537. [DOI] [PubMed] [Google Scholar]
- 34.Breier A, Malhotra AK, Pinals DA, Weisenfeld NI, Pickar D. Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. Am J Psychiatry. 1997;154(6):805–811. doi: 10.1176/ajp.154.6.805. [DOI] [PubMed] [Google Scholar]
- 35.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17(8):2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27(43):11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farber NB, Newcomer JW, Olney JW. The glutamate synapse in neuropsychiatric disorders. Focus on schizophrenia and Alzheimer’s disease. Prog Brain Res. 1998;116:421–437. doi: 10.1016/s0079-6123(08)60453-7. [DOI] [PubMed] [Google Scholar]
- 38.Neske GT, Patrick SL, Connors BW. Contributions of diverse excitatory and inhibitory neurons to recurrent network activity in cerebral cortex. J Neurosci. 2015;35(3):1089–1105. doi: 10.1523/JNEUROSCI.2279-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seamans J. Losing inhibition with ketamine. Nat Chem Biol. 2008;4(2):91–93. doi: 10.1038/nchembio0208-91. [DOI] [PubMed] [Google Scholar]
- 40.Kotermanski SE, Johnson JW. Mg2+ imparts NMDA receptor subtype selectivity to the Alzheimer’s drug memantine. J Neurosci. 2009;29(9):2774–2779. doi: 10.1523/JNEUROSCI.3703-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khlestova E, Johnson JW, Krystal JH, Lisman J. The Role of GluN2C-Containing NMDA Receptors in Ketamine’s Psychotogenic Action and in Schizophrenia Models. J Neurosci. 2016;36(44):11151–11157. doi: 10.1523/JNEUROSCI.1203-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12(3):529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 43.Perszyk RE, DiRaddo JO, Strong KL, Low CM, Ogden KK, Khatri A, et al. GluN2D-Containing N-methyl-d-Aspartate Receptors Mediate Synaptic Transmission in Hippocampal Interneurons and Regulate Interneuron Activity. Mol Pharmacol. 2016;90(6):689–702. doi: 10.1124/mol.116.105130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chowdhury GM, Zhang J, Thomas M, Banasr M, Ma X, Pittman B, et al. Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Mol Psychiatry. 2017;22(1):120–126. doi: 10.1038/mp.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Towers SK, Gloveli T, Traub RD, Driver JE, Engel D, Fradley R, et al. Alpha 5 subunit-containing GABAA receptors affect the dynamic range of mouse hippocampal kainate-induced gamma frequency oscillations in vitro. J Physiol. 2004;559(Pt 3):721–728. doi: 10.1113/jphysiol.2004.071191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fischell J, Van Dyke AM, Kvarta MD, LeGates TA, Thompson SM. Rapid Antidepressant Action and Restoration of Excitatory Synaptic Strength After Chronic Stress by Negative Modulators of Alpha5-Containing GABAA Receptors. Neuropsychopharmacology. 2015;40(11):2499–2509. doi: 10.1038/npp.2015.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zanos P, Nelson ME, Highland JN, Krimmel SR, Georgiou P, Gould TD, et al. A Negative Allosteric Modulator for alpha5 Subunit-Containing GABA Receptors Exerts a Rapid and Persistent Antidepressant-like Action without the Side Effects of the NMDA Receptor Antagonist Ketamine in Mice. eneuro. 2017 doi: 10.1523/ENEURO.0285-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carreno FR, Collins GT, Frazer A, Lodge DJ. Selective Pharmacological Augmentation of Hippocampal Activity Produces a Sustained Antidepressant-Like Response without Abuse-Related or Psychotomimetic Effects. Int J Neuropsychopharmacol. 2017 doi: 10.1093/ijnp/pyx003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pinault D. N-methyl d-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex. Biol Psychiatry. 2008;63(8):730–735. doi: 10.1016/j.biopsych.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Hong LE, Summerfelt A, Buchanan RW, O’Donnell P, Thaker GK, Weiler MA, et al. Gamma and delta neural oscillations and association with clinical symptoms under subanesthetic ketamine. Neuropsychopharmacology. 2010;35(3):632–640. doi: 10.1038/npp.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ehrlichman RS, Gandal MJ, Maxwell CR, Lazarewicz MT, Finkel LH, Contreras D, et al. N-methyl-d-aspartic acid receptor antagonist-induced frequency oscillations in mice recreate pattern of electrophysiological deficits in schizophrenia. Neuroscience. 2009;158(2):705–712. doi: 10.1016/j.neuroscience.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 52.Caixeta FV, Cornelio AM, Scheffer-Teixeira R, Ribeiro S, Tort AB. Ketamine alters oscillatory coupling in the hippocampus. Sci Rep. 2013;3:2348. doi: 10.1038/srep02348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ren Z, Pribiag H, Jefferson SJ, Shorey M, Fuchs T, Stellwagen D, et al. Bidirectional Homeostatic Regulation of a Depression-Related Brain State by Gamma-Aminobutyric Acidergic Deficits and Ketamine Treatment. Biol Psychiatry. 2016;80(6):457–468. doi: 10.1016/j.biopsych.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fuchs T, Jefferson SJ, Hooper A, Yee P-HP, Maguire J, Luscher B. Disinhibition of somatostatin-positive GABAergic interneurons results in an anxiolytic and antidepressant-like brain state. Molecular psychiatry. 2017;22(6):920–930. doi: 10.1038/mp.2016.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frankowska M, Filip M, Przegalinski E. Effects of GABAB receptor ligands in animal tests of depression and anxiety. Pharmacol Rep. 2007;59(6):645–655. [PubMed] [Google Scholar]
- 56.Slattery DA, Neumann ID, Cryan JF. Transient inactivation of the infralimbic cortex induces antidepressant-like effects in the rat. J Psychopharmacol. 2011;25(10):1295–1303. doi: 10.1177/0269881110368873. [DOI] [PubMed] [Google Scholar]
- 57.Pozzi L, Pollak Dorocic I, Wang X, Carlen M, Meletis K. Mice lacking NMDA receptors in parvalbumin neurons display normal depression-related behavior and response to antidepressant action of NMDAR antagonists. PLoS One. 2014;9(1):e83879. doi: 10.1371/journal.pone.0083879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fatt P, Katz B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- 59.Heuser JE, Reese TS, Dennis MJ, Jan Y, Jan L, Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sutton MA, Wall NR, Aakalu GN, Schuman EM. Regulation of dendritic protein synthesis by miniature synaptic events. Science. 2004;304(5679):1979–1983. doi: 10.1126/science.1096202. [DOI] [PubMed] [Google Scholar]
- 61.Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125(4):785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 62.Sutton MA, Taylor AM, Ito HT, Pham A, Schuman EM. Postsynaptic decoding of neural activity: eEF2 as a biochemical sensor coupling miniature synaptic transmission to local protein synthesis. Neuron. 2007;55(4):648–661. doi: 10.1016/j.neuron.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 63.Nosyreva E, Szabla K, Autry AE, Ryazanov AG, Monteggia LM, Kavalali ET. Acute suppression of spontaneous neurotransmission drives synaptic potentiation. J Neurosci. 2013;33(16):6990–7002. doi: 10.1523/JNEUROSCI.4998-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gideons ES, Kavalali ET, Monteggia LM. Mechanisms underlying differential effectiveness of memantine and ketamine in rapid antidepressant responses. Proc Natl Acad Sci U S A. 2014;111(23):8649–8654. doi: 10.1073/pnas.1323920111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zarate CA, Jr, Singh JB, Quiroz JA, De Jesus G, Denicoff KK, Luckenbaugh DA, et al. A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am J Psychiatry. 2006;163(1):153–155. doi: 10.1176/appi.ajp.163.1.153. [DOI] [PubMed] [Google Scholar]
- 66.Lenze EJ, Skidmore ER, Begley AE, Newcomer JW, Butters MA, Whyte EM. Memantine for late-life depression and apathy after a disabling medical event: a 12-week, double-blind placebo-controlled pilot study. Int J Geriatr Psychiatry. 2012;27(9):974–980. doi: 10.1002/gps.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferguson JM, Shingleton RN. An open-label, flexible-dose study of memantine in major depressive disorder. Clin Neuropharmacol. 2007;30(3):136–144. doi: 10.1097/WNF.0b013e3180314ae7. [DOI] [PubMed] [Google Scholar]
- 68.Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11(10):682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16(3):675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 70.Guo H, Lai L, Butchbach ME, Stockinger MP, Shan X, Bishop GA, et al. Increased expression of the glial glutamate transporter EAAT2 modulates excitotoxicity and delays the onset but not the outcome of ALS in mice. Hum Mol Genet. 2003;12(19):2519–2532. doi: 10.1093/hmg/ddg267. [DOI] [PubMed] [Google Scholar]
- 71.Miller OH, Yang L, Wang CC, Hargroder EA, Zhang Y, Delpire E, et al. GluN2B-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine. Elife. 2014;3:e03581. doi: 10.7554/eLife.03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang CC, Held RG, Chang SC, Yang L, Delpire E, Ghosh A, et al. A critical role for GluN2B-containing NMDA receptors in cortical development and function. Neuron. 2011;72(5):789–805. doi: 10.1016/j.neuron.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 73.Wang CC, Held RG, Hall BJ. SynGAP regulates protein synthesis and homeostatic synaptic plasticity in developing cortical networks. PLoS One. 2013;8(12):e83941. doi: 10.1371/journal.pone.0083941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gray JA, Shi Y, Usui H, During MJ, Sakimura K, Nicoll RA. Distinct modes of AMPA receptor suppression at developing synapses by GluN2A and GluN2B: single-cell NMDA receptor subunit deletion in vivo. Neuron. 2011;71(6):1085–1101. doi: 10.1016/j.neuron.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69(8):754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jimenez-Sanchez L, Campa L, Auberson YP, Adell A. The role of GluN2A and GluN2B subunits on the effects of NMDA receptor antagonists in modeling schizophrenia and treating refractory depression. Neuropsychopharmacology. 2014;39(11):2673–2680. doi: 10.1038/npp.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kiselycznyk C, Jury NJ, Halladay LR, Nakazawa K, Mishina M, Sprengel R, et al. NMDA receptor subunits and associated signaling molecules mediating antidepressant-related effects of NMDA-GluN2B antagonism. Behav Brain Res. 2015;287:89–95. doi: 10.1016/j.bbr.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28(6):631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- 80.Hashimoto K. Comments on “An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606 in patients with treatment-refractory major depressive disorder”. J Clin Psychopharmacol. 2009;29(4):411–412. doi: 10.1097/JCP.0b013e3181ace848. author reply 412. [DOI] [PubMed] [Google Scholar]
- 81.Hashimoto K, London ED. Further characterization of [3H]ifenprodil binding to sigma receptors in rat brain. Eur J Pharmacol. 1993;236(1):159–163. doi: 10.1016/0014-2999(93)90241-9. [DOI] [PubMed] [Google Scholar]
- 82.Hashimoto K, Ishiwata K. Sigma receptor ligands: possible application as therapeutic drugs and as radiopharmaceuticals. Curr Pharm Des. 2006;12(30):3857–3876. doi: 10.2174/138161206778559614. [DOI] [PubMed] [Google Scholar]
- 83.Stahl SM. The sigma enigma: can sigma receptors provide a novel target for disorders of mood and cognition? J Clin Psychiatry. 2008;69(11):1673–1674. doi: 10.4088/jcp.v69n1101. [DOI] [PubMed] [Google Scholar]
- 84.Hashimoto K. Sigma-1 receptors and selective serotonin reuptake inhibitors: clinical implications of their relationship. Cent Nerv Syst Agents Med Chem. 2009;9(3):197–204. doi: 10.2174/1871524910909030197. [DOI] [PubMed] [Google Scholar]
- 85.Ibrahim L, Diaz Granados N, Jolkovsky L, Brutsche N, Luckenbaugh DA, Herring WJ, et al. A Randomized, placebo-controlled, crossover pilot trial of the oral selective NR2B antagonist MK-0657 in patients with treatment-resistant major depressive disorder. J Clin Psychopharmacol. 2012;32(4):551–557. doi: 10.1097/JCP.0b013e31825d70d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sanacora G. What Are We Learning From Early-Phase Clinical Trials With Glutamate Targeting Medications for the Treatment of Major Depressive Disorder. JAMA psychiatry. 2016;73(7):651–652. doi: 10.1001/jamapsychiatry.2016.0780. [DOI] [PubMed] [Google Scholar]
- 87.Sutherland RJ. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci Biobehav Rev. 1982;6(1):1–13. doi: 10.1016/0149-7634(82)90003-3. [DOI] [PubMed] [Google Scholar]
- 88.Boulos LJ, Darcq E, Kieffer BL. Translating the Habenula-From Rodents to Humans. Biol Psychiatry. 81(4):296–305. doi: 10.1016/j.biopsych.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11(7):503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang D, Li Y, Feng Q, Guo Q, Zhou J, Luo M. Learning shapes the aversion and reward responses of lateral habenula neurons. Elife. :6. doi: 10.7554/eLife.23045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ji H, Shepard PD. Lateral Habenula Stimulation Inhibits Rat Midbrain Dopamine Neurons through a GABAA Receptor-Mediated Mechanism. The Journal of Neuroscience. 2007;27(26):6923–6930. doi: 10.1523/JNEUROSCI.0958-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brown PL, Palacorolla H, Brady D, Riegger K, Elmer GI, Shepard PD. Habenula-Induced Inhibition of Midbrain Dopamine Neurons Is Diminished by Lesions of the Rostromedial Tegmental Nucleus. J Neurosci. 2017;37(1):217–225. doi: 10.1523/JNEUROSCI.1353-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009 Feb 24;5132009:566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang Y, Wang H, Hu J, Hu H. Lateral habenula in the pathophysiology of depression. Curr Opin Neurobiol. 2017;48:90–96. doi: 10.1016/j.conb.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 95.Cui Y, Yang Y, Ni Z, Dong Y, Cai G, Foncelle A, et al. Astroglial Kir4. 1 in the lateral habenula drives neuronal bursts in depression. Nature. 2018;554(7692):323–327. doi: 10.1038/nature25752. [DOI] [PubMed] [Google Scholar]
- 96.Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, et al. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470(7335):535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lawson RP, Nord CL, Seymour B, Thomas DL, Dayan P, Pilling S, et al. Disrupted habenula function in major depression. Mol Psychiatry. 2017;22(2):202–208. doi: 10.1038/mp.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, et al. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature. 2018;554(7692):317–322. doi: 10.1038/nature25509. [DOI] [PubMed] [Google Scholar]
- 99.Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB. Ketamine and Other NMDA Antagonists: Early Clinical Trials and Possible Mechanisms in Depression. Am J Psychiatry. 2015;172(10):950–966. doi: 10.1176/appi.ajp.2015.15040465. [DOI] [PubMed] [Google Scholar]
- 100.Zarate CA, Jr, Mathews D, Ibrahim L, Chaves JF, Marquardt C, Ukoh I, et al. A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression. Biol Psychiatry. 2013;74(4):257–264. doi: 10.1016/j.biopsych.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sanacora G, Smith MA, Pathak S, Su HL, Boeijinga PH, McCarthy DJ, et al. Lanicemine: a low-trapping NMDA channel blocker produces sustained antidepressant efficacy with minimal psychotomimetic adverse effects. Mol Psychiatry. 2014;19(9):978–985. doi: 10.1038/mp.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sanacora G, Johnson MR, Khan A, Atkinson SD, Riesenberg RR, Schronen JP, et al. Adjunctive Lanicemine (AZD6765) in Patients with Major Depressive Disorder and History of Inadequate Response to Antidepressants: A Randomized, Placebo-Controlled Study. Neuropsychopharmacology. 2017;42(4):844–853. doi: 10.1038/npp.2016.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang B, Ren Q, Ma M, Chen QX, Hashimoto K. Antidepressant Effects of (+)-MK-801 and (-)-MK-801 in the Social Defeat Stress Model. Int J Neuropsychopharmacol. 2016 doi: 10.1093/ijnp/pyw080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Heresco-Levy U, Gelfin G, Bloch B, Levin R, Edelman S, Javitt DC, et al. A randomized add-on trial of high-dose D-cycloserine for treatment-resistant depression. Int J Neuropsychopharmacol. 2013;16(3):501–506. doi: 10.1017/S1461145712000910. [DOI] [PubMed] [Google Scholar]
- 105.Moskal JR, Burgdorf JS, Stanton PK, Kroes RA, Disterhoft JF, Burch RM, et al. The Development of Rapastinel (Formerly GLYX-13); A Rapid Acting and Long Lasting Antidepressant. Curr Neuropharmacol. 2017;15(1):47–56. doi: 10.2174/1570159X14666160321122703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang B, Zhang JC, Han M, Yao W, Yang C, Ren Q, et al. Comparison of R-ketamine and rapastinel antidepressant effects in the social defeat stress model of depression. Psychopharmacology (Berl) 2016;233(19–20):3647–3657. doi: 10.1007/s00213-016-4399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Burgdorf J, Zhang X-l, Weiss C, Gross A, Boikess SR, Kroes RA, et al. The long-lasting antidepressant effects of rapastinel (GLYX-13) are associated with a metaplasticity process in the medial prefrontal cortex and hippocampus. Neuroscience. 2015;308:202–211. doi: 10.1016/j.neuroscience.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu RJ, Duman C, Kato T, Hare B, Lopresto D, Bang E, et al. GLYX-13 Produces Rapid Antidepressant Responses with Key Synaptic and Behavioral Effects Distinct from Ketamine. Neuropsychopharmacology. 2017;42(6):1231–1242. doi: 10.1038/npp.2016.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Burgdorf J, Zhang XL, Nicholson KL, Balster RL, Leander JD, Stanton PK, et al. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology. 2013;38(5):729–742. doi: 10.1038/npp.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rajagopal L, Burgdorf JS, Moskal JR, Meltzer HY. GLYX-13 (rapastinel) ameliorates subchronic phencyclidine- and ketamine-induced declarative memory deficits in mice. Behav Brain Res. 2016;299:105–110. doi: 10.1016/j.bbr.2015.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Morris PJ, Moaddel R, Zanos P, Moore CE, Gould T, Zarate CA, et al. Synthesis and N-Methyl-d-aspartate (NMDA) Receptor Activity of Ketamine Metabolites. Organic Letters. 2017 doi: 10.1021/acs.orglett.7b02177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moaddel R, Abdrakhmanova G, Kozak J, Jozwiak K, Toll L, Jimenez L, et al. Sub-anesthetic concentrations of (R,S)-ketamine metabolites inhibit acetylcholine-evoked currents in alpha7 nicotinic acetylcholine receptors. Eur J Pharmacol. 2013;698(1–3):228–234. doi: 10.1016/j.ejphar.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kohrs R, Durieux ME. Ketamine: Teaching an Old Drug New Tricks. Anesthesia & Analgesia. 1998;87(5):1186–1193. doi: 10.1097/00000539-199811000-00039. [DOI] [PubMed] [Google Scholar]
- 114.Ebert B, Mikkelsen S, Thorkildsen C, Borgbjerg FM. Norketamine, the main metabolite of ketamine, is a non-competitive NMDA receptor antagonist in the rat cortex and spinal cord. Eur J Pharmacol. 1997;333(1):99–104. doi: 10.1016/s0014-2999(97)01116-3. [DOI] [PubMed] [Google Scholar]
- 115.Domino EF. Taming the ketamine tiger. 1965. Anesthesiology. 2010;113(3):678–684. doi: 10.1097/ALN.0b013e3181ed09a2. [DOI] [PubMed] [Google Scholar]
- 116.Zhang JC, Li SX, Hashimoto K. R (-)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav. 2014;116:137–141. doi: 10.1016/j.pbb.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 117.Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, et al. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 2015;5:e632. doi: 10.1038/tp.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fukumoto K, Toki H, Iijima M, Hashihayata T, Yamaguchi J-i, Hashimoto K, et al. Antidepressant Potential of (R)-Ketamine in Rodent Models: Comparison with (S)-Ketamine. Journal of Pharmacology and Experimental Therapeutics. 2017;361(1):9–16. doi: 10.1124/jpet.116.239228. [DOI] [PubMed] [Google Scholar]
- 119.Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, et al. Efficacy and Safety of Intranasal Esketamine Adjunctive to Oral Antidepressant Therapy in Treatment-Resistant Depression: A Randomized Clinical Trial. JAMA Psychiatry. 2017 doi: 10.1001/jamapsychiatry.2017.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zarate CA, Jr, Brutsche N, Laje G, Luckenbaugh DA, Venkata SL, Ramamoorthy A, et al. Relationship of ketamine’s plasma metabolites with response, diagnosis, and side effects in major depression. Biol Psychiatry. 2012;72(4):331–338. doi: 10.1016/j.biopsych.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zanos P, Moaddel R, Morris PJ, Wainer IW, Albuquerque EX, Thompson SM, et al. Reply to: Antidepressant Actions of Ketamine Versus Hydroxynorketamine. Biol Psychiatry. 2017;81(8):e69–e71. doi: 10.1016/j.biopsych.2016.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Leung LY, Baillie TA. Comparative pharmacology in the rat of ketamine and its two principal metabolites, norketamine and (Z)-6-hydroxynorketamine. J Med Chem. 1986;29(11):2396–2399. doi: 10.1021/jm00161a043. [DOI] [PubMed] [Google Scholar]
- 123.Singh NS, Zarate CA, Jr, Moaddel R, Bernier M, Wainer IW. What is hydroxynorketamine and what can it bring to neurotherapeutics? Expert Rev Neurother. 2014;14(11):1239–1242. doi: 10.1586/14737175.2014.971760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Carrier N, Kabbaj M. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology. 2013;70:27–34. doi: 10.1016/j.neuropharm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 125.Sarkar A, Kabbaj M. Sex Differences in Effects of Ketamine on Behavior, Spine Density, and Synaptic Proteins in Socially Isolated Rats. Biol Psychiatry. 2016;80(6):448–456. doi: 10.1016/j.biopsych.2015.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pham TH, Defaix C, Xu X, Deng SX, Fabresse N, Alvarez JC, et al. Common Neurotransmission Recruited in (R,S)-Ketamine and (2R,6R)-Hydroxynorketamine-Induced Sustained Antidepressant-like Effects. Biol Psychiatry. 2017 doi: 10.1016/j.biopsych.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 127.Fukumoto K, Toki H, Iijima M, Hashihayata T, Yamaguchi J-i, Hashimoto K, et al. Antidepressant potential of (R)-ketamine in rodent models: Comparison with (S)-ketamine. Journal of Pharmacology and Experimental Therapeutics. 2017 doi: 10.1124/jpet.116.239228. [DOI] [PubMed] [Google Scholar]
- 128.Yang C, Qu Y, Abe M, Nozawa D, Chaki S, Hashimoto K. (R)-Ketamine Shows Greater Potency and Longer Lasting Antidepressant Effects Than Its Metabolite (2R,6R)-Hydroxynorketamine. Biol Psychiatry. 2017;82(5):e43–e44. doi: 10.1016/j.biopsych.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 129.Suzuki K, Nosyreva E, Hunt KW, Kavalali ET, Monteggia LM. Effects of a ketamine metabolite on synaptic NMDAR function. Nature. 2017;546(7659):E1–E3. doi: 10.1038/nature22084. [DOI] [PubMed] [Google Scholar]
- 130.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. Zanos et al. reply. Nature. 2017;546(7659):E4–E5. doi: 10.1038/nature22085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8(2):101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- 132.Henley JM, Wilkinson KA. Synaptic AMPA receptor composition in development, plasticity and disease. Nat Rev Neurosci. 2016;17(6):337–350. doi: 10.1038/nrn.2016.37. [DOI] [PubMed] [Google Scholar]
- 133.Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33(1):18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- 134.Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22(3):238–249. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kocsis B. Differential role of NR2A and NR2B subunits in NMDA receptor antagonist-induced aberrant cortical gamma oscillations. Biological psychiatry. 2012;71(11):987–995. doi: 10.1016/j.biopsych.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. Int J Psychophysiol. 2000;38(3):315–336. doi: 10.1016/s0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- 137.Cunningham MO, Davies CH, Buhl EH, Kopell N, Whittington MA. Gamma oscillations induced by kainate receptor activation in the entorhinal cortex in vitro. J Neurosci. 2003;23(30):9761–9769. doi: 10.1523/JNEUROSCI.23-30-09761.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Muthukumaraswamy SD, Shaw AD, Jackson LE, Hall J, Moran R, Saxena N. Evidence that Subanesthetic Doses of Ketamine Cause Sustained Disruptions of NMDA and AMPA-Mediated Frontoparietal Connectivity in Humans. J Neurosci. 2015;35(33):11694–11706. doi: 10.1523/JNEUROSCI.0903-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhou W, Wang N, Yang C, Li XM, Zhou ZQ, Yang JJ. Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. European Psychiatry. 2014;29(7):419–423. doi: 10.1016/j.eurpsy.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 140.Koike H, Chaki S. Requirement of AMPA receptor stimulation for the sustained antidepressant activity of ketamine and LY341495 during the forced swim test in rats. Behav Brain Res. 2014;271:111–115. doi: 10.1016/j.bbr.2014.05.065. [DOI] [PubMed] [Google Scholar]
- 141.Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res. 2011;224(1):107–111. doi: 10.1016/j.bbr.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 142.Fukumoto K, Iijima M, Chaki S. Serotonin-1A receptor stimulation mediates effects of a metabotropic glutamate 2/3 receptor antagonist, 2S-2-amino-2-(1S,2S-2-carboxycycloprop-1-yl)-3-(xanth-9-yl)propanoic acid (LY341495), and an N-methyl-D-aspartate receptor antagonist, ketamine, in the novelty-suppressed feeding test. Psychopharmacology (Berl) 2014;231(11):2291–2298. doi: 10.1007/s00213-013-3378-0. [DOI] [PubMed] [Google Scholar]
- 143.Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B, et al. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology. 2013;38(9):1609–1616. doi: 10.1038/npp.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wolak M, Siwek A, Szewczyk B, Poleszak E, Pilc A, Popik P, et al. Involvement of NMDA and AMPA receptors in the antidepressant-like activity of antidepressant drugs in the forced swim test. Pharmacol Rep. 2013;65(4):991–997. doi: 10.1016/s1734-1140(13)71080-6. [DOI] [PubMed] [Google Scholar]
- 145.Bjorkholm C, Jardemark K, Schilstrom B, Svensson TH. Ketamine-like effects of a combination of olanzapine and fluoxetine on AMPA and NMDA receptor-mediated transmission in the medial prefrontal cortex of the rat. Eur Neuropsychopharmacol. 2015;25(10):1842–1847. doi: 10.1016/j.euroneuro.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 146.El Iskandrani KS, Oosterhof CA, El Mansari M, Blier P. Impact of subanesthetic doses of ketamine on AMPA-mediated responses in rats: An in vivo electrophysiological study on monoaminergic and glutamatergic neurons. J Psychopharmacol. 2015;29(7):792–801. doi: 10.1177/0269881115573809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang K, Xu T, Yuan Z, Wei Z, Yamaki VN, Huang M, et al. Essential roles of AMPA receptor GluA1 phosphorylation and presynaptic HCN channels in fast-acting antidepressant responses of ketamine. Sci Signal. 2016;9(458):ra123. doi: 10.1126/scisignal.aai7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nosyreva E, Autry AE, Kavalali ET, Monteggia LM. Age dependence of the rapid antidepressant and synaptic effects of acute NMDA receptor blockade. Frontiers in Molecular Neuroscience. 2014;7:94. doi: 10.3389/fnmol.2014.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012;64(2):238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Castren E, Kojima M. Brain-derived neurotrophic factor in mood disorders and antidepressant treatments. Neurobiol Dis. 2017;97(Pt B):119–126. doi: 10.1016/j.nbd.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 151.Castrén E, Võikar V, Rantamäki T. Role of neurotrophic factors in depression. Current Opinion in Pharmacology. 2007;7(1):18–21. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 152.Lindholm JSO, Castrén E. Mice with altered BDNF signaling as models for mood disorders and antidepressant effects. Frontiers in Behavioral Neuroscience. 2014;8(143) doi: 10.3389/fnbeh.2014.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hoshaw BA, Malberg JE, Lucki I. Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Res. 2005;1037(1–2):204–208. doi: 10.1016/j.brainres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 154.Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22(8):3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schmidt HD, Duman RS. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology. 2010;35(12):2378–2391. doi: 10.1038/npp.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Taliaz D, Loya A, Gersner R, Haramati S, Chen A, Zangen A. Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. J Neurosci. 2011;31(12):4475–4483. doi: 10.1523/JNEUROSCI.5725-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rantamaki T, Hendolin P, Kankaanpaa A, Mijatovic J, Piepponen P, Domenici E, et al. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cgamma signaling pathways in mouse brain. Neuropsychopharmacology. 2007;32(10):2152–2162. doi: 10.1038/sj.npp.1301345. [DOI] [PubMed] [Google Scholar]
- 158.Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23(1):349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bjorkholm C, Monteggia LM. BDNF - a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–79. doi: 10.1016/j.neuropharm.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Garcia LS, Comim CM, Valvassori SS, Reus GZ, Barbosa LM, Andreazza AC, et al. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(1):140–144. doi: 10.1016/j.pnpbp.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 161.Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol. 2014;18(1) doi: 10.1093/ijnp/pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314(5796):140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry. 2012;71(11):996–1005. doi: 10.1016/j.biopsych.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Laje G, Lally N, Mathews D, Brutsche N, Chemerinski A, Akula N, et al. Brain-Derived Neurotrophic Factor Val66Met Polymorphism and Antidepressant Efficacy of Ketamine in Depressed Patients. Biological psychiatry. 2012;72(11):e27–e28. doi: 10.1016/j.biopsych.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Taha E, Gildish I, Gal-Ben-Ari S, Rosenblum K. The role of eEF2 pathway in learning and synaptic plasticity. Neurobiol Learn Mem. 2013;105:100–106. doi: 10.1016/j.nlm.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 166.Chotiner JK, Khorasani H, Nairn AC, O’Dell TJ, Watson JB. Adenylyl cyclase-dependent form of chemical long-term potentiation triggers translational regulation at the elongation step. Neuroscience. 2003;116(3):743–752. doi: 10.1016/s0306-4522(02)00797-2. [DOI] [PubMed] [Google Scholar]
- 167.Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, et al. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3. 1 essential for mGluR-LTD. Neuron. 2008;59(1):70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Taha E, Gildish I, Gal-Ben-Ari S, Rosenblum K. The role of eEF2 pathway in learning and synaptic plasticity. Neurobiology of Learning and Memory. 2013;105:100–106. doi: 10.1016/j.nlm.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 169.Browne GJ, Proud CG. A novel mTOR-regulated phosphorylation site in elongation factor 2 kinase modulates the activity of the kinase and its binding to calmodulin. Mol Cell Biol. 2004;24(7):2986–2997. doi: 10.1128/MCB.24.7.2986-2997.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Redpath NT, Foulstone EJ, Proud CG. Regulation of translation elongation factor-2 by insulin via a rapamycin-sensitive signalling pathway. EMBO J. 1996;15(9):2291–2297. [PMC free article] [PubMed] [Google Scholar]
- 171.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361(1473):1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yoshii A, Constantine-Paton M. Post-synaptic BDNF-TrkB Signaling in Synapse Maturation, Plasticity and Disease. Developmental neurobiology. 2010;70(5):304–322. doi: 10.1002/dneu.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Duman RS, Li N, Liu RJ, Duric V, Aghajanian G. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012;62(1):35–41. doi: 10.1016/j.neuropharm.2011.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18(16):1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 175.Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33(2):67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Park SW, Lee JG, Seo MK, Lee CH, Cho HY, Lee BJ, et al. Differential effects of antidepressant drugs on mTOR signalling in rat hippocampal neurons. Int J Neuropsychopharmacol. 2014;17(11):1831–1846. doi: 10.1017/S1461145714000534. [DOI] [PubMed] [Google Scholar]
- 177.Paul RK, Singh NS, Khadeer M, Moaddel R, Sanghvi M, Green CE, et al. (R,S)-Ketamine metabolites (R,S)-norketamine and (2S,6S)-hydroxynorketamine increase the mammalian target of rapamycin (mTOR) function. Anesthesiology. 2014;121(1):149–159. doi: 10.1097/ALN.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yang C, Hu YM, Zhou ZQ, Zhang GF, Yang JJ. Acute administration of ketamine in rats increases hippocampal BDNF and mTOR levels during forced swimming test. Ups J Med Sci. 2013;118(1):3–8. doi: 10.3109/03009734.2012.724118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhang K, Yamaki VN, Wei Z, Zheng Y, Cai X. Differential regulation of GluA1 expression by ketamine and memantine. Behav Brain Res. 2017;316:152–159. doi: 10.1016/j.bbr.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 180.Popp S, Behl B, Joshi JJ, Lanz TA, Spedding M, Schenker E, et al. In search of the mechanisms of ketamine’s antidepressant effects: How robust is the evidence behind the mTor activation hypothesis. F1000Research. 2016;5(634) [Google Scholar]
- 181.Murrough JW. Ketamine for Depression: An Update. Biol Psychiatry. 2016;80(6):416–418. doi: 10.1016/j.biopsych.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 182.Zanos P, Gould TD. Intracellular Signaling Pathways Involved in (S)- and (R)-Ketamine Antidepressant Actions. Biol Psychiatry. 2018;83(1):2–4. doi: 10.1016/j.biopsych.2017.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Holubova K, Kleteckova L, Skurlova M, Ricny J, Stuchlik A, Vales K. Rapamycin blocks the antidepressant effect of ketamine in task-dependent manner. Psychopharmacology (Berl) 2016;233(11):2077–2097. doi: 10.1007/s00213-016-4256-3. [DOI] [PubMed] [Google Scholar]
- 184.Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126(5):955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 185.Jin T, George Fantus I, Sun J. Wnt and beyond Wnt: multiple mechanisms control the transcriptional property of beta-catenin. Cell Signal. 2008;20(10):1697–1704. doi: 10.1016/j.cellsig.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 186.Beurel E, Song L, Jope RS. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Molecular psychiatry. 2011;16(11):1068–1070. doi: 10.1038/mp.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Liu R-J, Fuchikami M, Dwyer JM, Lepack AE, Duman RS, Aghajanian GK. GSK-3 Inhibition Potentiates the Synaptogenic and Antidepressant-Like Effects of Subthreshold Doses of Ketamine. Neuropsychopharmacology. 2013;38(11):2268–2277. doi: 10.1038/npp.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378(6559):785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 189.Zhou W, Dong L, Wang N, Shi JY, Yang JJ, Zuo ZY, et al. Akt mediates GSK-3beta phosphorylation in the rat prefrontal cortex during the process of ketamine exerting rapid antidepressant actions. Neuroimmunomodulation. 2014;21(4):183–188. doi: 10.1159/000356517. [DOI] [PubMed] [Google Scholar]
- 190.Thompson SM, Kallarackal AJ, Kvarta MD, Van Dyke AM, LeGates TA, Cai X. An excitatory synapse hypothesis of depression. Trends Neurosci. 2015;38(5):279–294. doi: 10.1016/j.tins.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Witkin JM, Monn JA, Schoepp DD, Li X, Overshiner C, Mitchell SN, et al. The Rapidly Acting Antidepressant Ketamine and the mGlu2/3 Receptor Antagonist LY341495 Rapidly Engage Dopaminergic Mood Circuits. J Pharmacol Exp Ther. 2016;358(1):71–82. doi: 10.1124/jpet.116.233627. [DOI] [PubMed] [Google Scholar]
- 192.Li Y, Zhu ZR, Ou BC, Wang YQ, Tan ZB, Deng CM, et al. Dopamine D2/D3 but not dopamine D1 receptors are involved in the rapid antidepressant-like effects of ketamine in the forced swim test. Behav Brain Res. 2015;279:100–105. doi: 10.1016/j.bbr.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 193.Gigliucci V, O’Dowd G, Casey S, Egan D, Gibney S, Harkin A. Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology (Berl) 2013;228(1):157–166. doi: 10.1007/s00213-013-3024-x. [DOI] [PubMed] [Google Scholar]
- 194.Can A, Zanos P, Moaddel R, Kang HJ, Dossou KS, Wainer IW, et al. Effects of Ketamine and Ketamine Metabolites on Evoked Striatal Dopamine Release, Dopamine Receptors, and Monoamine Transporters. J Pharmacol Exp Ther. 2016;359(1):159–170. doi: 10.1124/jpet.116.235838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sleigh J, Harvey M, Voss L, Denny B. Ketamine – More mechanisms of action than just NMDA blockade. Trends in Anaesthesia and Critical Care. 2014;4(2):76–81. [Google Scholar]
- 196.Loix S, De Kock M, Henin P. The anti-inflammatory effects of ketamine: state of the art. Acta Anaesthesiol Belg. 2011;62(1):47–58. [PubMed] [Google Scholar]
- 197.Bowdle TA, Radant AD, Cowley DS, Kharasch ED, Strassman RJ, Roy-Byrne PP. Psychedelic effects of ketamine in healthy volunteers: relationship to steady-state plasma concentrations. Anesthesiology. 1998;88(1):82–88. doi: 10.1097/00000542-199801000-00015. [DOI] [PubMed] [Google Scholar]
- 198.Pomarol-Clotet E, Honey GD, Murray GK, Corlett PR, Absalom AR, Lee M, et al. Psychological effects of ketamine in healthy volunteers. Phenomenological study. Br J Psychiatry. 2006;189:173–179. doi: 10.1192/bjp.bp.105.015263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Coull JT, Morgan H, Cambridge VC, Moore JW, Giorlando F, Adapa R, et al. Ketamine perturbs perception of the flow of time in healthy volunteers. Psychopharmacology (Berl) 2011;218(3):543–556. doi: 10.1007/s00213-011-2346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kalsi SS, Wood DM, Dargan PI. The epidemiology and patterns of acute and chronic toxicity associated with recreational ketamine use. Emerg Health Threats J. 2011;4:7107. doi: 10.3402/ehtj.v4i0.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wolff K, Winstock AR. Ketamine : from medicine to misuse. CNS Drugs. 2006;20(3):199–218. doi: 10.2165/00023210-200620030-00003. [DOI] [PubMed] [Google Scholar]