Supplemental Digital Content is Available in the Text.
Keywords: Tanezumab, Osteoarthritis, Bunionectomy, Analgesia, Safety, Nerve growth factor
Abstract
Introduction:
The neurotrophin nerve growth factor has a demonstrated role in pain transduction and pathophysiology.
Objectives:
Two randomized, double-blind, placebo-controlled, phase 1 studies were conducted to evaluate safety, tolerability, and analgesic efficacy of single doses of tanezumab, a humanized anti–nerve growth factor monoclonal antibody, in chronic or acute pain.
Methods:
In the first study (CL001), patients with moderate to severe pain from osteoarthritis (OA) of the knee received a single intravenous infusion of tanezumab (3–1000 μg/kg) or placebo in a dose-escalation (part 1; N = 42) or parallel-arm (part 2; N = 79) study design. The second study (CL002) was a placebo-controlled dose-escalation (tanezumab 10–1000 μg/kg; N = 50) study in patients undergoing bunionectomy surgery.
Results:
Adverse event rates were generally similar across treatments. Most adverse events were generally mild to moderate in severity and no patients discontinued as a result of adverse events. Adverse events of abnormal peripheral sensation were more common with higher doses of tanezumab (≥100 μg/kg) than with placebo. These were generally mild to moderate in severity. Tanezumab provided up to 12 weeks of effective analgesia for OA knee pain, with statistically significant improvements at doses ≥100 μg/kg (P < 0.05). By contrast, no trend for analgesic activity was found when tanezumab was administered 8 to 16 hours before bunionectomy.
Conclusions:
The demonstration of a favorable safety profile and clinical efficacy in OA pain supports clinical development of tanezumab as a potential treatment for chronic pain conditions.
1. Introduction
The successful management of acute and chronic pain remains a significant medical challenge. For patients experiencing acute pain, the therapeutic goal is total and rapid pain relief with healing of tissue.6 Chronic pain management is complicated, as underlying changes in pain sensation may result in ongoing pain perception, even after the damage is healed, so complete relief of pain is uncommon.6 Some therapies for relieving pain may not provide complete efficacy and can be associated with unwanted complications including dependency, safety, or tolerability issues.11,12,16,29,35,37
New pain therapies must demonstrate improved efficacy and/or safety, and one approach to achieve this goal is to target specific pain mediators. The neurotrophin, nerve growth factor (NGF) has a demonstrated role in pain transduction and pathophysiology.36 Although NGF has a critical role in early neural development,18 in adults this role changes to other functions including neuronal plasticity, hypersensitization to noxious stimuli, and pain signalling.15,36 Exogenous NGF administration causes rapid and long-lasting hyperalgesia and local allodynia.18,36 Elevated NGF levels have been associated with acute and chronic pain conditions and injured and inflamed tissues.26
Tanezumab is a humanized anti-NGF monoclonal antibody with high specificity and affinity for NGF.1 Tanezumab decreases NGF activity by preventing interaction between NGF and its high-affinity (TrkA) and low-affinity (p75) receptors.1 Two phase 1 studies (CL001 and CL002) were conducted to assess the safety, tolerability, and analgesic efficacy of a single intravenous (IV) dose of tanezumab in chronic and acute pain. In chronic pain (study CL001), moderate to severe knee osteoarthritis (OA) pain was used for assessing analgesic safety and efficacy. The acute pain model (study CL002) was acute postoperative bunionectomy pain. Both patient populations were deemed appropriate for first-in-human studies to assess safety while potentially allowing for preliminary assessment of efficacy.5,13,28,31
2. Methods
Two randomized, double-blind, placebo-controlled, phase 1 studies with similar designs were conducted in compliance with the Declaration of Helsinki and all International Conference on Harmonization Good Clinical Practice guidelines. The studies were conducted at study centers (5 and 1 for study CL001 and CL002, respectively) in the United States. Study protocols and informed consent documentation were reviewed and approved by institutional review boards. Written informed consent was obtained from each patient before initiation of protocol-specified procedures. After written informed consent was obtained and eligibility established, the study site assigned the patient's randomization number; based on this, the pharmacist assigned treatment using a list prepared by an unblinded statistician.
2.1. Study CL001-tanezumab in osteoarthritis
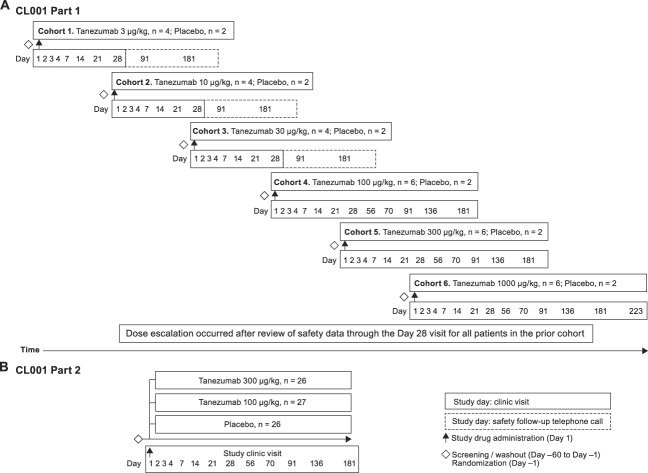
Study CL001 (original Rinat study number [Pfizer study number A4091006]) was conducted in 2 parts. Part 1 was a placebo-controlled dose escalation (tanezumab 3–1000 μg/kg) with 6 cohorts of patients (Fig. 1A). Part 2 was a placebo-controlled, parallel-arm comparison of tanezumab 100 or 300 μg/kg with doses selected based on an interim analysis of safety and efficacy data from part 1 (Fig. 1B).
Figure 1.
CL001 study design (A) part 1 sequential, single-dose, dose-escalation, placebo-controlled, randomized study and (B) part 2 parallel-arm, placebo-controlled, randomized study.
2.1.1. Study population
Appendix Text 1, available online as supplemental digital content at http://links.lww.com/PR9/A16.
2.1.2. Study design
Patients underwent screening, completing electronic diary entries 4 times daily for 7 days before randomization. Eligible patients were required to discontinue all pain medication (cyclooxygenase [COX]-2 inhibitors, nonsteroidal anti-inflammatory drugs, and opioid analgesics) at least 14 days before tanezumab or placebo and for the study duration; aspirin ≤325 mg/d was allowed for cardiac prophylaxis (Appendix Text 2, available online as supplemental digital content at http://links.lww.com/PR9/A16).
In part 1, patients were assigned to 1 of 6 sequential dose cohorts (3, 10, 30, 100, 300, or 1000 μg/kg). Four patients received tanezumab in cohorts 1 to 3 and 6 patients in cohorts 4 to 6; 2 additional patients in each cohort were randomly assigned to placebo treatment. In part 2, patients were randomly assigned to receive tanezumab 100 μg/kg, tanezumab 300 μg/kg, or placebo (in a 1:1:1 ratio). No dosage modifications were allowed in either part of the study. Tanezumab or placebo was administered through slow IV injection over 3 to 5 minutes for doses of ≤10 μg/kg and through infusion at 100 mL/h for doses of ≥30 μg/kg on study day 1.
After discharge on day 2, patients were to return for study visits for safety and efficacy assessments, routine laboratory tests, and blood sampling. Patients in part 1 cohorts 1 to 3 (3, 10, or 30 μg/kg) visited on days 3, 4, 5, 7, 14, 21, and 28 (termination visit) with safety follow-up telephone calls on days 91 and 181. Patients in part 1 cohorts 4 and 5 and part 2 (100 or 300 μg/kg) visited on days 3, 4, 7, 14, 21, 28, 42, 56, 70, 91, 136, and 181 (termination visit). Patients in part 1 cohort 6 (1000 μg/kg) visited on days 3, 4, 7, 14, 21, 28, 42, 56, 70, 91, 136, 181, and 223 (termination visit).
2.1.3. Safety evaluations
Detailed queries on the nature, onset, duration, severity, outcome, and any relationship of events to study drug were made for all adverse events (AEs). Any serious AEs (SAEs) (such as those resulting in hospitalization or death, or life-threatening) were reported to the sponsor within 24 hours of investigator awareness. Safety assessments included physical and neurologic examinations, laboratory assessments, and 12-lead ECG. The Hopkins Verbal Learning Test–Revised4 was also conducted at screening, baseline (day 1), day 1 (at 6 hours postinfusion), and prespecified study visits. Patients receiving tanezumab 3 to 300 μg/kg or placebo were monitored for safety for at least 180 days; those receiving tanezumab 1000 μg/kg or placebo were monitored for at least 223 days.
2.1.4. Efficacy evaluations
The primary efficacy endpoint for parts 1 and 2 was the visual analogue scale (VAS) sum of pain intensity difference (SPID) for current pain in the index knee for days 2 to 14. Secondary endpoints were SPID for current pain in the index knee for other time points, SPID for walking knee pain, change from baseline in average Western Ontario and McMaster Universities Osteoarthritis (WOMAC) index scores, WOMAC subscale scores, and daily rescue medication use.3
Current index knee pain, index knee pain during walking, and WOMAC were evaluated using a validated electronic VAS (0–100 scale; higher scores denoted greater pain).25 Current knee pain was recorded 4 times daily and index knee pain during walking over the past 24 hours was recorded once daily. The WOMAC index consisted of 24 questions in 3 subscales: pain (5 questions), stiffness (2 questions), and physical function (17 questions),3 completed at office visits. Rescue medication use was recorded daily. Pain and WOMAC scores were recorded for 28, 181, or 223 days after injection for patients receiving tanezumab 3 to 30 μg/kg, 100 to 300 μg/kg, or 1000 μg/kg, respectively. At the time the studies were conducted (2004–2005), electronic diaries for recording pain and rescue medication use were relatively new, enabling real-time data capture in a naturalistic setting.
2.1.5. Statistical analysis
Appendix Text 3, available online as supplemental digital content at http://links.lww.com/PR9/A16.
2.2. Study CL002-tanezumab in bunionectomy
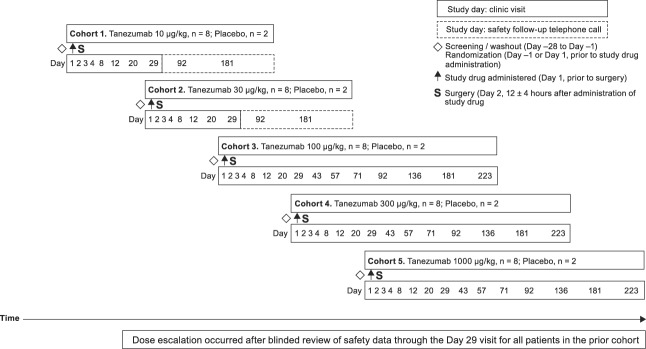
Study CL002 (original Rinat study number [Pfizer study number A4091007]) was a placebo-controlled dose-escalation (tanezumab 10–1000 μg/kg) study with a single administration of tanezumab in 5 cohorts of patients (Fig. 2). A planned tanezumab 2000-μg/kg dose cohort was not performed because of a high frequency of AEs of abnormal peripheral sensation among patients treated with tanezumab 1000 μg/kg.
Figure 2.
CL002 single-dose, dose-escalation, placebo-controlled, randomized study design.
2.2.1. Study population
Appendix Text 4, available online as supplemental digital content at http://links.lww.com/PR9/A16.
2.2.2. Study design
The study consisted of a screening period of up to 28 days (for discontinuation and washout of prohibited pain medications); research unit admission for eligible patients for study drug administration (day 1), surgery (day 2), and postoperative observations (days 1−4); outpatient follow-up (through day 29 for patients receiving tanezumab 10 and 30 μg/kg, through day 181 for patients receiving tanezumab 100 and 300 μg/kg, and through day 223 for patients receiving tanezumab 1000 μg/kg); and safety extension telephone contact (days 92 and 181 for patients receiving tanezumab 10 and 30 μg/kg) to assess potential late AEs. Postoperative study visits were conducted on days 8, 12, 20, and 29 for patients receiving tanezumab 10 and 30 μg/kg, continued on days 43, 57, 71, 92, 136, and 181 for patients receiving tanezumab 100 and 300 μg/kg, and through day 223 for patients receiving tanezumab 1000 μg/kg. Safety and efficacy assessments, routine laboratory tests, and blood samples were obtained during these visits.
Fifty eligible patients were randomized to receive tanezumab or placebo (vehicle) in a 4:1 design in 1 of 5 sequential dose cohorts (tanezumab 10, 30, 100, 300, and 1000 μg/kg). A randomization code list for assigning tanezumab or placebo was prepared by an unblinded statistician. Study drug or placebo was administered through slow IV injection over 3 to 5 minutes (cohort 1) or infused at 100 mL/h (cohorts 2–5), 8 to 16 hours before surgery; a timing based on previous observations in part 1 of CL001 and previous nonclinical studies.33
The surgical procedure consisted of primary unilateral first metatarsal bunionectomy, with or without internal fixation, and with no collateral procedures, performed under regional anesthesia with lidocaine (Mayo block), propofol sedation, and prophylactic antibiotic treatment. Patients were on bed rest for 48 hours and observed before discharge. No dosage modifications were allowed unless signs of an infusion reaction occurred. Any signs of an allergic reaction resulted in permanent cessation of infusion.
Patients were advised to refrain from rescue medication use until completion of the 4-hour postoperative pain assessments (Appendix Text 5, available online as supplemental digital content at http://links.lww.com/PR9/A16).
2.2.3. Safety evaluations
Observed or volunteered AEs, severity, and investigator's opinion of relationship to study treatment were recorded. Any SAE was reported to the sponsor within 24 hours of investigator awareness. Safety and tolerability assessments included physical and neurologic examinations, vital signs, laboratory assessments, infusion-related reactions, immunogenicity evaluations, 12-lead ECG, cardiac telemetry, and pulse oximetry during drug administration and surgery, wound healing, and postoperative radiograph of the foot. The Hopkins Verbal Learning Test–Revised was administered at screening, before study drug administration on day 1, and on days 12, 29, 92, 136, 181, and 223 depending on cohort assignment. All patients were monitored for safety at each protocol-specified postoperative visit.
2.2.4. Efficacy evaluations
The pharmacodynamic activity of tanezumab was evaluated by changes in postoperative pain compared with placebo. Efficacy endpoints were determined through 3 measures: a 4-point categorical (Likert) pain scale (none [0], mild [1], moderate [2], and severe [3]); a VAS pain scale ranging from 0 to 100 (higher scores denoted greater pain); and an 11-point numerical rating scale ranging from 0 (none) to 10 (worst imaginable) recorded pain during the preceding 24 hours. Other efficacy measures included Patient's Global Evaluation (PGE) of study medication (poor [0], fair [1], good [2], very good [3], and excellent [4]) and rescue medication use. Evaluations of pain intensity using the categorical pain scale and VAS were conducted at 30 minutes, 2, 4, 6, 8, 12, 16, 24, 28, 32, 36, 40, and 48 hours after completion of surgery, and at the time of rescue medication use. After discharge, patients recorded pain assessments and rescue medication use. Pain intensity was recorded 4 times daily for days 4 to 11 and once in the morning of day 12. Assessment for worst pain and least pain using the numerical rating scale was evaluated once daily on mornings of days 4 to 12. Daily PGE of study medication was evaluated once daily on mornings of days 3 to 12.
2.2.5. Statistical analysis
Appendix Text 6, available online as supplemental digital content at http://links.lww.com/PR9/A16.
3. Results
3.1. Safety
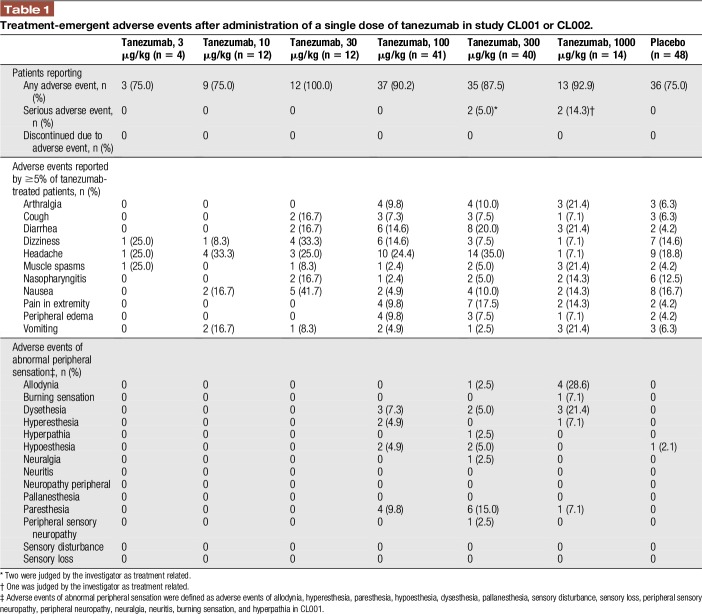
For studies CL001 and CL002 combined, the number of patients reporting AEs was generally similar across treatments, but incidence of treatment-related AEs was higher with tanezumab treatment than placebo (Table 1). Most AEs were mild or moderate and resolved before completion of the studies. No patients discontinued because of AEs, but 3 tanezumab patients (Study CL002) reported 4 SAEs considered related to treatment by the investigator: 3 events of convulsions in 2 patients receiving 300 μg/kg (2 convulsions were considered pseudoseizures and were reported by 1 patient) and 1 event of allodynia (1000 μg/kg) (Appendix Text 7, available online as supplemental digital content at http://links.lww.com/PR9/A16).
Table 1.
Treatment-emergent adverse events after administration of a single dose of tanezumab in study CL001 or CL002.
For both parts 1 and 2 of study CL001, the most frequent AEs in the tanezumab groups were headache and diarrhea. For study CL002, the most frequent AEs in the tanezumab groups were nausea, dizziness, and headache (Appendix Tables 1–3, available online as supplemental digital content at http://links.lww.com/PR9/A16).
Adverse events of abnormal peripheral sensation (such as dysesthesia, allodynia, paresthesia, and hyperesthesia) were more common in patients who had received tanezumab than placebo-treated patients (Appendix Text 8, available online as supplemental digital content at http://links.lww.com/PR9/A16).
3.2. Study CL001
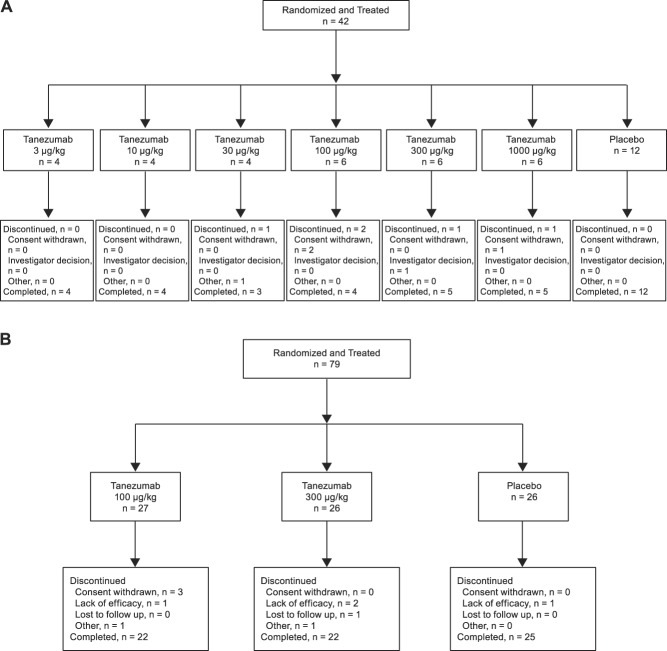
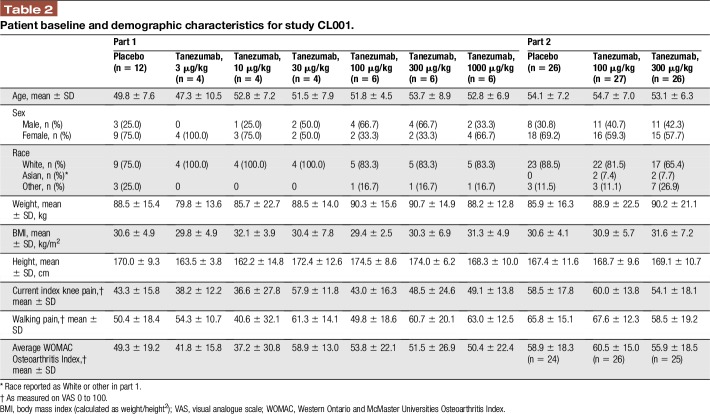
A total of 121 patients were screened for eligibility and assigned to treatment in study CL001 (N = 42 in part 1; N = 79 in part 2; Fig. 3A, B). Patients assigned to treatments received IV study medication and were included in the modified intention to treat analysis. Most patients completed the study (part 1: 83.3% of tanezumab-treated patients and 100% of placebo-treated patients; part 2: 83.0% of tanezumab-treated patients and 96.2% of placebo-treated patients). Patient demographics and baseline characteristics were similar across groups, although more females (61.9%) than males (38.1%) participated (Table 2).
Figure 3.
Patient disposition in study CL001 (A) part 1 and (B) part 2.
Table 2.
Patient baseline and demographic characteristics for study CL001.
3.2.1. Efficacy
3.2.1.1. Part 1
Mean daily current index knee pain showed an initial decrease from baseline in all tanezumab groups (Fig. 4). For days 2 to 14, differences in SPID for current knee pain from placebo were statistically significant (unadjusted P = 0.0093–0.0480) for tanezumab doses ≥30 μg/kg but not for doses <30 μg/kg (P > 0.05). For days 2 to 28, the 100-μg/kg dose was the only dose to result in statistically significant differences from placebo (unadjusted P = 0.0361), but all doses assessed over days 2 to 84 (100, 300, and 1000 μg/kg) resulted in statistically significant differences from placebo (unadjusted P = 0.0006–0.0079).
Figure 4.
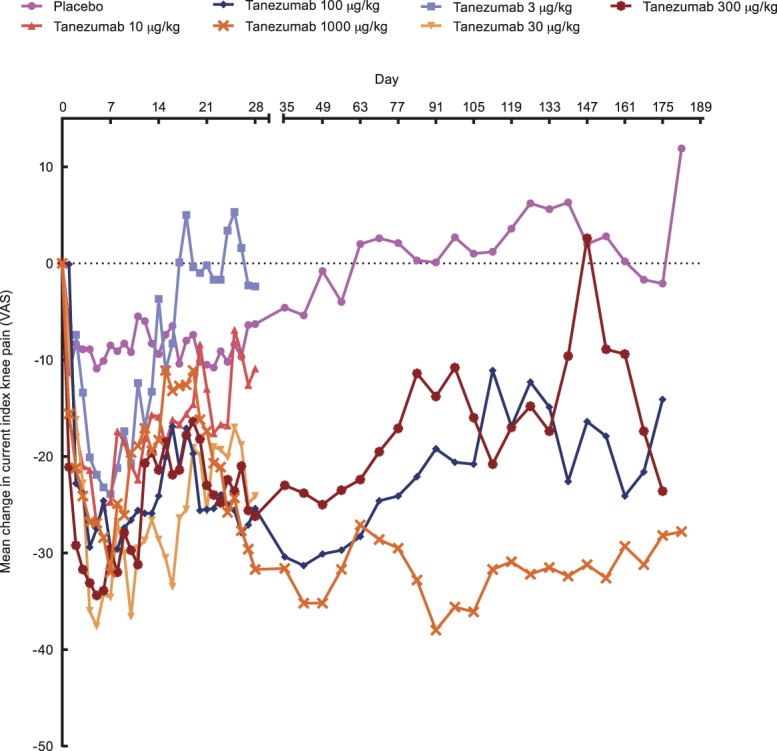
Current index knee pain: mean change from baseline visual analogue scale (VAS) in study CL001, part 1. Overall, knee pain was measured on VAS 0 to 100 where higher scores equal greater pain.
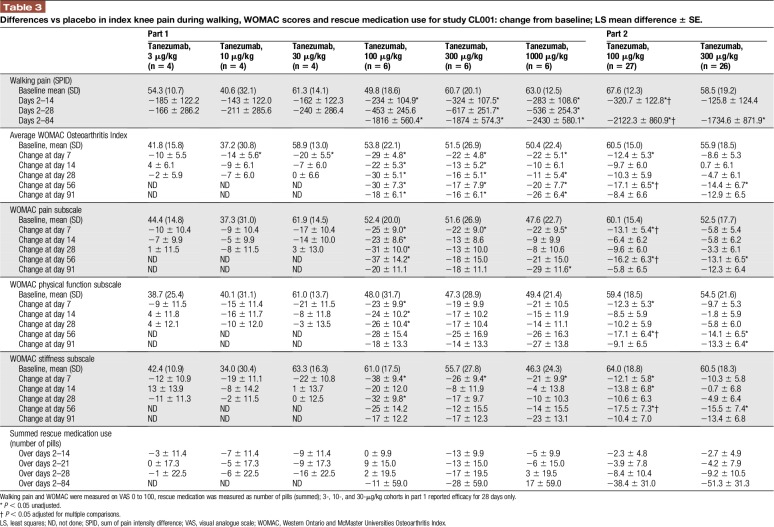
Knee pain during walking was reduced with tanezumab, with statistically significant differences from placebo for doses of ≥100 μg/kg (Table 3). The percentage of patients with ≥30% reduction in walking pain during days 2 to 84 were statistically greater with tanezumab 100, 300, or 1000 μg/kg (66.7%, 83.3%, and 83.3%, respectively, vs placebo (16.7%) P = 0.0062 to 0.0338; analysis not performed with tanezumab ≤30 μg/kg). Tanezumab 100 μg/kg resulted in statistically greater percentages of patients reporting ≥50% and ≥70% reduction in walking pain compared with placebo (tanezumab 100 μg/kg: 66.7% for both; placebo: 16.7% and 8.3%, respectively; P = 0.0338 and 0.0091).
Table 3.
Differences vs placebo in index knee pain during walking, WOMAC scores and rescue medication use for study CL001: change from baseline; LS mean difference ± SE.
Greater improvement in average WOMAC scores and subscales was noted with higher tanezumab doses (≥100 μg/kg; Table 3). Rescue medication use (number of pills taken) was similar across treatments with no difference vs placebo (unadjusted P = 1.000–0.1819).
3.2.1.2. Part 2
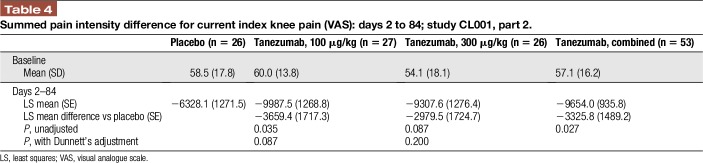
For the primary endpoint, SPID for VAS in current knee pain for days 2 to 14 for the combined treatment groups, no statistically significant difference was found vs placebo (P = 0.1416). Both doses of tanezumab led to greater reduction in current index knee pain vs placebo. When individual doses were compared with placebo, the least squares (LS) mean difference in SPID for current knee pain vs placebo with tanezumab 100 μg/kg was statistically significant for days 2 to 14 (−481.0; unadjusted P = 0.0355) and days 2 to 84 (−3659.4; unadjusted P = 0.0351), but not with tanezumab 300 μg/kg (days 2–14: −92.3, unadjusted P = 0.6854; days 2–84: −2979.5, unadjusted P = 0.0866, Table 4). Differences were not significant when adjusted for multiple comparisons.
Table 4.
Summed pain intensity difference for current index knee pain (VAS): days 2 to 84; study CL001, part 2.
For walking pain in the index knee, the LS mean difference vs placebo was statistically significant over days 2 to 14 with tanezumab 100 μg/kg (unadjusted P = 0.0101 and adjusted P = 0.0267; Table 3), but not for tanezumab 300 μg/kg (unadjusted P = 0.3138 and adjusted P = 0.6029). Over days 2 to 84, both doses of tanezumab resulted in statistically significant differences in LS mean walking pain vs placebo (tanezumab 100 μg/kg: unadjusted P = 0.0151 and adjusted P = 0.0391; tanezumab 300 μg/kg: unadjusted P = 0.0489 and adjusted P = 0.1184).
Single doses of tanezumab 100 μg/kg or tanezumab 300 μg/kg improved the average WOMAC score and all WOMAC subscale scores (Table 3). Statistically significant LS mean differences from placebo (unadjusted comparisons) were noted on day 7 for all WOMAC subscale scores and the average WOMAC index score with tanezumab 100 μg/kg (unadjusted P = 0.0163–0.0395), but not with tanezumab 300 μg/kg (unadjusted P > 0.05 for all). At day 56, LS mean differences from placebo were statistically significant with both doses (tanezumab 100 μg/kg: unadjusted P = 0.0083–0.0179 and adjusted P = 0.0214–0.0447; tanezumab 300 μg/kg: unadjusted P = 0.0330–0.0457 and adjusted P > 0.05 for all). Patients treated with tanezumab reported lower daily rescue medication use vs placebo group over days 2 to 84; this difference was not statistically significant (P > 0.05).
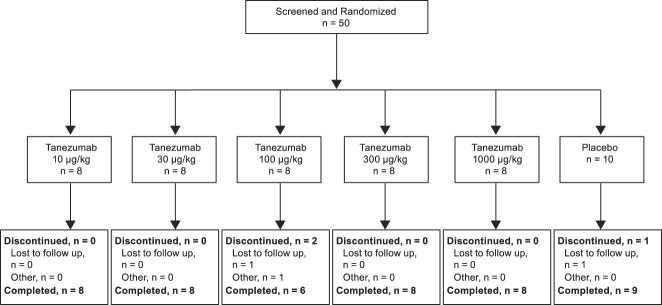
3.3. Study CL002
Fifty patients were screened for eligibility in study CL002 (Fig. 5). All fifty patients received IV study drug (or placebo) and were included in the modified intention to treat analysis set. Most completed the study (94.0%). Patient demographics and baseline characteristics were similar across groups, although more females (90.0%) than males (10.0%) participated (Table 5).
Figure 5.
Patient disposition in study CL002.
Table 5.
Patient baseline and demographic characteristics for study CL002.
3.3.1. Efficacy
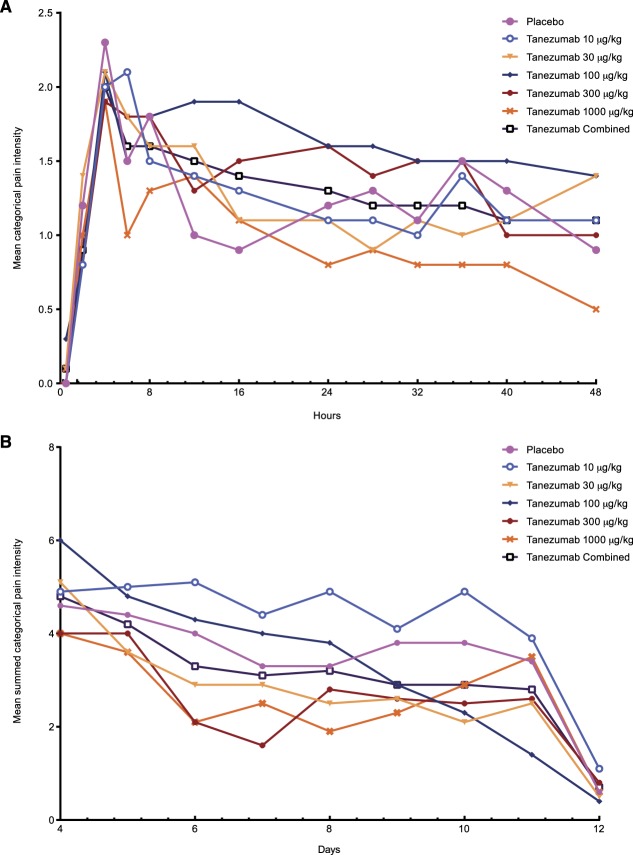
When tanezumab was administered 8 to 16 hours before bunionectomy, pain intensity was similar among all treatments and no difference between active (tanezumab) and placebo treatments was found, regardless of the pain measurement scale used (Fig. 6). No dose response was noted and no significant differences in the PGE were found.
Figure 6.
(A) Mean categorical pain intensity (0–48 hours) and (B) summed categorical pain intensity (days 4–12) after bunionectomy in study CL002.
The time to first rescue medication use, number of patients in each treatment who required rescue medication, number of days patients reported that they did not use rescue medication, and number of times patients used them were similar across treatments. Reported pain intensity at the time of first rescue medication use was not different across treatments.
4. Discussion
These first-in-human dosing studies provided support for the anti-NGF monoclonal antibody tanezumab as a treatment for chronic pain and justify further clinical studies. In both parts of study CL001, a single IV infusion of tanezumab reduced chronic OA pain and provided analgesic activity, with statistically significant results in secondary endpoints at the highest doses. This statistical significance was noted, despite a relatively small number of patients, suggesting tanezumab has robust clinical efficacy in OA. Numeric improvements in OA pain and function were also noted in tanezumab doses ≤30 μg/kg, although no statistical differences were seen. This may reflect low numbers or the shorter follow-up period; tanezumab at 30 μg/kg or lower in subsequent, larger studies with longer follow-up resulted in significant improvements in pain, function, and global assessments in hip or knee OA.2,8,9,14,25,27,31,32,34 Comparing the results from later studies using a fixed-dose regimen with the current studies (using a dosing regimen adjusted for body weight), tanezumab 100 μg/kg is approximately equivalent to 10 mg as a fixed dose.21
Infusion of tanezumab 8 to 16 hours before bunionectomy surgery did not result in significant efficacy. A number of factors may explain the apparent lack of efficacy in this acute pain condition. The presence of significant pain in the control group of study CL002 was of short duration and the surgery produced a small pain signal. Furthermore, it may take longer than the 8 to 16 hours for NGF to become a significant factor in the generation of an acute pain signal. In study CL001, maximal efficacy of tanezumab was achieved several days after administration. Thus, administration of tanezumab on the day before surgery in CL002 may have been too close to the time of surgery to result in a significant reduction in postsurgical pain, especially given the relatively rapid reduction of pain with placebo. In addition, baseline pain was reduced in some tanezumab-treated patients leading to further confounding. As a result of the limited size of this study and its design, tanezumab cannot be ruled out as a possible treatment for acute pain when administered under different conditions.
In both studies, tanezumab generally exhibited good safety and tolerability. No alterations in memory status, assessed by the Hopkins Verbal Learning Test–Revised scores were observed with tanezumab treatment, consistent with other studies.22,25,27,32 The overall rate for AEs was similar in tanezumab and placebo groups. In addition, no complications of anesthesia were noted during bunionectomy. In addition, tanezumab did not impact the safety and tolerability of opioid analgesics used after bunionectomy.
All patients healed normally after bunionectomy. Similarly, in animal models of pain, anti-NGF therapy resulted in reduction of pain behaviors, but did not impact normal bone healing in mice or rats after bone fracture.20,24,30
Adverse events such as dysesthesia, allodynia, paresthesia, and hyperesthesia were seen with the highest doses of tanezumab (≥100 μg/kg), generally mild to moderate in severity, initially observed by week 2 after infusion, and resolved by week 4. The estimated half-life of tanezumab is approximately 21 days in humans.19,21 Thus, AEs of abnormal peripheral sensation resolved, regardless of high tanezumab concentrations during weeks 2 to 4. Although tanezumab 1000 μg/kg was associated with more frequent occurrence of AEs, a larger proportion being rated as severe, and a higher rate of AEs of abnormal peripheral sensation (5 of 10 patients in CL002), none of the AEs were irreversible or led to disability. In addition, although review of all data for both studies led to the recommendation that enrollment could continue, a protocol amendment included the termination of enrollment in CL001 part 2 because of the reporting of 2 unexpected SAEs in CL002, namely pseudoseizure and seizure—although these SAEs were determined to be unrelated to study treatment. Over the course of both studies, the occurrence of AEs did not reach safety stop criteria (defined as 2 patients in a cohort experiencing grade 3 or 4 toxicities, SAEs, grade 2 or higher peripheral neuropathy, wound dehiscence, or untoward events during anesthesia). No maximum tolerable dose was established.
These studies provide guidance for further clinical development of tanezumab. The 1000-μg/kg dose was associated with a high frequency of dysesthesia; therefore, subsequent studies have since used doses lower than 1000 μg/kg. Because of the statistically significant improvements in pain and function seen in patients with OA, a longer, phase 2 dose-ranging trial was conducted in moderate to severe OA of the knee.25 The results of that study, and its open-label extension with repeated dosing, indicated that tanezumab infusion results in improvements in pain and function with statistical and clinical significance and few safety concerns.25,32 The results of the phase 1 studies reported here also provided direction for the use of tanezumab as a treatment for other pain conditions. Phase 2 trials of tanezumab resulted in demonstration of proof of concept with significant improvement in painful diabetic neuropathy and chronic low back pain.7,17,22,23
The clinical development of tanezumab is focused on the treatment of chronic pain because of the lack of significant efficacy in the relief of acute pain reported here. Although tanezumab administered on the day before the surgery did not result in significant reduction in pain intensity, it may be possible that tanezumab could be used for the treatment or prevention of postoperative pain if it is sustained or administration occurs earlier. Alternatively, tanezumab may have a role in the treatment of acute pain during rehabilitation. In addition, it has been suggested that tanezumab may provide effective reduction of skeletal pain resulting from trauma.10 Nerve growth factor–responsive neurons innervating tissue are necessary (but not sufficient) for a pain state to respond to tanezumab. For tanezumab treatment to be effective, the pain state must also be, to some extent, dependent on NGF signaling through those fibers. There are likely situations in which NGF signaling is not the relevant pathway that causes the aberrant pain state.26 Further investigation into these areas is warranted.
These first-in-human studies of tanezumab in chronic and acute pain demonstrated the safety and tolerability of this anti-NGF monoclonal antibody. Clinical efficacy was shown in patients with chronic pain but not in patients with acute pain. These studies have guided the clinical development of tanezumab. Trials of longer duration in larger populations should fully elucidate the safety, tolerability, efficacy, and clinical potential.
Disclosures
P.A. Walicke, F. Hefti, R. Bales, S.-P. Lu, and D.L. Shelton were employees of Rinat Neuroscience at the time of the study. J.L. Ruckle was Medical Director at Radiant Research at the time of the study; and is currently at Pacific Pharma Group LLC. M.T. Brown and C.R. West are employees of Pfizer and hold stock and/or stock options in Pfizer.
These studies were funded by Rinat Neuroscience, which was acquired by Pfizer in April 2006. Editorial support was provided by Penny Gorringe, MSc, and Joseph Oleynek, of Engage Scientific Solutions and funded by Pfizer.
Acknowledgments
The authors acknowledge the guidance of Arnon Rosenthal throughout the early development of tanezumab.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A16.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
References
- [1].Abdiche YN, Malashock DS, Pons J. Probing the binding mechanism and affinity of tanezumab, a recombinant humanized anti-NGF monoclonal antibody, using a repertoire of biosensors. Protein Sci 2008;17:1326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Balanescu AR, Feist E, Wolfram G, Davignon I, Smith MD, Brown MT, West CR. Efficacy and safety of tanezumab added on to diclofenac sustained release in patients with knee or hip osteoarthritis: a double-blind, placebo-controlled, parallel-group, multicentre phase III randomised clinical trial. Ann Rheum Dis 2014;73:1665–72. [DOI] [PubMed] [Google Scholar]
- [3].Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833–40. [PubMed] [Google Scholar]
- [4].Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test—revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol 1998;12:43–55. [Google Scholar]
- [5].Bensen WG, Fiechtner JJ, McMillen JI, Zhao WW, Yu SS, Woods EM, Hubbard RC, Isakson PC, Verburg KM, Geis GS. Treatment of osteoarthritis with celecoxib, a cyclooxygenase-2 inhibitor: a randomized controlled trial. Mayo Clin Proc 1999;74:1095–105. [DOI] [PubMed] [Google Scholar]
- [6].Borenstein DG. Beginning the treatment of musculoskeletal pain. Postgrad Med 2002;112(1 suppl):8–12. [DOI] [PubMed] [Google Scholar]
- [7].Bramson C, Herrmann DN, Carey W, Keller D, Brown MT, West CR, Verburg KM, Dyck PJ. Exploring the role of tanezumab as a novel treatment for the relief of neuropathic pain. Pain Med 2015;16:1163–76. [DOI] [PubMed] [Google Scholar]
- [8].Brown MT, Murphy FT, Radin DM, Davignon I, Smith MD, West CR. Tanezumab reduces osteoarthritic knee pain: results of a randomized, double-blind, placebo-controlled phase III trial. J Pain 2012;13:790–8. [DOI] [PubMed] [Google Scholar]
- [9].Brown MT, Murphy FT, Radin DM, Davignon I, Smith MD, West CR. Tanezumab reduces osteoarthritic hip pain: results of a randomized, double-blind, placebo-controlled phase III trial. Arthritis Rheum 2013;65:1795–803. [DOI] [PubMed] [Google Scholar]
- [10].Cattaneo A. Tanezumab, a recombinant humanized mAb against nerve growth factor for the treatment of acute and chronic pain. Curr Opin Mol Ther 2010;12:94–106. [PubMed] [Google Scholar]
- [11].Chou R, Atlas SJ, Stanos SP, Rosenquist RW. Nonsurgical interventional therapies for low back pain: a review of the evidence for an American Pain Society clinical practice guideline. Spine 2009;34:1078–93. [DOI] [PubMed] [Google Scholar]
- [12].Chou R, Loeser JD, Owens DK, Rosenquist RW, Atlas SJ, Baisden J, Carragee EJ, Grabois M, Murphy DR, Resnick DK, Stanos SP, Shaffer WO, Wall EM. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine 2009;34:1066–77. [DOI] [PubMed] [Google Scholar]
- [13].Desjardins PJ, Shu VS, Recker DP, Verburg KM, Woolf CJ. A single preoperative oral dose of valdecoxib, a new cyclooxygenase-2 specific inhibitor, relieves post-oral surgery or bunionectomy pain. Anesthesiology 2002;97:565–73. [DOI] [PubMed] [Google Scholar]
- [14].Ekman EF, Gimbel JS, Bello AE, Smith MD, Keller DS, Annis KM, Brown MT, West CR, Verburg KM. Efficacy and safety of intravenous tanezumab for the symptomatic treatment of osteoarthritis: 2 randomized controlled trials versus naproxen. J Rheumatol 2014;41:2249–59. [DOI] [PubMed] [Google Scholar]
- [15].Frade JM, Barde YA. Nerve growth factor: two receptors, multiple functions. Bioessays 1998;20:137–45. [DOI] [PubMed] [Google Scholar]
- [16].Gan TJ, Habib AS, Miller TE, White W, Apfelbaum JL. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin 2014;30:149–60. [DOI] [PubMed] [Google Scholar]
- [17].Gimbel JS, Kivitz AJ, Bramson C, Nemeth MA, Keller DS, Brown MT, West CR, Verburg KM. Long-term safety and effectiveness of tanezumab as treatment for chronic low back pain. PAIN 2014;155:1793–801. [DOI] [PubMed] [Google Scholar]
- [18].Hefti FF, Rosenthal A, Walicke PA, Wyatt S, Vergara G, Shelton DL, Davies AM. Novel class of pain drugs based on antagonism of NGF. Trends Pharmacol Sci 2006;27:85–91. [DOI] [PubMed] [Google Scholar]
- [19].Hurst SI, Arends R, Hughey DB, Vergara G, Shelton D, Liras J. Interspecies scaling of tanezumab, an anti-NGF antibody, and comparison of the projected human pharmacokinetic parameters to single-dose clinical data. Presented at: Annual Scientific Meeting of the American Association of Pharmaceutical Scientists (AAPS); November 8–12, 2009; Los Angeles, CA. Available at: http://abstracts.aaps.org/published/. Accessed December 18, 2014.
- [20].Jimenez-Andrade JM, Martin CD, Koewler NJ, Freeman KT, Sullivan LJ, Halvorson KG, Barthold CM, Peters CM, Buus RJ, Ghilardi JR, Lewis JL, Kuskowski MA, Mantyh PW. Nerve growth factor sequestering therapy attenuates non-malignant skeletal pain following fracture. PAIN 2007;133:183–96. [DOI] [PubMed] [Google Scholar]
- [21].Jonsson EN, Xie R, Marshall SF, Arends RH. Population pharmacokinetics of tanezumab in phase 3 clinical trials for osteoarthritis pain. Br J Clin Pharmacol 2016;81:688–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Katz N, Borenstein DG, Birbara C, Bramson C, Nemeth MA, Smith MD, Brown MT. Efficacy and safety of tanezumab in the treatment of chronic low back pain. PAIN 2011;152:2248–58. [DOI] [PubMed] [Google Scholar]
- [23].Kivitz AJ, Gimbel JS, Bramson C, Nemeth MA, Keller DS, Brown MT, West CR, Verburg KM. Efficacy and safety of tanezumab versus naproxen in the treatment of chronic low back pain. PAIN 2013;154:1009–21. [DOI] [PubMed] [Google Scholar]
- [24].Koewler NJ, Freeman KT, Buus RJ, Herrera MB, Jimenez-Andrade JM, Ghilardi JR, Peters CM, Sullivan LJ, Kuskowski MA, Lewis JL, Mantyh PW. Effects of a monoclonal antibody raised against nerve growth factor on skeletal pain and bone healing after fracture of the C57BL/6J mouse femur. J Bone Miner Res 2007;22:1732–42. [DOI] [PubMed] [Google Scholar]
- [25].Lane NE, Schnitzer TJ, Birbara CA, Mokhtarani M, Shelton DL, Smith MD, Brown MT. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med 2010;363:1521–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mantyh PW, Koltzenburg M, Mendell LM, Tive L, Shelton DL. Antagonism of nerve growth factor-TrkA signaling and the relief of pain. Anesthesiology 2011;115:189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nagashima H, Suzuki M, Araki S, Yamabe T, Muto C. Preliminary assessment of the safety and efficacy of tanezumab in Japanese patients with moderate to severe osteoarthritis of the knee: a randomized, double-blind, dose-escalation, placebo-controlled study. Osteoarthritis Cartilage 2011;19:1405–12. [DOI] [PubMed] [Google Scholar]
- [28].Pollak R, Raymond GA, Jay RM, Hillstrom HJ, Mahan KT, Riff D, Jacobs EL, Brown MT, Verburg KM. Analgesic efficacy of valdecoxib for acute postoperative pain after bunionectomy. J Am Podiatr Med Assoc 2006;96:393–407. [DOI] [PubMed] [Google Scholar]
- [29].Roelofs PD, Deyo RA, Koes BW, Scholten RJ, van Tulder MW. Non-steroidal anti-inflammatory drugs for low back pain. Cochrane Database Syst Rev 2008:CD000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sabsovich I, Wei T, Guo TZ, Zhao R, Shi X, Li X, Yeomans DC, Klyukinov M, Kingery WS, Clark JD. Effect of anti-NGF antibodies in a rat tibia fracture model of complex regional pain syndrome type I. PAIN 2008;138:47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Schnitzer TJ, Ekman EF, Spierings EL, Greenberg HS, Smith MD, Brown MT, West CR, Verburg KM. Efficacy and safety of tanezumab monotherapy or combined with non-steroidal anti-inflammatory drugs in the treatment of knee or hip osteoarthritis pain. Ann Rheum Dis 2015;74:1202–11. [DOI] [PubMed] [Google Scholar]
- [32].Schnitzer TJ, Lane NE, Birbara C, Smith MD, Simpson SL, Brown MT. Long-term open-label study of tanezumab for moderate to severe osteoarthritic knee pain. Osteoarthritis Cartilage 2011;19:639–46. [DOI] [PubMed] [Google Scholar]
- [33].Shelton DL, Vergara G, Pons J, Ho WH, Stratton J. Effect of PF04383119 on post-incisional pain following IV or IP administration. Anesthesiology 2008;109:A1539. [Google Scholar]
- [34].Spierings EL, Fidelholtz J, Wolfram G, Smith MD, Brown MT, West CR. A phase III placebo- and oxycodone-controlled study of tanezumab in adults with osteoarthritis pain of the hip or knee. PAIN 2013;145:1603–12. [DOI] [PubMed] [Google Scholar]
- [35].Trescot AM, Helm S, Hansen H, Benyamin R, Glaser SE, Adlaka R, Patel S, Manchikanti L. Opioids in the management of chronic non-cancer pain: an update of American Society of the interventional pain Physicians' (ASIPP) guidelines. Pain Physician 2008;11(2 suppl):S5–62. [PubMed] [Google Scholar]
- [36].Watson JJ, Allen SJ, Dawbarn D. Targeting nerve growth factor in pain: what is the therapeutic potential? BioDrugs 2008;22:349–59. [DOI] [PubMed] [Google Scholar]
- [37].Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, Dougados M, Hochberg M, Hunter DJ, Kwoh K, Lohmander LS, Tugwell P. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage 2008;16:137–62. [DOI] [PubMed] [Google Scholar]