Supplemental Digital Content is available in the text.
Abstract
Background:
Both synthetic and bioprosthetic meshes play important roles in surgical procedures such as ventral hernia repair. Although sometimes used interchangeably, these devices have inherently different properties. We therefore sought to better understand how these materials interact with the host environment to optimize surgical techniques and to improve outcomes.
Methods:
Synthetic mesh (polypropylene, Prolene) or bioprosthetic mesh (acellular fetal/neonatal bovine dermis, SurgiMend) was implanted intraperitoneally into rats lateral to a ventral incision in a novel intra-abdominal implant model. Two variables were modified with each material: (1) tight or loose tissue apposition, altered by modifying suture placement; and (2) abdominal wall injury, altered by selective abrasion of the peritoneal lining. After 5 weeks, the meshes and abdominal wall were evaluated grossly and histologically. The analysis focused on the degree of inflammatory response, neovascularization, and mesh adherence to the surrounding tissues.
Results:
Synthetic mesh adhered to the abdominal wall and visceral organs, regardless of the degree of apposition or tissue injury, due to a foreign body–mediated inflammatory reaction. In areas of noninjured peritoneal lining, SurgiMend was adherent peri-suture. Neovascularization entered the mesh from these apposition points and spread outward. In areas of denuded peritoneal lining, the adherent and vascularized areas were significantly greater and not merely coincident with suture placement.
Conclusions:
The inflammatory and wound healing responses with bioprosthetic mesh seem fundamentally different from synthetic mesh. Understanding these differences may lead to varied outcomes in adherence and vascularization of the materials, and ultimately the efficacy of hernia repair. Additionally, these differences highlight the need for further basic research to optimize mesh selection for surgical technique.
INTRODUCTION
Biomaterials derived from processed extracellular matrix, commonly referred to as “biologic or bioprosthetic meshes,” are widely used in clinical surgical practice. Their uses include tissue repair and reinforcement in implant-based breast reconstruction, hernia repair, tendon augmentation, dural repair, and wound healing.1 Following implantation, these materials exhibit different properties from one another, and from the numerous antecedent synthetic mesh materials.1–3 However, many surgeons will simply substitute bioprosthetic meshes for synthetic meshes, using the same surgical repair techniques established and optimized for synthetic mesh, but with varied outcomes.4,5
This tenant is especially true in hernia repair. Whereas some synthetic and bioprosthetic materials have demonstrated clear success in reinforcement of hernia repairs,4 others have been associated with high rates of hernia recurrence.5 Factors contributing to this variation in clinical outcome may include the type of mesh,4 the surgical technique,6,7 and patient comorbidities.8 Mesh type and surgical techniques are interdependent, with changes to one possibly affecting the other. Therefore, a better understanding of the unique postimplantation characteristics of these materials may improve clinical outcomes, by selecting for techniques and surgical approaches that are optimized to them.
SurgiMend (Integra LifeSciences) is an acellular scaffold derived from fetal or neonatal bovine dermis, and originally developed as a matrix or scaffold for tissue engineering.1 SurgiMend (Integra LifeSciences) has been used successfully in large abdominal wall reconstructions, in addition to other clinical applications.4,7,9,10 Small animal models that simulate ventral hernia repair, with SurgiMend (Integra LifeSciences) placed as an underlay, have demonstrated the persistence of a vascularized, dense fibrous connective tissue and a lack of bowel adhesions, despite direct contact with healthy, uninjured bowel.11,12 Whereas the SurgiMend (Integra LifeSciences) was adherent to the peritoneal surface of the anterior abdominal wall in areas of injury or suture placement, regions extending peripherally were not.11 This is consistent with the author’s (D.M.A.) clinical experience where similar observations have been made under computed tomography imaging conducted as part of routine surveillance for cancer recurrence (Fig. 1). Regardless of other parameters, synthetic polymer meshes become adherent by “scarring into place” following a well-characterized foreign body encapsulation response. A lack of foreign body reaction to SurgiMend (Integra LifeSciences) has been demonstrated previously.1,12
Fig. 1.
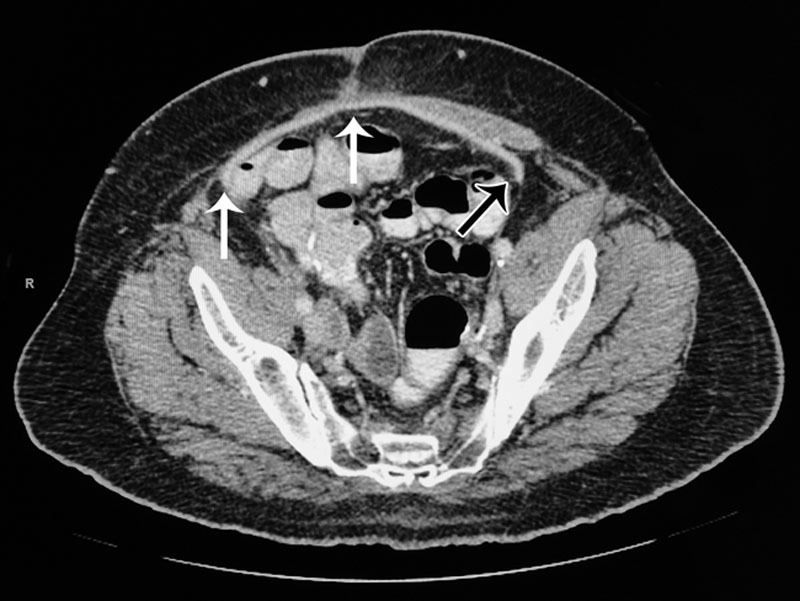
CT image of a 66-year-old male with a history of advanced prostate cancer requiring pelvic exenteration that was reconstructed with a right rectus abdominis muscle flap, and then developed EC fistulae postoperatively. One year after exenteration, the patient underwent repair of the fistulae and an abdominal wall reconstruction consisting of left-sided anterior component separation and underlay reinforcement with SurgiMend 4.0 (Integra LifeSciences) (white arrows). The image was taken 4 months postoperatively where a region of the SurgiMend (Integra LifeSciences) distal to the suture line on the patient’s left side seems not to be adherent to the abdominal wall (black arrow). CT indicates computed tomography.
The goal of this study is to improve our understanding of bioprosthetic material behavior following implantation to optimize surgical techniques and outcomes. For example, most hernia repairs fail at the musculotendinous junction, which changes over time as the materials remodel at that interface following implantation.13 From previous human clinical and animal model experience, we suspect that adherence and revascularization between SurgiMend (Integra LifeSciences) and surrounding tissues are linked to close apposition with each other and/or association with injured healing areas. We hypothesize that in the absence of a classic foreign body response, adherence, revascularization, and cell repopulation are modulated by localized injury and tissue apposition. To test these variables, a novel intra-abdominal implant (IAI) model was created and characterized.
METHODS
IAI Model and Study Design
The IAI model is not a hernia repair but rather consists of 2 cm × 2 cm2 of material implanted intra-abdominally, lateral to a midline incision (Fig. 2). A total of 4 conditions were investigated: (1) limited mesh apposition versus (2) tight mesh apposition; and (3) uninjured versus (4) injured peritoneum (Fig. 2). Mesh apposition was controlled using prolene sutures: 1 central suture for limited apposition or 4 corner sutures for tight apposition, against the anterior abdominal wall. Injury was performed by surgical abrasion of the peritoneal lining. A single, 5-week time point was investigated for each condition. Acellular neonatal bovine dermis (SurgiMend 2.0; Integra Life Sciences, NJ) or a control polypropylene mesh (Prolene; Ethicon, Sommerville, NJ) was implanted. Conditions were repeated in replicates of 5 for SurgiMend (Integra LifeSciences) and 3 for the Prolene (Ethicon) control.
Fig. 2.
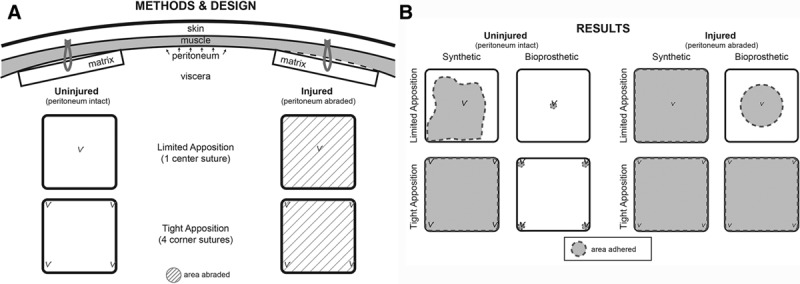
A, Study design for implantation of mesh into rat peritoneum. Through a vertical midline incision, 2 pieces of mesh were placed within each animal. Care was taken in avoid any suture remaining intraperitoneally to avoid adhesion and/or vascular contributions from the viscera. The peritoneum was sharply abraded where indicated. Animals were evaluated after 5 weeks. B, Schematic of Results. The synthetic mesh was typically adherent under any condition. The bioprosthetic mesh was adherent only at the areas of suture fixation when peritoneal lining was left intact, whereas adherence was significantly greater in the areas of denuded lining.
Animals
Adult, male, Sprague-Dawley rats were obtained from Charles River Laboratories (Wilmington, MA) weighing between 245 and 300 g. A total of 16 animals were used in this study. Two identical implants were placed per animal, in a pairwise comparison of injured versus uninjured abdominal wall, using the surgical techniques outlined below. Following surgery, animals were housed in individual cages, monitored daily, and fed ad libitum.
Surgical Techniques
Each animal was anesthetized with isoflurane. The animal was placed supine, its abdomen shaved, and prepped in povidone iodine. A ventral midline incision was made, and skin flaps were elevated laterally to expose the myofascial abdominal wall. A 4-cm incision was made through the linea alba to enter the peritoneum. On one side of each animal, a 2 cm × 2 cm2 was marked and the peritoneal lining was sharply abraded. The contralateral side was left uninjured. Two squares of hydrated mesh were inserted and sutured to the areas of abrasion or noninjury. Sutures (4-0 Prolene; Ethicon, Sommerville, NJ) were placed in either the center or the 4 corners of each implanted mesh. Sutures were placed transmyofascially through a partial thickness of the SurgiMend (Integra LifeSciences), with knots tied on the subcutaneous plane, to minimize exposure of the suture to the intraperitoneal contents. The midline abdominal muscle and skin were closed in layers with interrupted prolene suture and skin staples, respectively. Skin staples were removed after 7 days.
Tissue Harvest
Five weeks after implantation, animals were euthanized and the implant sites were photographed. Each implant was characterized as either adherent or nonadherent to the peritoneal surface, based on the percentage of the implant surface that could not easily be lifted from the abdominal wall. Categories of adherence included the following: less than 25%, between 25% and 75%, and greater than 75%. The composite of implant, muscle, and skin was lastly excised and placed into 10% buffered formalin for histological analysis.
Histology
After 1 week of fixation in 10% buffered formalin, specimens were bisected diagonally. The specimens were placed into cassettes, dehydrated, paraffin embedded, sectioned 5 microns thick, and stained with hematoxylin and eosin.
Statistics
Results were analyzed by contingency tables using two-tailed Fisher’s exact test because the sample sizes were small (N ≤ 5). Differences between categorical variables were considered significant if P < 0.05. Calculations were performed using statistical analysis tools provided by In-Silico (Hamburg, Germany).
RESULTS
Adherence with Synthetic Mesh
Synthetic mesh implanted against the peritoneal surface was explanted at 5 weeks, and the results were summarized in Table 1. In a majority of the explants, regardless of the condition, the synthetic mesh was adherent to the abdominal wall in >75% of the total surface area. With limited apposition, some areas of the synthetic mesh had curled away from the abdominal wall and were instead adherent to other internal organs (Fig. 3,). Scar tissue had grown through the large pores of the Prolene (Ethicon) mesh, encapsulating the implant (Fig. 3). With tight apposition, the synthetic mesh was not only adherent to the abdominal wall but also often embedded within the muscle itself (Fig. 4). Adhesions to the omentum and bowel were routinely observed with synthetic mesh, but not with bioprosthetic mesh (Table 1).
Table 1.
Summary of Adherent Area and Bowel Adhesions with Bioprosthetic or Synthetic Mesh
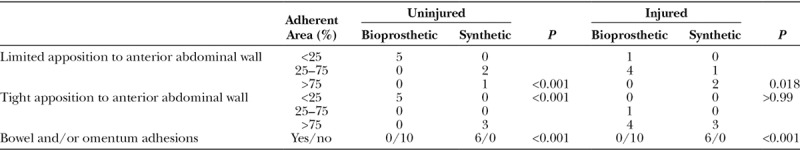
Fig. 3.
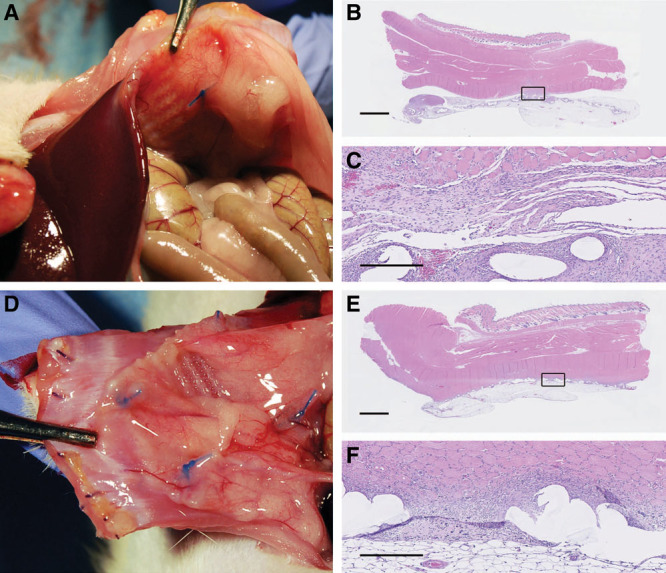
Synthetic mesh with limited apposition was generally adherent to the abdominal wall, and to often to other visceral organs: limited apposition, no injury (A), and limited apposition, with injury (D). Histologically, a classic foreign body inflammatory response was noted, often extending into the muscle, and encapsulating the synthetic plastic fibers of the mesh: limited apposition, no injury (B, C), and limited apposition, with injury (E, F). Black squares in images B and E are magnified and shown in images C and F, respectively. Scale bars = 2.5 mm in B and E, 500 µm in E and F.
Fig. 4.
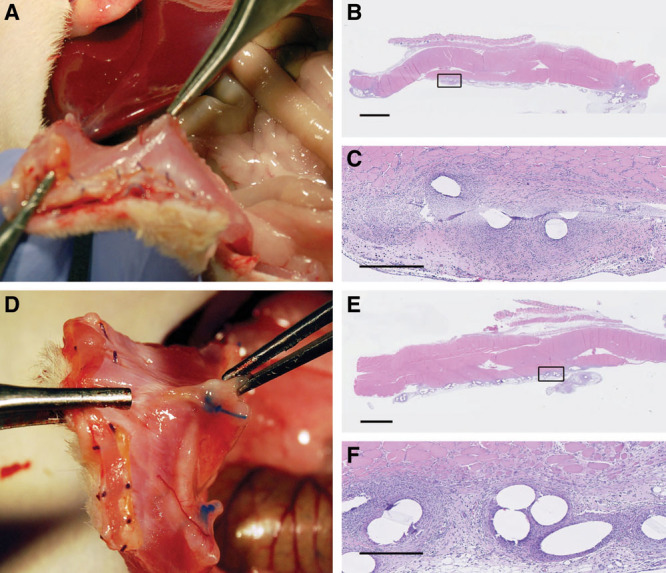
Synthetic mesh with tight apposition was adherent to the abdominal wall and often embedded into the abdominal muscle regardless of injury: tight apposition, no injury (A), and tight apposition, with injury (D). Histologically, a classic foreign body inflammatory response was noted, extending into the muscle, and encapsulating the synthetic plastic fibers of the mesh: tight apposition, no injury (B, C), and tight apposition, with injury (E, F). Black squares in images B and E are magnified and shown in images C and F, respectively. Scale bars = 2.5 mm in B and E, 500 µm in E and F.
Adherence with Bioprosthetic Mesh
The implanted bioprosthetic mesh was explanted at 5 weeks, and the results were summarized in Table 1. Without injury to the peritoneum, adherence of the bioprosthetic to the abdominal wall was focused at the sutures. In limited apposition, an area of attachment and vascularization originated from the center (Fig. 5); with tight apposition, this attachment/vascularization occurred in the corner regions (Fig. 6,) (see video, Supplemental Digital Content 1, This video is available in the “Related Videos” section of the Full-Text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A699). In conditions of injury, the area of tissue adherence increased significantly (Table 1). With injury but limited apposition, this adherence traversed about 50% of the mesh surface area, but never extended completely to the edge of the implant. With injury and tight apposition, the entire surface of the matrix became adherent to the abdominal wall. No bowel or omental adhesions were observed to the bioprosthetic mesh, and this was statistically significant when compared with synthetic mesh (Table 1). These results are summarized graphically in Figure 2.
Fig. 5.
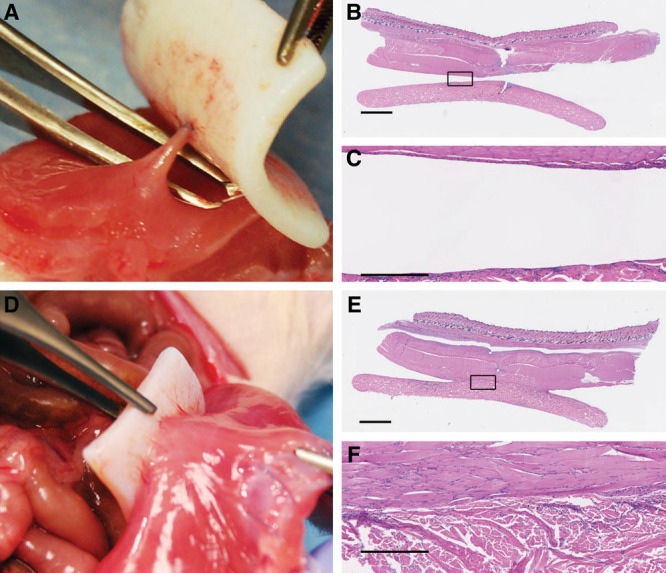
Bioprosthetic mesh in limited apposition without injury (A) was adherent only in an area adjacent to the suture. Revascularization originated from this location of injury as well. With injury and limited apposition, the area of attachment extended laterally, but did not extend all the way to the edges (D), suggesting a competition between repair of the peritoneal lining and host tissue ingrowth from the muscle. Histologically, the bioprosthetic was repopulated with host cells, but without indications of a classic foreign body response, including a lack of foreign body giant cells or encapsulation: limited apposition, no injury (B, C), and limited apposition, with injury (E, F). In areas remaining in contact, the muscle is directly against the bioprosthetic without encapsulation or significant inflammation. Black squares in images B and E are magnified and shown in images C and F, respectively. Scale bars = 2.5 mm in B and E, 500 µm in E and F.
Fig. 6.
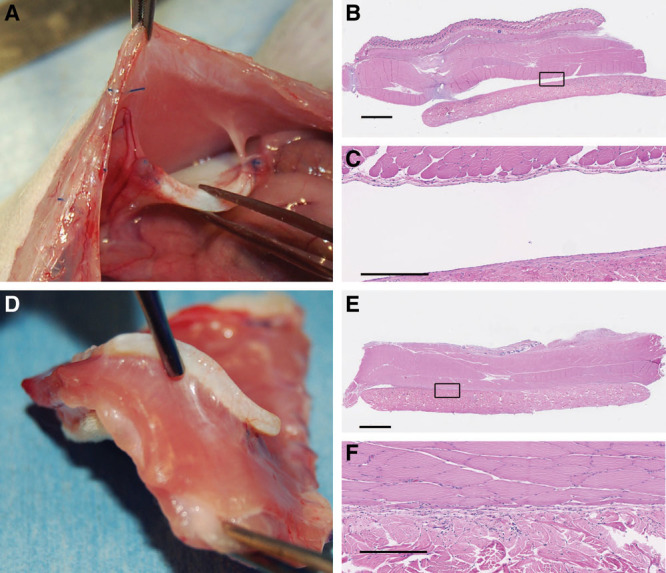
Bioprosthetic mesh in tight apposition with uninjured (A) and injured (D) abdominal muscle. Adherence is limited to the perisuture area without injury (A), but the bioprosthetic is totally adherence across the surface with in tight apposition with injured muscle (D). Histologically, the bioprosthetic was repopulated with host cells, but without indications of a classic foreign body response, including a lack of foreign body giant cells or encapsulation: tight apposition, no injury (B, C), and tight apposition, with injury (E, F). In areas remaining in contact with injured muscle, the muscle is directly against the bioprosthetic without encapsulation or significant inflammation. Black squares in images B and E are magnified and shown in images C and F, respectively. Scale bars = 2.5 mm in B and E, 500 µm in E and F.
Video Graphic 1.
See video, Supplemental Digital Content 5, which displays a demonstration of one animal demonstrating the condition of bioprosthetic mesh in tight apposition to uninjured and injured abdominal muscle. Bioprosthetic adherence is limited to the perisuture area without injury to the peritoneum but adherent across the surface in tight apposition with peritoneal abrasion. This video is available in the “Related Videos” section of the Full-Text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A699.
Histology
At the 5-week time point investigated, the Prolene (Ethicon) mesh was associated with an inflammatory response consistent with classic foreign body encapsulation (Figs. 3, 4). The mesh was surrounded by fibrous connective tissue including loose collagen fibers, inflammatory cells (macrophages, foreign body giant cells), and fibroblasts. The bioprosthetic mesh, regardless of condition, was repopulated with host vasculature and cells, predominantly fibroblasts but less frequently macrophages, mast cells, and other inflammatory cells (Figs. 5, 6). Cell density and capillary/blood vessel deposition were grossly noted, but not quantitated. In the uninjured conditions, cell density appeared qualitatively greater in regions closer to the suture(s) and lesser in areas distant from them. Capillaries and blood vessels were present through the entire 2 mm thickness of the implant, but more sparse or absent in regions farther from the suture at this time point. With injury, the matrix was tightly apposed to the abdominal wall, but without histological evidence of a strong inflammatory response or foreign body encapsulation response. Qualitatively, the density of cells and blood vessels within the SurgiMend (Integra LifeSciences) appeared greater with peritoneal injury. In the injured, limited apposition condition, the peritoneum regenerated at the periphery up to the region of mesh adherence, and at this interface, mesothelial cells continued around to cover the exposed surface of the SurgiMend (Integra LifeSciences). In injured, tight apposition conditions, the mesothelial lining also covered the exposed surface of the SurgiMend (Integra LifeSciences).
DISCUSSION
Most hernia repairs fail at the musculotendinous junction, which changes over time as materials remodel at that interface following implantation.13 The goal of this study was to understand the behavior of bioprosthetic meshes so that we may modify surgical techniques to improve patient outcomes. Toward this end, an IAI model was employed to compare SurgiMend (Integra LifeSciences), a bioprosthetic mesh, to Prolene (Ethicon), a permanent synthetic mesh. The results demonstrate several important findings.
First, the amount of adherence of this bioprosthetic mesh to the abdominal wall is facilitated by injury of the mesothelial lining. Limited injury occurs with suture placement and is more extensive with surgical abrasion. Without injury or suture placement, adhesion between the bioprosthetic mesh and the peritoneal lining or visceral contents should not occur. In contrast, synthetic mesh will adhere irrespective of injury or suture placement, and results in foreign body encapsulation under all conditions.
Second, adherence of both bioprosthetic and synthetic meshes is strongly associated with tissue apposition. Even with mesothelial lining injury, the bioprosthetic mesh was nonadherent across the entire surface of the implant if the implant was not maintained in tight tissue apposition. The peritoneal mesothelial lining will repair itself from its cut edges, underneath a nonadherent mesh. This results in a central region in which the matrix adheres to the abdominal wall, and a peripheral region where it does not. Interestingly, the SurgiMend (Integra LifeSciences) may have also prevented bowel from adhering to these injured areas, until such time that repair of the injured areas could occur. In contrast, the synthetic mesh was encapsulated and strongly adherent to any tissue maintained in apposition, including uninjured peritoneum and in some cases bowel or omentum.
Third, revascularization originates from areas of injury with bioprosthetic mesh. The source blood vessels were seen specifically near areas of suture placement and extending outward within the SurgiMend (Integra LifeSciences) in the uninjured series. In the injured series, vascularization was less focused but did include the entirety of the adherent portions. Continued angiogenesis toward the periphery occurred even in cases with limited injury and apposition. It is unclear at this time if inherent signaling with the SurgiMend (Integra LifeSciences) contributes to this neovascularization, but will be the focus of future studies. In contrast, the foreign body response to the synthetic mesh was present throughout the implant and demonstrated scar formation consistent with this classic injury response.
The inflammatory and wound healing responses associated with SurgiMend seem fundamentally different from those of Prolene (Ethicon) mesh. However, it is unclear if these responses can be extrapolated to other bioprosthetic and synthetic materials. Additional questions arise from these data, including (1) Which cell types are specifically involved and are they host tissue specific? (2) How do these results affect the surgical application of these materials and ultimately the clinical results? (3) Are there ways to utilize these principles to modify or improve surgical techniques to get desired outcomes? (4) How are these materials “remodeled” after implantation, and to what effect on clinical outcomes? The animal model used in this study can easily be adapted for these studies and are now ongoing.
Several limitations of this study exist as well. Neonatal bovine dermis was the source material for this study, but other materials are commonly used in hernia repair.1 SurgiMend (Integra LifeSciences) is known to exhibit greater mechanical strength compared to adult porcine dermal matrices,14 but its comparative revascularization and remodeling characteristics remain less well defined. Also, the mechanisms of bioprosthetic mesh adherence to host tissue, the signals that govern and coordinate host cell infiltration, and the eventual remodeling that ensues remain unknown. Further studies are currently in progress to help answer these questions. Also, the factors that govern tissue healing in rats may differ from humans, and immediate extrapolation to human surgical procedures may be premature.
Taken together, these results suggest that, depending on the location of the implanted bioprosthetic mesh, important differences may exist in the biology of hernia repair. Well-described techniques for hernia repair include placing mesh as an onlay, as an inlay (within the rectus sheath and/or preperitoneal plane), and as an underlay within the peritoneum. If an onlay or inlay is used, limited suture apposition of the bioprosthetic mesh may be sufficient for vascularization due to the large raw surface area present (although the mechanical properties and surgical tenants of hernia repair may still require additional suturing). If an underlay is used, increased rate and extent of vascularization may occur if additional suturing is performed, and/or the peritoneal lining is denuded where the bioprosthetic mesh is in contact with the abdominal wall. Such techniques may allow the bioprosthetic mesh to integrate and vascularize more quickly, which in turn may increase resistance to infection, important in patients who are at higher risk for such a complication. The strength of the hernia repair may also be increased faster and to a greater degree, and less dependent on the sutures placed at the musculotendinous junction, with faster/greater adherence of the mesh to the myofascial abdominal wall. Seroma formation may also be reduced with greater adherence of the mesh to the abdominal wall, decreased dead space, and less inflammatory response. It should be stated, however, that increasing the rate of vascularization and adherence of a mesh has unknown effects on its long-term remodeling and longevity of the matrix itself. It is important that future studies are designed to further characterize these properties.
The history and clinical experience with synthetic mesh materials are extensive and have defined a set of expectations for behavior upon implantation. For the surgeon accustomed to using synthetic meshes in hernia repair, using a bioprosthetic mesh may require an additional change in techniques than the simple substitution of materials itself. In addition to patient conditions and comorbidities, the inherent biology of the matrix and its possible effects on clinical outcomes needs to be considered. Adherence, neovascularization, and ultimately remodeling of the bioprosthetic matrix will be affected by several additional variables that need to be considered: whether or not the bioprosthetic is in tight apposition to injured, activated capillaries, whether the mesothelial surfaces are intact or violated, and possibly the distance from an invading blood supply. In conclusion, the results of this study demonstrate clear differences in these variables between bioprosthetic and synthetic mesh materials and highlight the need for further applied research to help surgeons both select appropriate mesh materials, and to apply these materials in the most appropriate manner to achieve quality clinical outcomes for their patients.
ACKNOWLEDGMENTS
The authors thank Lara Reyelt for her assistance in conducting the surgical procedures.
Supplementary Material
Footnotes
Published online 25 May 2018.
This study was funded by the Integra LifeSciences.
Disclosure: Dr. Adelman is a consultant and received research funding from Integra LifeSciences. Dr. Cornwell is an employee of Integra LifeSciences. The Article Processing Charge was paid for by the Integra LifeSciences.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Cornwell KG, Landsman A, James KS. Extracellular matrix biomaterials for soft tissue repair. Clin Podiatr Med Surg. 2009;26:507–523.. [DOI] [PubMed] [Google Scholar]
- 2.Deeken CR, Eliason BJ, Pichert MD, et al. Differentiation of biologic scaffold materials through physicomechanical, thermal, and enzymatic degradation techniques. Ann Surg. 2012;255:595–604.. [DOI] [PubMed] [Google Scholar]
- 3.Shah BC, Tiwari MM, Goede MR, et al. Not all biologics are equal! Hernia. 2011;15:165–171.. [DOI] [PubMed] [Google Scholar]
- 4.Clemens MW, Selber JC, Liu J, et al. Bovine versus porcine acellular dermal matrix for complex abdominal wall reconstruction. Plast Reconstr Surg. 2013;131:71–79.. [DOI] [PubMed] [Google Scholar]
- 5.Blatnik J, Jin J, Rosen M. Abdominal hernia repair with bridging acellular dermal matrix–an expensive hernia sac. Am J Surg. 2008;196:47–50.. [DOI] [PubMed] [Google Scholar]
- 6.Jin J, Rosen MJ, Blatnik J, et al. Use of acellular dermal matrix for complicated ventral hernia repair: does technique affect outcomes? J Am Coll Surg. 2007;205:654–660.. [DOI] [PubMed] [Google Scholar]
- 7.Booth JH, Garvey PB, Baumann DP, et al. Primary fascial closure with mesh reinforcement is superior to bridged mesh repair for abdominal wall reconstruction. J Am Coll Surg. 2013;217:999–1009.. [DOI] [PubMed] [Google Scholar]
- 8.Breuing K, Butler CE, Ferzoco S, et al. Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery. 2010;148:544–558.. [DOI] [PubMed] [Google Scholar]
- 9.Lineaweaver W. Bovine fetal collagen implants improve success rates in abdominal component reconstructions. Paper/Talk presented at: Annual Scientific Conference of the Southeastern Society of Plastic and Reconstructive Surgeons; 2011; Naples, FL. [Google Scholar]
- 10.Janfaza M, Martin M, Skinner R. A preliminary comparison study of two noncrosslinked biologic meshes used in complex ventral hernia repairs. World J Surg. 2012;36:1760–1764.. [DOI] [PubMed] [Google Scholar]
- 11.Cornwell KG, Greenburg AG, James KS. A generative tissue fabricated with SurgiMend has a mesothelial lining limiting adhesion formation in a model of large ventral hernia repair. Paper/Talk presented at: American Hernia Society; 2010. [Google Scholar]
- 12.Cornwell KG, Zhang F, Lineaweaver W. Bovine fetal collagen reinforcement in a small animal model of hernia with component repair. J Surg Res. 2016;201:416–424.. [DOI] [PubMed] [Google Scholar]
- 13.Campbell KT, Burns NK, Rios CN, et al. Human versus non-cross-linked porcine acellular dermal matrix used for ventral hernia repair: comparison of in vivo fibrovascular remodeling and mechanical repair strength. Plast Reconstr Surg. 2011;127:2321–2332.. [DOI] [PubMed] [Google Scholar]
- 14.Adelman DM, Selber JC, Butler CE. Bovine versus porcine acellular dermal matrix: a comparison of mechanical properties. Plast Reconstr Surg Glob Open. 2014;2:e155. [DOI] [PMC free article] [PubMed] [Google Scholar]


