Summary:
The pathogenesis of Dupuytren’s disease (DD) remains unclear although there is increasing evidence supporting the role of stem cells in this and other fibrotic conditions. This review examines the role of DD tissue-associated embryonic stem cells (ESCs) and mesenchymal stem cells (MSCs), and circulating fibrocytes and circulating MSCs, in the biology of DD. It is exciting to infer that dysfunction of an upstream ESC-like population within the affected tissue leads to the downstream development and proliferation of aberrant myofibroblasts through a putative MSC intermediate. This ESC-like population may be a potential novel therapeutic target through modulation of the renin-angiotensin system. Furthermore, circulating CD34+ fibrocytes and MSCs either derived from the bone marrow, peripheral blood cells, or DD-associated ESC-like population, may serve as potential additional extra-palmar reservoirs that undergo endothelial-to-mesenchymal transition, eventually giving rise to the aberrant myofibroblasts. Further studies examining the relative roles of these stem cells and the precise regulatory pathways that govern them may lead to novel therapy that targets these populations.
INTRODUCTION
Dupuytren’s disease (DD) is a fibro-proliferative disorder characterized by progressive palmar fascia fibrosis1–3 with a variable prevalence among racial groups and an increased incidence in males of Northern European ancestry.1,2 Despite known risk factors, the pathogenesis of DD remains unclear.
Palmer fascia fibrosis in DD causes progressive fixed flexion contracture of the digits, resulting in functional impairment. Management options of DD include fasciectomy as the mainstay of treatment, and more recently, collagenase injections.3 Fasciectomy is associated with a risk of injury to the neurovascular bundle and recurrence rates of up to 70%.4
Previous studies of DD have implicated an aberrant myofibroblast proliferation suggesting a mesenchymal origin.5,6 We have recently demonstrated a primitive population of cells expressing embryonic stem cell (ESC) markers, localized to the endothelium of the microvessels in DD nodules and cords.7 We have also demonstrated that these ESC-like cells express components of the renin-angiotensin system (RAS), namely pro-renin receptor, angiotensin-converting enzyme (ACE), and angiotensin II receptor 1 and angiotensin II receptor 2.8 We infer that this primitive population putatively gives rise to an MSC intermediate, which in turn, produce the downstream myofibroblasts characteristic of DD7 (Fig. 1).
Fig. 1.
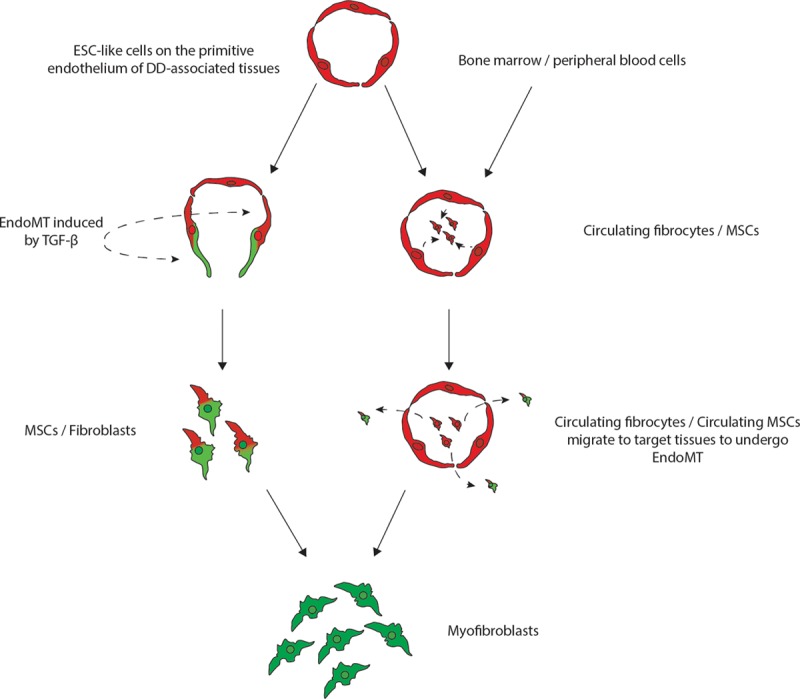
A proposed model demonstrating the potential sources of stem cells that give rise to the myofibroblasts observed in Dupuytren’s disease: (1) DD tissue-associated embryonic stem cell (ESC)-like cells localized to the endothelium of the microvessels that undergo endothelial-mesenchymal transition (EndoMT) induced by cytokines such as TGF-β1, giving rise to myofibroblasts through a MSC intermediate; or (2) circulating fibrocytes and circulating MSCs that originate from the bone marrow or the peripheral blood cells or from the primitive endothelium of the microvessels in DD-associated tissue, and migrate to DD sites and differentiate into myofibroblasts, via an Endo-MT.
This review presents current evidence of the role of stem cells in the pathogenesis of DD.
MYOFIBROBLASTS
The development of nodules and cords in DD has been attributed to an aberrant proliferation of myofibroblasts, the dominant cell type within DD.9 Myofibroblasts expresses types I and III collagen but are distinct from fibroblasts by their expression of α-SMA.10 It has been proposed that myofibroblasts normally differentiate from fibroblasts through a proto-myofibroblast intermediate, regulated by transforming growth factor-β1 (TGF-β1) and platelet-derived growth factor.9,10 There is also increasing evidence showing MSCs as the potential origin of aberrant myofibroblasts observed in DD.6
MESENCHYMAL STEM CELLS
Mesenchymal stem cells (MSCs) are fibroblast-like cells that are multipotent and express mesenchymal markers CD73, CD90, and CD105.11 These MSCs are devoid of hematopoetic stem cell markers CD45, CD34, or CD19 and are derived from the bone marrow,12 adipose tissue,13 and peripheral or umbilical cord blood.14 The multipotency of MSCs are defined by their ability to differentiate into multiple cell lineages such as adipocytes, osteocytes, chondrocytes, and fibroblasts.11–13
A recent study has demonstrated the presence of a MSC population that expresses surface markers CD73, CD90, CD105, but not CD34, within DD tissue.6 These MSCS are localized to the surrounding fat and overlying skin and are proposed to be a potential source of aberrant myofibroblasts observed in DD (Fig. 1).5,6 The proposed role of this MSC population within the surrounding tissue in DD is further supported by reduced recurrence rates following dermofasciectomy in which the skin overlying the cords and nodules is also excised.15
The terminology proposed by the International Society for Cellular Therapy does not differentiate MSCs from fibroblasts. Fibroblasts, like MSCs, also express cell markers CD73 and CD105, and whether these 2 populations are homogenous or distinct cell populations, remains to be investigated in DD.11,16
EMBRYONIC STEM CELL–LIKE CELLS
We have demonstrated an ESC-like population localized to the endothelium of the microvessels in DD cords and nodules that express ESC markers NANOG, pSTAT3, SALL4, and OCT4.8
NANOG is a homeoprotein linked to self-renewal and pluripotency.17–19 Intrinsic to the function of ESCs, the absence of NANOG leads to a loss in pluripotency and results in differentiation.18,19 STAT3 is a transcription factor and plays a role in cellular regulation pathways. Unlike NANOG, STAT3 is an independent mediator of pluripotency in stem cells.20,21 SALL4 is a regulator of NANOG and is also required for maintaining stem cell pluripotency.22,23 The transcription factor SALL4 binds to the NANOG gene and upregulates NANOG expression.22,23 In addition, SALL4 regulates OCT4, a POU transcription factor required for the maintenance of the pluripotent potential in ESCs.19,24,25
Through the process of endothelial-to-mesenchymal transition (EndoMT) the primitive population on the endothelium of the microvessels in DD that expresses ESC markers are proposed to give rise to an MSC intermediate, which in turn, gives rise to the downstream myofibroblasts (Fig. 1). Additionally, these ESC-like cells express components of the RAS, namely pro-renin receptor, ACE, and ATIIR1 and ATIIR2, implicating RAS dysfunction in the ultimate development of aberrant myofibroblasts.8 This is supported by similar findings in keloid scar.26 Although a causal relationship has yet to be established, the improvement of fibrotic conditions such as keloid scar following administration of low-dose enalapril, an ACE inhibitor, supports a role for the RAS in this condition.27,28
CIRCULATING FIBROCYTES AND CIRCULATING MSCS
The role of circulating cancer stem cells (CSCs) in cancer has also recently been reported.29–31 These circulating CSCs express the surface marker CD34 and undergo an epithelial to mesenchymal transition, attributed to the ability of cancer to metastasize.32,33 Circulating CSCs, like hematopoetic stem cells, are found in the peripheral blood, and they have the ability to migrate to target organs.34,35
The role of circulating fibrocytes in fibrosis has also been reported recently.35 Circulating fibrocytes are fibroblast progenitors that migrate to distant tissue sites where they contribute to inflammation and fibrosis.35 The origin of these circulating fibrocytes remains unclear and may include the bone marrow or peripheral blood cells.34,35 It is exciting to speculate that these circulating fibrocytes that express CD34, migrate from the peripheral circulation to target organs, leading to fibroblast proliferation and fibrosis in DD35,36 (Fig. 1). These CD34+ circulating fibrocytes have also been implicated in tissue repair, wound healing, and contributes to keloid scar and hypertrophic scar.37–42 The ability of these circulating fibrocytes to differentiate into myofibroblasts, further supports their role in fibrosis in DD.43–45
Circulating MSCs have also been reported to play a potential role in fibrosis.38,48 Circulating MSCs, are a subset of MSCs, that exist in the peripheral circulation, and like circulating fibrocytes, they increase in number and migrate to target organs during tissue injury and inflammation.46–48 The similarity in function and structure between the circulating CD34+ fibrocytes and the circulating MSCs suggests that these cell types may act as additional reservoirs that give rise to the aberrant myofibroblasts observed in DD.44,45 Whether these circulating fibrocytes and circulating MSCs are the same population, potentially originating from the DD-associated primitive endothelium and/or from other sites, such as the bone marrow through an EndoMT process, and subsequently migrate to the site of DD (Fig. 1), remains the topic of future research.
ENDOTHELIAL-TO-MESENCHYMAL TRANSITION
Endothelial-mesenchymal transition is characterized by the loss of expression of endothelial surface markers CD31 and VE-cadherin, and the expression of mesenchymal components α-SMA and type I collagen.43,44,49,50 In early human development, the cardiac valves and septum have been proposed to arise from endoMT, whereby the surrounding endothelium gives rise to endocardial cushions through a mesenchymal intermediate.48 Similar findings have also been shown in the development of hematopoietic cells from a hemogenic endothelial phenotype.52
In addition to its aforementioned role in embryological development, endoMT has also been shown to contribute to the development of pulmonary,44 cardiac,49 and renal fibrosis.53
EndoMT is initiated by cytokines such as TGF-β.54–56 Although the exact mechanism is yet to be elicited, TGF-β has been shown to upregulate transcription factors Snail,52 Slug,54 and Twist,54 which leads to the downstream regression of endothelial cell surface markers, and the expression of mesenchymal markers.55–58
We postulate that cyotkines, such as TGF-β, expressed by the tissues surrounding the putative primitive phenotypic endothelium, induces differentiation of the ESC-like cells to form a MSC intermediate via an endoMT, which ultimately give rise to aberrant myofibroblasts (Fig. 1). Currently, there remains no consensus on whether MSCs and fibroblasts are phenotypically distinguishable populations within DD.16 Furthermore, the mechanism by which MSCs differentiate into myofibroblasts in DD remains unclear, and may result from direct differentiation of MSCs into myofibroblasts, or via a fibroblast intermediate.59
DISCUSSION
There is increasing evidence supporting the role of DD tissue-associated ESC-like cells and MSCs, and circulating fibrocytes and circulating MSCs, in DD. We have described the characteristics and potential source and the role of each of these cell populations and how they may relate to one another. We propose that dysfunction of the ESC-like cells on the endothelium of the microvessels gives rise to the downstream aberrant myofibroblasts, through an MSC intermediate.7 This ESC-like population expresses components of the RAS,8 and therefore may be a novel therapeutic target through modulation of the RAS using existing medications. We also propose that circulating CD34+ fibrocytes and circulating MSCs may serve as additional extra-palmar reservoirs that migrate to target organs and differentiate into aberrant myofibroblasts through EndoMT.
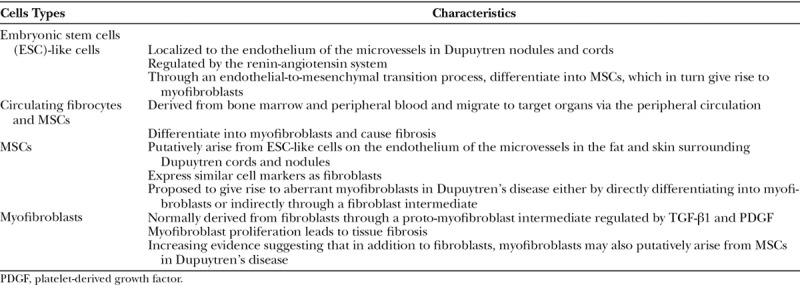
The key cell types in DD and their characteristics are presented in Table 1. A better understanding of the role of stem cells in DD may potentially lead to the development of targeted therapy for this enigmatic condition.
Table 1.
Stem Cells that Putatively Give Rise to Myofibroblasts in Dupuytren’s Disease
Footnotes
Published online 10 May 2018.
Disclosure: T.I. and S.T.T. are inventors of a provisional patent application Treatment of Fibrotic Conditions (PCT/NZ2016/050187). The authors are otherwise not aware of any commercial associations or financial relationships that might pose or create a conflict of interest with information presented in any submitted manuscript. The Article Processing Charge was paid for by the Gillies McIndoe Research Institute’s internal fund.
REFERENCES
- 1.Hindocha S, McGrouther DA, Bayat A. Epidemiological evaluation of Dupuytren’s disease incidence and prevalence rates in relation to etiology. Hand (N Y). 2009;4:256–269.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geoghegan JM, Forbes J, Clark DI, et al. Dupuytren’s disease risk factors. J Hand Surg Br. 2004;29:423–426.. [DOI] [PubMed] [Google Scholar]
- 3.Riester S, van Wijnen A, Rizzo M, Kakar S. Pathogenesis and treatment of Dupuytren disease. JBJS Rev. 2014;2:e2 doi:10.2106/JBJS.RVW.M.00072. [DOI] [PubMed] [Google Scholar]
- 4.Becker GW, Davis TR. The outcome of surgical treatments for primary Dupuytren’s disease—a systematic review. J Hand Surg Eur Vol. 2010;35:623–626.. [DOI] [PubMed] [Google Scholar]
- 5.Hindocha S, Iqbal SA, Farhatullah S, et al. Characterization of stem cells in Dupuytren’s disease. Br J Surg. 2011;98:308–315.. [DOI] [PubMed] [Google Scholar]
- 6.Iqbal SA, Manning C, Syed F, et al. Identification of mesenchymal stem cells in perinodular fat and skin in Dupuytren’s disease: a potential source of myofibroblasts with implications for pathogenesis and therapy. Stem Cells Dev. 2012;21:609–622.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koh SP, On N, Brasch HD, et al. Embryonic stem cell-like population in Dupuytren’s disease. Plast Reconstr Surg Glob Open. 2016;4:e1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.On N, Koh SP, Brasch HD, et al. Embryonic stem cell-like population in Dupuytren’s disease expresses components of the Renin-Angiotensin System. Plast Reconstr Surg Glob Open. 2017;5:e1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bisson MA, McGrouther DA, Mudera V, et al. The different characteristics of Dupuytren’s disease fibroblasts derived from either nodule or cord: expression of alpha-smooth muscle actin and the response to stimulation by TGF-beta1. J Hand Surg Br. 2003;28:351–356.. [DOI] [PubMed] [Google Scholar]
- 10.Tomasek JJ, Gabbiani G, Hinz B, et al. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363.. [DOI] [PubMed] [Google Scholar]
- 11.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317.. [DOI] [PubMed] [Google Scholar]
- 12.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49.. [DOI] [PubMed] [Google Scholar]
- 13.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee OK, Kuo TK, Chen WM, et al. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–1675.. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong JR, Hurren JS, Logan AM. Dermofasciectomy in the management of Dupuytren’s disease. J Bone Joint Surg Br. 2000;82:90–94.. [DOI] [PubMed] [Google Scholar]
- 16.Haniffa MA, Collin MP, Buckley CD, et al. Mesenchymal stem cells: the fibroblasts’ new clothes? Haematologica. 2009;94:258–263.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitsui K, Tokuzawa Y, Itoh H, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642.. [DOI] [PubMed] [Google Scholar]
- 18.Chambers I, Silva J, Colby D, et al. Nanog safeguards pluripotency and mediates germline development. Nature. 2007;450:1230–1234.. [DOI] [PubMed] [Google Scholar]
- 19.Loh YH, Wu Q, Chew JL, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–440.. [DOI] [PubMed] [Google Scholar]
- 20.Zhao W, Ji X, Zhang F, et al. Embryonic stem cell markers. Molecules. 2012;17:6196–6236.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Humphrey RK, Beattie GM, Lopez AD, et al. Maintenance of pluripotency in human embryonic stem cells is STAT3 independent. Stem Cells. 2004;22:522–530.. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Tam WL, Tong GQ, et al. Sall4 modulates embryonic stem cell pluripotency and early embryonic development by the transcriptional regulation of Pou5f1. Nat Cell Biol. 2006;8:1114–1123.. [DOI] [PubMed] [Google Scholar]
- 23.Wu Q, Chen X, Zhang J, et al. Sall4 interacts with Nanog and co-occupies Nanog genomic sites in embryonic stem cells. J Biol Chem. 2006;281:24090–24094.. [DOI] [PubMed] [Google Scholar]
- 24.Huangfu D, Osafune K, Maehr R, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26:1269–1275.. [DOI] [PubMed] [Google Scholar]
- 25.Nichols J, Zevnik B, Anastassiadis K, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391.. [DOI] [PubMed] [Google Scholar]
- 26.Grant C, Chudakova DA, Itinteang T, et al. Expression of embryonic stem cell markers in keloid-associated lymphoid tissue. J Clin Pathol. 2016;69:643–646.. [DOI] [PubMed] [Google Scholar]
- 27.Iannello S, Milazzo P, Bordonaro F, et al. Low-dose enalapril in the treatment of surgical cutaneous hypertrophic scar and keloid—two case reports and literature review. MedGenMed. 2006;8:60. [PMC free article] [PubMed] [Google Scholar]
- 28.Uzun H, Bitik O, Hekimoğlu R, et al. Angiotensin-converting enzyme inhibitor enalapril reduces formation of hypertrophic scars in a rabbit ear wounding model. Plast Reconstr Surg. 2013;132:361e–371e.. [DOI] [PubMed] [Google Scholar]
- 29.Munro MJ, Wickremesekera SK, Peng L, et al. Cancer stem cells in colorectal cancer: a review. J Clin Pathol. 2018;71:110–116.. [DOI] [PubMed] [Google Scholar]
- 30.Itinteang T, Dunne JC, Chibnall AM, et al. Cancer stem cells in moderately differentiated oral tongue squamous cell carcinoma express components of the renin-angiotensin system. J Clin Pathol. 2016;69:942–945.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bradshaw A, Wickremsekera A, Tan ST, et al. Cancer stem cell hierarchy in glioblastoma multiforme. Front Surg. 2016;3:21 doi:10.3389/fsurg.2016.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273.. [DOI] [PubMed] [Google Scholar]
- 34.Kuznetsov SA, Mankani MH, Gronthos S, et al. Circulating skeletal stem cells. J Cell Biol. 2001;153:1133–1140.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abe R, Donnelly SC, Peng T, et al. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562.. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi K, Mulliken JB, Kozakewich HP, et al. Cellular markers that distinguish the phases of hemangioma during infancy and childhood. J Clin Invest. 1994;93:2357–2364.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andersson-Sjöland A, de Alba CG, Nihlberg K, et al. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40:2129–2140.. [DOI] [PubMed] [Google Scholar]
- 38.Strieter RM, Keeley EC, Hughes MA, et al. The role of circulating mesenchymal progenitor cells (fibrocytes) in the pathogenesis of pulmonary fibrosis. J Leukoc Biol. 2009;86:1111–1118.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehrad B, Burdick MD, Zisman DA, et al. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun. 2007;353:104–108.. [DOI] [PubMed] [Google Scholar]
- 40.Iqbal SA, Sidgwick GP, Bayat A. Identification of fibrocytes from mesenchymal stem cells in keloid tissue: a potential source of abnormal fibroblasts in keloid scarring. Arch Dermatol Res. 2012;304:665–671.. [DOI] [PubMed] [Google Scholar]
- 41.Metz CN. Fibrocytes: a unique cell population implicated in wound healing. Cell Mol Life Sci. 2003;60:1342–1350.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grieb G, Steffens G, Pallua N, et al. Circulating fibrocytes—biology and mechanisms in wound healing and scar formation. Int Rev Cell Mol Biol. 2011;291:1–19.. [DOI] [PubMed] [Google Scholar]
- 43.Piera-Velazquez S, Li Z, Jimenez SA. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol. 2011;179:1074–1080.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jimenez SA, Piera-Velazquez S. Endothelial to mesenchymal transition (EndoMT) in the pathogenesis of systemic sclerosis-associated pulmonary fibrosis and pulmonary arterial hypertension. Myth or reality? Matrix Biol. 2016;51:26–36.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abu El-Asrar AM, Struyf S, Van Damme J, et al. Circulating fibrocytes contribute to the myofibroblast population in proliferative vitreoretinopathy epiretinal membranes. Br J Ophthalmol. 2008;92:699–704.. [DOI] [PubMed] [Google Scholar]
- 46.Rochefort GY, Delorme B, Lopez A, et al. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells. 2006;24:2202–2208.. [DOI] [PubMed] [Google Scholar]
- 47.Tondreau T, Meuleman N, Delforge A, et al. Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: proliferation, Oct4 expression, and plasticity. Stem Cells. 2005;23:1105–1112.. [DOI] [PubMed] [Google Scholar]
- 48.Xu L, Li G. Circulating mesenchymal stem cells and their clinical implications. J Orthop Translat. 2014;2:1–7.. doi:10.1016/j.jot.2013.11.002. [Google Scholar]
- 49.Goumans MJ, van Zonneveld AJ, ten Dijke P. Transforming growth factor beta-induced endothelial-to-mesenchymal transition: a switch to cardiac fibrosis? Trends Cardiovasc Med. 2008;18:293–298.. [DOI] [PubMed] [Google Scholar]
- 50.Piera-Velazquez S, Jimenez SA. Molecular mechanisms of endothelial to mesenchymal cell transition (EndoMT) in experimentally induced fibrotic diseases. Fibrogenesis Tissue Repair. 2012;5:S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Markwald RR, Fitzharris TP, Manasek FJ. Structural development of endocardial cushions. Am J Anat. 1977;148:85–119.. [DOI] [PubMed] [Google Scholar]
- 52.Dejana E, Hirschi KK, Simons M. The molecular basis of endothelial cell plasticity. Nat Commun. 2017;8:14361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011;7:684–696.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ema M, Faloon P, Zhang WJ, et al. Combinatorial effects of Flk1 and Tal1 on vascular and hematopoietic development in the mouse. Genes Dev. 2003;17:380–393.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeisberg EM, Potenta S, Xie L, et al. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123–10128.. [DOI] [PubMed] [Google Scholar]
- 56.Zeisberg EM, Tarnavski O, Zeisberg M, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961.. [DOI] [PubMed] [Google Scholar]
- 57.Stenmark KR, Frid M, Perros F. Endothelial-to-mesenchymal transition: an evolving paradigm and a Promising Therapeutic Target in PAH. Circulation. 2016;133:1734–1737.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heldin CH, Vanlandewijck M, Moustakas A. Regulation of EMT by TGFβ in cancer. FEBS Lett. 2012;586:1959–1970.. [DOI] [PubMed] [Google Scholar]
- 59.Lee CH, Moioli EK, Mao JJ. Fibroblastic differentiation of human mesenchymal stem cells using connective tissue growth factor. Conf Proc IEEE Eng Med Biol Soc. 2006;1:775–778.. [DOI] [PMC free article] [PubMed] [Google Scholar]


