Supplemental Digital Content is available in the text.
Abstract
Background:
Cellulite can be seen on the skin in widespread alterations of the skin surface and dimpling. The purpose of this study was to assess the effectiveness and safety of the manual subcision technique to treat dimpling from cellulite, using a specific class IIA medical device (Celluerase).
Methods:
The multi-center observational study assessed 200 women treated in a single session for different dimpling, using manual subcision administered by Celluerase. Aesthetic outcomes were evaluated by the authors, and the patients assessed satisfaction levels.
Results:
Two hundred women between 20 and 55 years were treated. The medical evaluation of patients saw improvements with an average score of 8.1, whereas the subjective evaluation by patients gave an average improvement score of 7.8. Adverse events were reported.
Discussion:
Women have septa orientation at right angels to the skin surface, and those with cellulite have an irregular septa conformation, with some septa being hypertrophic-thickened, and others being narrowed-lysed. Magnetic resonance imaging has confirmed that cellulite depressions are associated with a significant increase of thickness of underlying subcutaneous fibrous septa. Subcision has immediate results because it eliminates traction on the skin.
Conclusion:
The study has shown the effectiveness and safety of the manual subcision in the treatment of dimpling. The device used, designed specifically for this technique, has shown itself to be very helpful and effective in terms of practical use, aesthetic outcome and safety, with various advantages compared with other commonly used devices.
INTRODUCTION
Cellulite can be seen on the skin in many different ways, to the extent that with so many clinical presentations, it is impossible to have a valid and specific clinical classification.1 However, from a treatment viewpoint, it is possible to recognize 2 typical types of lesion: widespread alteration of the skin’s texture and dimpling.
Widespread alteration of the skin’s texture (Fig. 1), otherwise referred to as lumpy-bumpy appearance, orange peel, cottage cheese, or mattress aspect,2 is the most frequent type of lesion from cellulite. It almost always occurs in women with cellulite, with no specific differences in incidence among various populations (obese, normal weight, underweight, sportswomen, young, elderly, etc.). The skin texture is irregular, uneven and no longer smooth. It has pitting and eversions that are difficult to define and of minimal size. Generally, this occurs in the whole area affected by cellulite (trochanteric area, buttocks, and back of thighs), even if it can also extend to less frequently affected areas (front of thighs, knees, calves, inner thighs), and it has irregular and nonconstant swings in terms of severity (more or less visible).
Fig. 1.
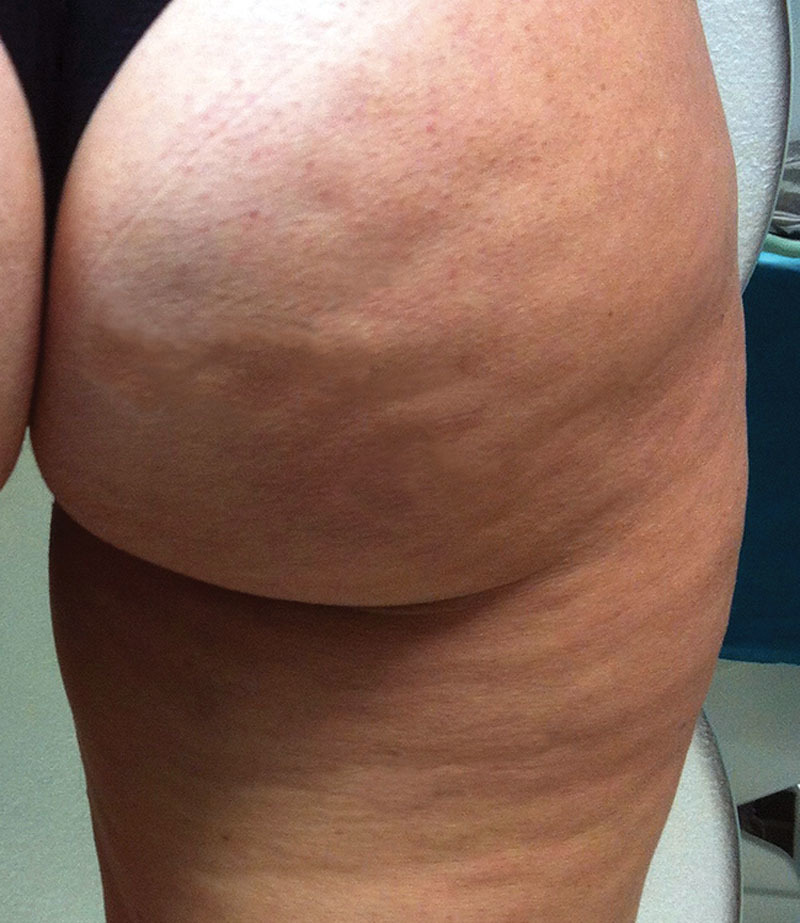
A 45-year-old woman with widespread alterations to skin texture (orange peel).
Dimpling (Fig. 2) consists of single inward-facing lesions. These differ in terms of number, shape (round, linear, oval), dimension, location, and extension.3 It is not in relation to the amount of widespread alteration on the skin’s surface, and unlike this, it is stable, meaning there are no periodic swings. These lesions were incorrectly associated with a more advanced stage of cellulite, but in fact they are caused by the hypertrophic fibrous septa of the extracellular matrix of superficial subcutaneous adipose tissue, and which retract the skin’s surface (Fig. 3)4–10 causing it to be inward facing.
Fig. 2.
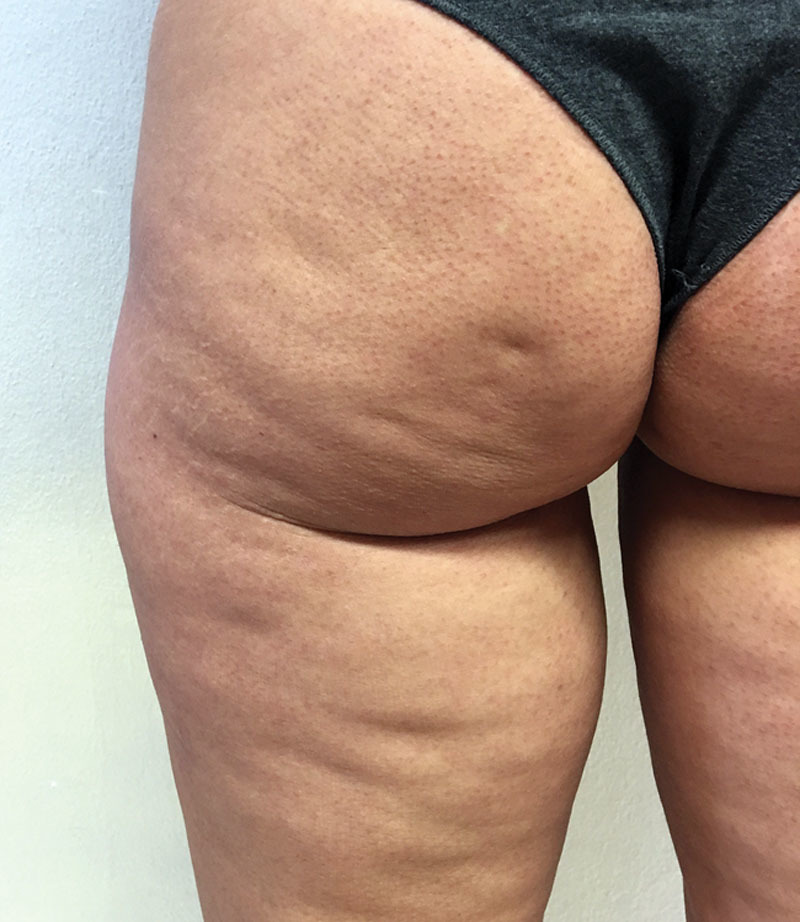
A 42-year-old woman with dimpling in the buttock area and on the back of the thighs, an excellent candidate for subcision.
Fig. 3.
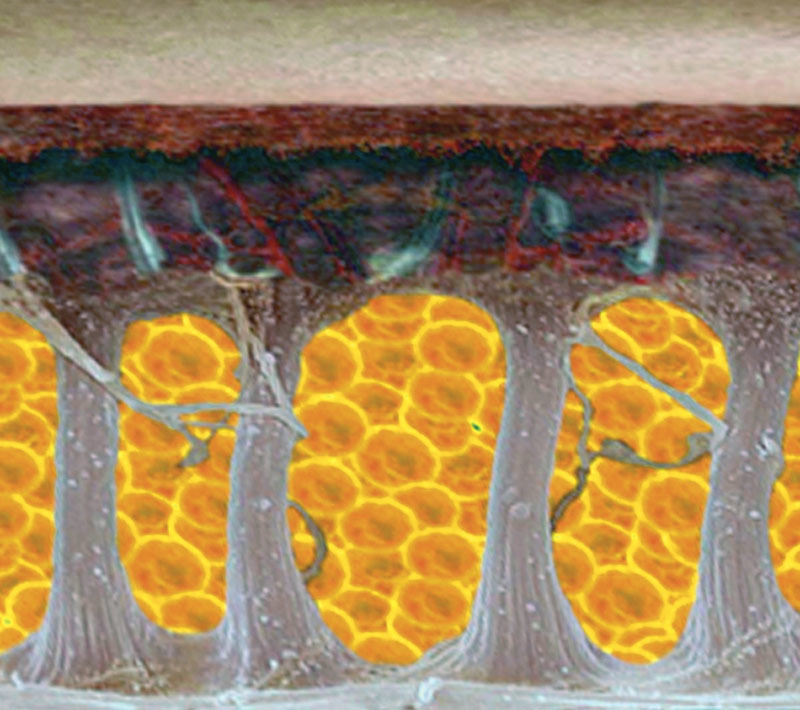
Graphic representation of the variation in subcutaneous connective tissue in a woman without cellulite and with cellulite. In all women, the fibrous septa are mainly at right angles to the skin’s surface. In cellulite, they are variable in size, thickened, and lysed. The thickened fibrous septa cause the skin to be retracted toward the muscle fiber, causing dimpling.
Over the years, numerous treatment prospects have been proposed for cellulite: topical therapy,11 mesotherapy,12–14 lymphatic or vacuum-assisted massage,15–18 acoustic wave therapy,19,20 light therapy, external noninvasive lasers,21 and radiofrequency.22 These have not always been effective, have involved a high number of sessions, and the results have been brief in duration, all leading to an increasingly lower use of these methods, and a loss in their popularity.23 To date, there is no single exclusive, effective treatment for the different types of cellulite24: synergetic use of several noninvasive or minimally invasive methods, repeated in cycles, seems to be the best choice when it comes to widespread skin texture alteration.25,26 The surgical technique of subcision, involving mechanical cutting of the retracting hypertrophic septa has revealed itself to be a very effective and lasting way to treat dimpling.27,28 Originally described by Orentreich and Orentreich29 and then by Hexsel and Mazzucco,30 the surgical procedure is aimed at each individual lesion and immediately leads to a change in the skin’s surface (see discussion). Subcision can be divided23 into manual, vacuum-assisted,31 and laser-assisted methods.10, 32–34
The purpose of this study was to assess the effectiveness and safety of the manual subcision technique to treat dimpling from cellulite, using a specific class IIA medical device.
MATERIALS AND METHODS
The multi-center observational study assessed 200 women treated in a single session between September 2016 and November 2017 for different dimpling, using manual subcision administered by celluerase. Written informed consent was obtained from each patient.
Patient Selection
The patients presented with various forms, degrees, and amounts of dimpling on the buttocks, trochanteric area, and backs of the thighs. They did not present with any exclusion criteria for the treatment: psychological35,36 (indecisive or immature personalities, anxiety, dismorphophobia, with fictitious disorders, or family members disapproving the treatment), minors, over 60 years old, pregnancy, women who are lactating, obesity, allergic reactions to local anesthetics, severe autoimmune diseases, acute infections in progress, immunosuppressive diseases with weakened immune systems, organ diseases uncompensated, with functional deficits or in acute phase (diabetes, kidney failure, liver disease, severe dyslipidemia, dysthyroidism, and so on), using anticoagulants, antiplatelets, with hemorrhagic diathesis or platelet disease. Local contraindications for the treatment were acute skin diseases (continued solutions, wounds, local infection, acute dermatological lesions), recent surgery on the site, or medical and aesthetic treatments (eg, liposculpture, intralipotherapy, cryotherapy, and so on), scarring problems (atrophic/hypertrophic scars and keloids), superficial venous diseases in the area to be treated.
Device
The instrument used was a microsurgical blade with 2 cutters and a gauge of 19 G × 30 mm, specifically designed for this technique (class IIA Medical Device - Celluerase).
Posttreatment Management
Antibiotic prophylaxis was always carried out and commenced 24 hours before treatment: except in specific cases, short-term antibiotic therapy was administered (azithromycin 500 mg administered orally, once a day for 3 days).
After the treatment, the use of restraining elastic sheaths was compulsory for 1 week 24 hours per day and highly recommended during the first month. These improve the results, reduce the risk of adverse events (seroma, extensive hematoma, paradox results, skin laxity), reduce recovery time, and improve aesthetic outcome. Lymphatic drainage or pressure therapies were also advised to reduce the postoperative phase and accelerate a full recovery37,38 (2 sessions per week for 2–3 weeks posttreatment starting from the third day after the treatment). No other therapies or techniques were allowed in the treated area throughout the study. After treatment, pain killers were allowed although generally not necessary.
Technique
The manual technique (see video, Supplemental Digital Content 1, which shows step-by-step the manual technique of subcision. This video is available in the “Related Videos” section of the Full-Text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A766) of subcision initially involves marking out the visible inward-facing lesions with the patient in an orthostatic resting position with tangential overhead lighting (Fig. 4). Subsequently, with the patient lying on their stomach, the lesions on the surface subcutaneous tissue are infiltrated, immediately below the derma (Fig. 5), using a mix of: 90% local anesthetic at 0.5:100, 8% sodium bicarbonate at 8.4:100 and 2% adrenaline 1:1,000.39–41 After waiting 10’ to achieve a good level of ischemization in the area to be treated, the skin is pierced with the device at an angle of around 60°, about 5–10 mm from the margin of the largest axis in the drawing. Once past the derma, it will be possible to perceive a sudden loss of resistance to the forward movement, and once at the surface of the subcutaneous tissue (5–8 mm approximately), circular movements are made parallel to the skin’s surface, moving clockwise and counterclockwise so as to cut the septa perpendicular to the skin’s surface (Fig. 6). These movements are varied in direction until it is no longer possible to feel the fibrous tendrils that obstruct movement (there must be no resistance to the movement of the device). The procedure is repeated from several access points until the whole area to be treated has been covered. The free hand carries out a gravity manoeuvre (5–10 cm cranially to the lesion, pushing the tissue in a cranium-caudal direction) to highlight any residual adherence. If necessary, treat the areas that still show partial retraction again. At the end, the area is manually compressed for about 5’ to stop any dripping from the skin access point and limit the size of the hematoma. Bandaging and elastic sheath are then applied.
Fig. 4.
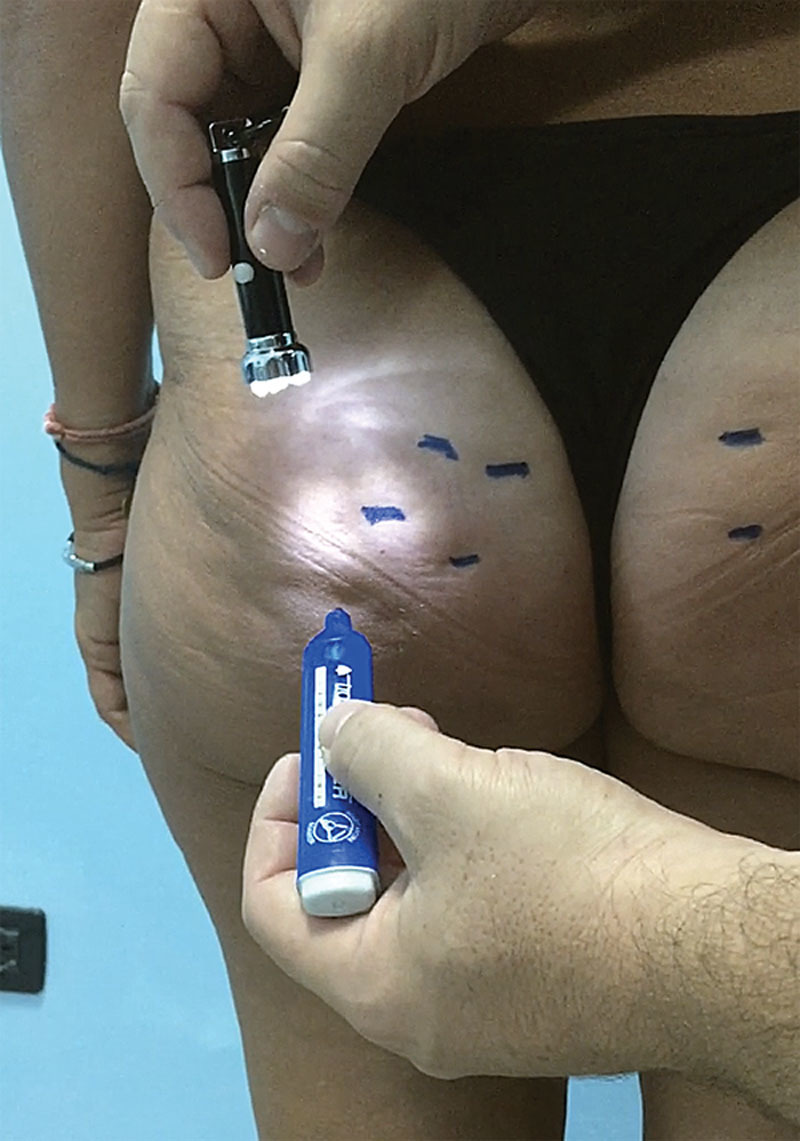
The dimpling to be treated is marked with the patient in the standing position. An auxiliary light from above is useful in highlighting these lesions. Those who can only be seen with muscle contraction or by handling the area must not be treated.
Fig. 5.
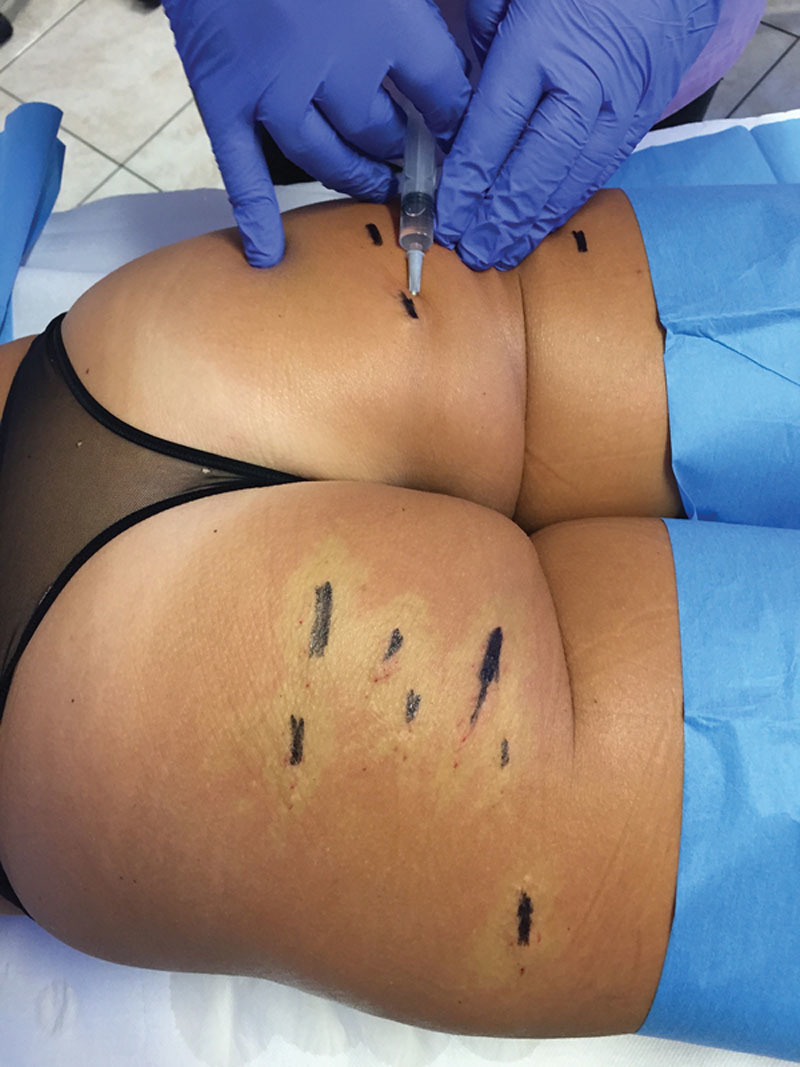
Infiltration with anesthetic solution is carried out on the lesion to be treated and which is marked beforehand. After a few minutes, the infiltrated areas will look lighter in color due to a passing ischemia caused by the adrenaline (see right buttock).
Fig. 6.
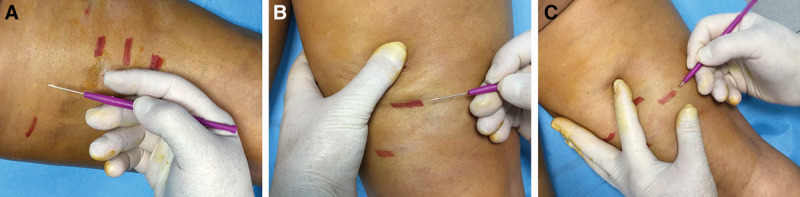
The cutting edges of the device are kept parallel to the skin’s surface, keeping the index fingertip in contact with the formation detected on the device (A). The device is inserted 5 mm from the lateral margin of the dimpling (B) at an angle of about 60°. The movement continues as far as the hypodermis (C), which is perceived when there is no more resistance to the movement itself. The operator makes circular movements—clockwise and counterclockwise—parallel to the surface of the skin, moving laterally and forward. The physician will easily perceive the cutting of the hypertrophic septa.
Video Graphic 1.
See video, Supplemental Digital Content 1, which shows step-by-step the manual technique of subcision. Including marking out the dimpling lesions with tangential overhead lighting, infiltration with anesthetic solution in a superficial right plane and posttreatment management. This video is available in the “Related Videos” section of the Full-Text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A766.
Evaluation of the Results
Aesthetic outcomes were evaluated by the authors in 200 patients treated by them directly, using preoperative and postoperative photographic documentation. Each case was assessed by 2 doctors who did not perform the treatment. Each doctor was asked to assess the results and to express a value from 0 to 10 (where 0 is “no result at all” and 10 is “the best result achievable”). Subject satisfaction also was evaluated: their level was rated by filling out an anonymous form (the results were rated from 0 to 10, where 0 is “no result at all” and 10 is “the best result achievable”). The postoperation evaluations were performed 6 months after treatment. Patients evaluated with variations of ±3% of initial body weight were not considered in this study. Adverse events were assessed directly by the doctor who performed the treatment, monitoring patients through to complete resolution or stabilization of the problem.
RESULTS
Two hundred women between 20 and 55 years (34 years and 3 months on average) were treated. The medical evaluation of patients saw improvements with an average score of 8.1 (range, 7.3–9.4). The subjective evaluation of patients saw improvements with an average score of 7.8 (range, 5.8–9.5). The study showed no statistically significant differences in results between different groups in terms of age and body mass index. In patients with localized fat deposits and/or flabby skin tone in the areas affected by cellulite, the improvements were significantly lower compared with the overall average (the medical evaluation was 7.6 and the subjective evaluation 7.1). The adverse events from the technique were bruising (200), numbness (172), pain after procedure (106), blood dripping in the first 12 hours (121), nodules lasting less than 1 month (29), hyperpigmentation from hemosideric deposition (13), permanent nodules lasting more than 1 month (8), paradox/bulging effect (5), skin irregularities (5), seroma (3), localized sensory disorders (1).
DISCUSSION
Cellulite is a common term used to identify a disease on the superficial subcutaneous adipose tissue located most commonly on the outer thighs, posterior thighs, and buttocks of the majority of postpubertal females, and mainly characterized by skin surface irregularities and other symptoms (feeling of heaviness in the limbs, pain, hypoesthesia, localized cold feeling, and so on).42 There are many mechanisms responsible for cellulite and in part, they are yet to be recognized: hyperpolymerization matrix mucopolysaccharides,43 alteration of microcirculation,44 enzymatic and mechanic proteolysis of interlobular septa,9 adipocytes hypertrophy,45 hypoxia and inflammation.46 The histological characteristics of cellulite have been cleared by high-resolution image (high frequency ultrasound and high-resolution magnetic resonance).47 In women, the extracellular matrix is less present compared with in men and therefore, the layout of the fibrous septa is mainly at right angles to the skin’s surface, whereas in men it is more randomized.1,8,48 This means a significant anatomical difference between the 2 sexes, which would seem, for the most part, to be the reason why it is almost exclusively women who are subject to cellulite. Although fibrous septa can be found parallel to the skin surface, tilted at 45°, or perpendicular to the skin surface, females with cellulite have a greater percentage of perpendicular septa compared with males or females without cellulite.47 Moreover, in women with cellulite, the perpendicular septa are uneven, some being hypertrophic (responsible for dimpling) and others, thinned.46 Some authors, referring to the model of the uterine endometrium, attribute this specific characteristic to the lytic action of the metalloproteinases that cyclically vary according to the serum concentration of estrogens9 (essential to the uterus to regenerate the endometrium in case of a lack of fertilization). Magnetic resonance imaging has confirmed that cellulite depressions are associated with a significant increase in the thickness of underlying subcutaneous fibrous septa. Greater tension on these fibrous septa from standing, pinching, or active muscle contraction (due to communication with the underlying musculoaponeurotic system) worsens their clinical appearance, whereas they tend to disappear when tension is minimized with the patient lying down.
The treatment of dimpling with subcision brings immediate results via several mechanisms (Fig. 7):
Fig. 7.
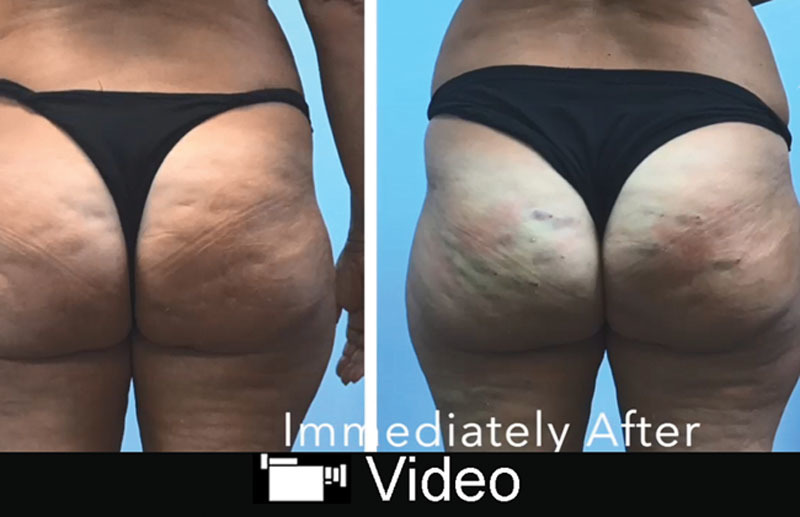
Images pre- (A) and immediately posttreatment (B). The treated areas will look swollen, bruised, and irregular, but they are already free from retraction.
- it eliminates the traction on the skin from the retracting septa;
- it redistributes subcutaneous tension forces, mitigating fat protrusion, and reallocating fat lobules into the spaces created by the procedure.
- it creates hematoma that in turn boost the formation of new connective tissue.30 It is therefore useless and also counterproductive to use fillers to keep the skin’s surface smooth: fillers prevent the tissue from regenerating and also lead to the return of the initial condition without any benefit.49–51
Using the Celluerase device, specifically designed for this method, has several advantages compared with other devices used for subcision:
- it avoids making an access point with probable permanent scarring, and is less traumatic (as opposed to dovetail cannula, laser fiber).
- it cuts procedure times, because it is more effective in cutting the septa, thanks to its 2-sided convex cutting surface (as against hypodermic needles, Nokor needles, dovetail cannula);
- it has a greater precision with minimal tissue damage thanks to the ergonomic grip and cutting surface that provide excellent control when cutting septa that are at right angles to the skin’s surface (as opposed to cannula, needles, laser fibers);
- it reduces fibrosis, that is, the build up of type I collagen fibers and therefore, it does not have a negative biological effect on the subcutaneous fat layer, which is already altered by cellulite (versus laser fibers);
- it reduces the risk of seroma and organized hematoma, avoiding large-scale disconnection (as opposed to vacuum-assisted devices);
- it is cost effective (compared with vacuum-assisted devices and laser fibers).
We should stress that the Celluerase is used for manual subcision and depends on an operator, and therefore, it requires training with a suitable learning curve to achieve the right experience before use.
CONCLUSIONS
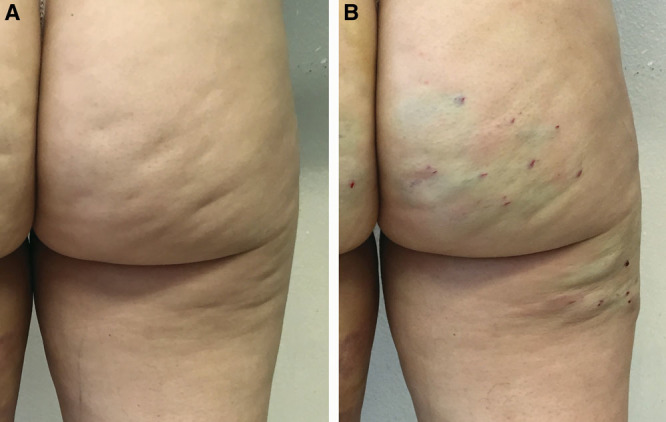
In aesthetic terms, cellulite has different effects on the skin’s appearance, with dimpling being the most evident. Of the possible presumed treatment options, many of these are lacking in scientific support and standardized clinical studies, or their results are ephemeral and not significant. Subcision is an established therapy that can lead to significant improvement in the clinical appearance of cellulite with a low adverse event profile. The study has shown the effectiveness and safety of manual subcision in the treatment of dimpling (Figs. 8, 9). The device used, designed specifically for this technique, has shown itself to be very helpful and effective in terms of practical use, aesthetic outcome and safety, with various advantages compared with other commonly used devices. Nevertheless, extending the study to a higher number of patients would be statistically more significant.
Fig. 8.
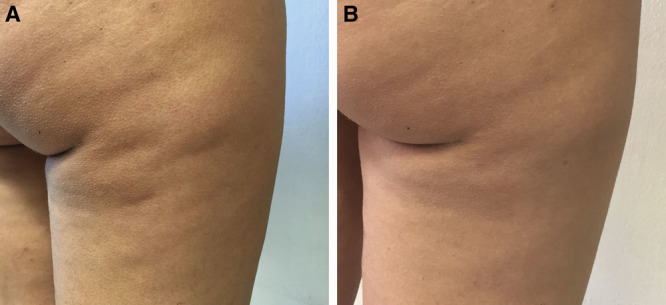
A 33-year-old woman treated with 1 subcision session using Celluerase (A). Result at 1 month (B).
Fig. 9.
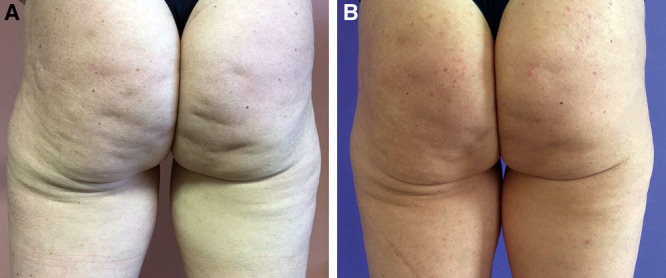
A 42-year-old woman treated with 1 subcision session using Celluerase (A). Result at 3 months (B).
Supplementary Material
Footnotes
Published online 18 May 2018.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Nürnberger F, Müller G. So-called cellulite: an invented disease. J Dermatol Surg Oncol. 1978;4:221–229.. [DOI] [PubMed] [Google Scholar]
- 2.Rossi AB, Vergnanini AL. Cellulite: a review. J Eur Acad Dermatol Venereol. 2000;14:251–262.. [DOI] [PubMed] [Google Scholar]
- 3.Hexsel DM. Body Repair. Women’s Dermatology. 2001:Nova Iorque, N.Y.: Parthenon Publishing; 586–595.. [Google Scholar]
- 4.Querleux B. Magnetic resonance imaging and spectroscopy of skin and subcutis. J Cosmet Dermatol. 2004;3:156–161.. [DOI] [PubMed] [Google Scholar]
- 5.Mirrashed F, Sharp JC, Krause V, et al. Pilot study of dermal and subcutaneous fat structures by MRI in individuals who differ in gender, BMI, and cellulite grading. Skin Res Technol. 2004;10:161–168.. [DOI] [PubMed] [Google Scholar]
- 6.Lucassen GE, van der Sluys WL, van Herk JJ, et al. The effectiveness of massage treatment on cellulite as monitored by ultrasound imaging. Skin Res Technol. 1997;3:154. [DOI] [PubMed] [Google Scholar]
- 7.Quatresooz P, Xhauflaire-Uhoda E, Piérard-Franchimont C, et al. Cellulite histopathology and related mechanobiology. Int J Cosmet Sci. 2006;28:207–210.. [DOI] [PubMed] [Google Scholar]
- 8.Hexsel DM, Abreu M, Rodrigues TC, et al. Side-by-side comparison of areas with and without cellulite depressions using magnetic resonance imaging. Dermatol Surg. 2009;35:1471–1477.. [DOI] [PubMed] [Google Scholar]
- 9.Pugliese PT. The pathogenesis of cellulite: a new concept. J Cosmet Dermatol. 2007;6:140–142.. [DOI] [PubMed] [Google Scholar]
- 10.Omi T, Sato S, Kawana S. Ultrastructural assessment of cellulite morphology: clues to a therapeutic strategy? Laser Ther. 2013;22:131–136.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turati F, Pelucchi C, Marzatico F, et al. Efficacy of cosmetic products in cellulite reduction: systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2014;28:1–15.. [DOI] [PubMed] [Google Scholar]
- 12.Rotunda AM, Avram MM, Avram AS. Cellulite: is there a role for injectables? J Cosmet Laser Ther. 2005;7:147–154.. [DOI] [PubMed] [Google Scholar]
- 13.Caruso MK, Roberts AT, Bissoon L, et al. An evaluation of mesotherapy solutions for inducing lipolysis and treating cellulite. J Plast Reconstr Aesthet Surg. 2008;61:1321–1324.. [DOI] [PubMed] [Google Scholar]
- 14.Sivagnanam G. Mesotherapy—the French connection. J Pharmacol Pharmacother. 2010;1:4–8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Godoy JMP, de Godoy ACP, Godoy MFG. Considering the hypothesis of the pathophysiology of cellulite in its treatment. Dermatol Reports. 2017;9:7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang P, Wiseman J, Jacoby T, et al. Noninvasive mechanical body contouring: (Endermologie) a one-year clinical outcome study update. Aesthetic Plast Surg. 1998;22:145–153.. [DOI] [PubMed] [Google Scholar]
- 17.Gold MH. Cellulite—an overview of non-invasive therapy with energy-based systems. J Dtsch Dermatol Ges. 2012;10:553–558.. [DOI] [PubMed] [Google Scholar]
- 18.Ortonne JP, Queille-Roussel C, Duteil L, et al. Treatment of cellulite: effectiveness and sustained effect at 6 months with endermologie demonstrated by several quantitative evaluation methods. Nouv Dermatol. 2004;23:261–269.. [Google Scholar]
- 19.Schlaudraff KU, Kiessling MC, Császár NB, et al. Predictability of the individual clinical outcome of extracorporeal shock wave therapy for cellulite. Clin Cosmet Investig Dermatol. 2014;7:171–183.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knobloch K, Joest B, Krämer R, et al. Cellulite and focused extracorporeal shockwave therapy for non-invasive body contouring: a randomized trial. Dermatol Ther (Heidelb). 2013;3:143–155.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson JD, Goldman MP. Laser, light, and energy devices for cellulite and lipodystrophy. Clin Plast Surg. 2011;38:463–74, vii.. [DOI] [PubMed] [Google Scholar]
- 22.Khan MH, Victor F, Rao B, et al. Treatment of cellulite: Part II. Advances and controversies. J Am Acad Dermatol. 2010;62:373–384.; quiz 385. [DOI] [PubMed] [Google Scholar]
- 23.Friedmann DP, Vick GL, Mishra V. Cellulite: a review with a focus on subcision. Clin Cosmet Investig Dermatol. 2017;10:17–23.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zerini I, Sisti A, Cuomo R, et al. Cellulite treatment: a comprehensive literature review. J Cosmet Dermatol. 2015;14:224–240.. [DOI] [PubMed] [Google Scholar]
- 25.Kapoor R, Shome D, Ranjan A. Use of a novel combined radiofrequency and ultrasound device for lipolysis, skin tightening and cellulite treatment. J Cosmet Laser Ther. 2017;19:266–274.. [DOI] [PubMed] [Google Scholar]
- 26.Wanner M, Avram M. An evidence-based assessment of treatments for cellulite. J Drugs Dermatol. 2008;7:341–345.. [PubMed] [Google Scholar]
- 27.Hexsel D, Dal Forno T, Hexsel C, et al. Magnetic resonance imaging of cellulite depressed lesions successfully treated by subcision. Dermatol Surg. 2016;42:693–696.. [DOI] [PubMed] [Google Scholar]
- 28.Kaminer MS, Coleman WP, 3rd, Weiss RA, et al. Multicenter pivotal study of vacuum-assisted precise tissue release for the treatment of cellulite. Dermatol Surg. 2015;41:336–347.. [DOI] [PubMed] [Google Scholar]
- 29.Orentreich DS, Orentreich N. Subcutaneous incisionless (subcision) surgery for the correction of depressed scars and wrinkles. Dermatol Surg. 1995;21:543–549.. [DOI] [PubMed] [Google Scholar]
- 30.Hexsel DM, Mazzuco R. Subcision: a treatment for cellulite. Int J Dermatol. 2000;39:539–544.. [DOI] [PubMed] [Google Scholar]
- 31.Kaminer MS, Coleman WP, 3rd, Weiss RA, et al. A multicenter pivotal study to evaluate tissue stabilized-guided subcision using the Cellfina device for the treatment of cellulite with 3-year follow-up. Dermatol Surg. 2017;43:1240–1248.. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki GH. Single treatment of grades II and III cellulite using a minimally invasive 1,440-nm pulsed Nd:YAG laser and side-firing fiber: an institutional review board-approved study with a 24-month follow-up period. Aesthetic Plast Surg. 2013;37:1073–1089.. [DOI] [PubMed] [Google Scholar]
- 33.DiBernardo BE, Sasaki GH, Katz BE, et al. A multicenter study for cellulite treatment using a 1440-nm Nd:YAG wavelength Laser with side-firing fiber. Aesthet Surg J. 2016;36:335–343.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katz B. Quantitative & qualitative evaluation of the efficacy of a 1440 nm Nd:YAG laser with novel bi-directional optical fiber in the treatment of cellulite as measured by 3-dimensional surface imaging. J Drugs Dermatol. 2013;12:1224–1230.. [PubMed] [Google Scholar]
- 35.Gorney M, Martello J. Patient selection criteria. Clin Plast Surg. 1999;26:37–40, vi.. [PubMed] [Google Scholar]
- 36.Martello J, Bailey CW., Jr. Doctor-patient relationship. The consultation. Clin Plast Surg. 1999;26:53–5, vi.. [PubMed] [Google Scholar]
- 37.Bayrakci Tunay V, Akbayrak T, Bakar Y, et al. Effects of mechanical massage, manual lymphatic drainage and connective tissue manipulation techniques on fat mass in women with cellulite. J Eur Acad Dermatol Venereol. 2010;24:138–142.. [DOI] [PubMed] [Google Scholar]
- 38.de Godoy JM, Groggia MY, Ferro Laks L, et al. Intensive treatment of cellulite based on physiopathological principles. Dermatol Res Pract. 2012;2012:834280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Namias A, Kaplan B. Tumescent anesthesia for dermatologic surgery. Cosmetic and noncosmetic procedures. Dermatol Surg. 1998;24:755–758.. [PubMed] [Google Scholar]
- 40.McCalmont TH, Leshin B. Lask GP, Moy RL. Preoperative evaluation of the cutaneous surgery patient. In: Principles and Techniques of Cutaneous Surgery. 1996:New York, N.Y.: McGraw-Hill; 101–112.. [Google Scholar]
- 41.Hoffman BB, Lefkowitz RJ. Hardman JG, Limbird LE, Mollinoff PB. Catecholamines, sympathomimetic drugs and adrenergic receptor antagonists. In: Goodman & Gilman’s Pharmacological Basis of Therapeutics. 1996:New York, N.Y.: McGraw-Hill; 199–248.. [Google Scholar]
- 42.Rossi AM, Katz BE. A modern approach to the treatment of cellulite. Dermatol Clin. 2014;32:51–59.. [DOI] [PubMed] [Google Scholar]
- 43.Lotti T, Ghersetich I, Grappone C, et al. Proteoglycans in so-called cellulite. Int J Dermatol. 1990;29:272–274.. [DOI] [PubMed] [Google Scholar]
- 44.Merlen JF, Curri SB, Sarteel AM. [Cellulitis, a conjunctive microvascular disease]. Phlebologie. 1979;32:279–282.. [PubMed] [Google Scholar]
- 45.Shoham N, Gefen A. Mechanotransduction in adipocytes. J Biomech. 2012;45:1–8.. [DOI] [PubMed] [Google Scholar]
- 46.Halberg N, Khan T, Trujillo ME, et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29:4467–4483.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Querleux B, Cornillon C, Jolivet O, et al. Anatomy and physiology of subcutaneous adipose tissue by in vivo magnetic resonance imaging and spectroscopy: relationships with sex and presence of cellulite. Skin Res Technol. 2002;8:118–124.. [DOI] [PubMed] [Google Scholar]
- 48.Rosenbaum M, Prieto V, Hellmer J, et al. An exploratory investigation of the morphology and biochemistry of cellulite. Plast Reconstr Surg. 1998;101:1934–1939.. [DOI] [PubMed] [Google Scholar]
- 49.Lemperle G, Morhenn V, Charrier U. Human histology and persistence of various injectable filler substances for soft tissue augmentation. Aesthetic Plast Surg. 2003;27:354–366.; discussion 367. [DOI] [PubMed] [Google Scholar]
- 50.Boraldi F, Croce MA, Quaglino D, et al. Cell-matrix interactions of in vitro human skin fibroblasts upon addition of hyaluronan. Tissue Cell. 2003;35:37–45.. [DOI] [PubMed] [Google Scholar]
- 51.Croce MA, Dyne K, Boraldi F, et al. Hyaluronan affects protein and collagen synthesis by in vitro human skin fibroblasts. Tissue Cell. 2001;33:326–331.. [DOI] [PubMed] [Google Scholar]


