Abstract
Rationale:
TAFRO syndrome is a systemic inflammatory disorder characterized by thrombocytopenia, anasarca, fever, reticulin fibrosis, renal dysfunction, and organomegaly. In contrast to that in multicentric Castleman disease, interleukin-6 targeting strategies seem ineffective in some TAFRO syndrome cases; however, the optimal treatment remains unclear. Here, we report 2 cases of TAFRO syndrome, where 1 with cardiomyopathy, successfully treated with tacrolimus. This is the first case report of successful treatment with tacrolimus in TAFRO syndrome.
Patient concerns:
Both patients (cases 1 and 2) developed fever, anasarca, thrombocytopenia, renal dysfunction, and mild hepatosplenomegaly.
Diagnoses:
In both patients, lymph node pathology revealed mixed type Castleman disease-like features, and bone marrow showed reticulin myelofibrosis. TAFRO syndrome was diagnosed based on the patients’ laboratory, clinical, and pathologic findings. In case 2, we observed a rare complication of cardiomyopathy with no evidence of takotsubo cardiomyopathy or viral myocarditis.
Interventions and outcomes:
In case 1, tocilizumab combined with glucocorticoids was ineffective and caused septic shock; additionally, cyclosporine A was discontinued because of hepatotoxicity. However, tacrolimus was effective in resolving TAFRO syndrome without any adverse events. In case 2, tacrolimus completely reversed TAFRO syndrome and was also effective in cardiomyopathy.
Lessons:
This report suggests that tacrolimus is potentially effective and safe as an initial treatment and a glucocorticoid-sparing agent. Our literature review shows that calcineurin inhibitors, including tacrolimus, may be effective in TAFRO syndrome. Since previous studies indicate a role of Th1 inflammation in TAFRO syndrome pathogenesis, tacrolimus may, therefore, be effective in treating TAFRO syndrome.
Keywords: calcineurin inhibitors, cardiomyopathy, cyclosporine A, multicentric Castleman disease, tacrolimus, TAFRO syndrome
1. Introduction
TAFRO syndrome was first described in Japan in 2010 as a unique variant of multicentric Castleman disease (MCD) with an aggressive clinical course and comprised thrombocytopenia, anasarca, fever, reticulin fibrosis, renal dysfunction, and organomegaly (TAFRO).[1] MCD constitutes a heterogeneous group of lymphoproliferative disorders characterized by excessive systemic inflammatory features, including frequent fever, generalized peripheral lymphadenopathy, hepatosplenomegaly, polyclonal hypergammaglobulinemia, and elevated levels of serum C-reactive protein (CRP), interleukin-6 (IL-6), and vascular endothelial growth factor (VEGF). These clinical manifestations of MCD are possibly driven by excessive proinflammatory hypercytokinemia, particularly in association with elevated levels of IL-6. On the contrary, MCD is strongly associated with human herpesvirus-8 (HHV-8), which infects B cells and expresses a viral homolog of IL-6; TAFRO syndrome is considered as a subgroup of HHV-8-negative MCD or idiopathic MCD group.[2]
Although IL-6 levels in TAFRO syndrome are elevated, many of its features differ considerably from classical MCD features, including severe thrombocytopenia and absence of hypergammaglobulinemia,[3] which is difficult to explain owing to hyper-IL-6 syndrome because IL-6 overexpression typically results in thrombocytosis and hypergammaglobulinemia. In addition, IL-6 targeting strategies seem to be ineffective for some TAFRO syndrome cases, whereas they are highly effective for MCD (91% complete response rate).[4] These findings suggest that not only IL-6 but also other proinflammatory conditions may play roles in the pathogenesis of TAFRO syndrome.[5] Furthermore, the combination of glucocorticoids and tocilizumab, an anti-IL-6 receptor antibody, increases the risk of severe infections.[6] In terms of effectiveness and adverse events, IL-6-targeting agents may not be the best choice for TAFRO syndrome; however, the optimal treatment remains unclear.
To the best of our knowledge, this is the first report of 2 cases of TAFRO syndrome successfully treated with tacrolimus, where 1 with a rare complication of cardiomyopathy, which was also completely resolved after treatment. We also discuss the relationship between the action mechanism of tacrolimus and possible pathogenesis of TAFRO syndrome.
2. Case reports
2.1. Case 1
A 68-year-old Japanese woman without medical history was admitted to our hospital with a 4-week history of abdominal distension and fever of 38.1°C. Physical examination revealed swollen cervical and axillary lymph nodes (<1 cm in diameter) and abdominal tenderness. Laboratory studies revealed anemia (hemoglobin, 7.3 g/dL); thrombocytopenia (38,000/μL); reduced immunoglobulin G (IgG, 770 mg/dL); elevated levels of alkaline phosphatase (ALP, 720 U/L), soluble interleukin-2 receptor (sIL-2R, 3060 U/mL), and CRP (2.7 mg/dL); and renal dysfunction (serum creatinine 1.6 mg/dL) with microhematuria. Test results for autoantibodies, including antinuclear antibody (ANA) and antineutrophil cytoplasmic antibody (ANCA); and viruses, including HHV-8 and human immunodeficiency virus (HIV), were negative. Computed tomography (CT) revealed systemic lymphadenopathy, bilateral pleural effusion, massive ascites, and hepatosplenomegaly. IL-6 and VEGF levels in serum (24 and 390 pg/mL, respectively) and in ascitic fluid (1800 and 63 pg/mL, respectively) were elevated. Cervical lymph node biopsy revealed atrophic germinal centers, expanded mantle zones, and proliferated high endothelial venules and small number of plasma cells in the interfollicular zones (Fig. 1A, B). Bone marrow biopsy revealed hyperplasia of megakaryocytes and reticulin fibrosis. These symptoms and histopathologic findings met the diagnostic criteria for TAFRO syndrome.[3,7]
Figure 1.
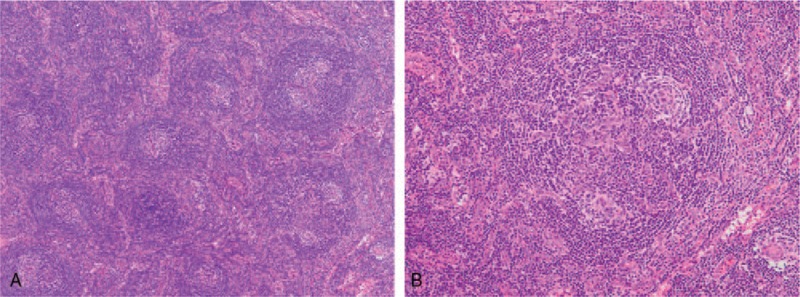
Histologic findings of the cervical lymph node. (A) Lymph node with proliferated high endothelial venules and small number of plasma cells in the interfollicular zone. Hematoxylin and eosin staining (40× magnification). (B) Atrophic germinal centers with enlarged nuclei of high endothelial cells and expanded mantle zones. Hematoxylin and eosin staining (100× magnification).
Initial treatment with 60 mg/day (1 mg/kg/day) of oral prednisolone gradually improved renal dysfunction but failed to resolve thrombocytopenia and massive ascites, requiring frequent platelet transfusion, romiplostim administration, and ascites drainage (Fig. 2). Additional treatment with 8 mg/kg of tocilizumab failed to improve the symptoms; moreover, the patient developed septic shock from empyema caused by methicillin-sensitive Staphylococcus aureus, necessitating the discontinuation of tocilizumab. Additionally, 150 mg/day (2.5 mg/kg/day) of cyclosporine A had to be discontinued within a week because of hepatotoxicity. Referring to literature demonstrating the effectiveness of calcineurin inhibitor,[8,9] we administered 4 mg/day (0.07 mg/kg/day) of tacrolimus targeting 5 to 10 ng/mL of trough concentrations, after obtaining informed consent from the patient. Tacrolimus improved renal dysfunction and thrombocytopenia rapidly without requiring platelet transfusion or romiplostim administration. Abdominal distension gradually subsided without ascites drainage: CT showed complete ascites resolution 4 weeks after tacrolimus initiation, and glucocorticoids were tapered off rapidly. The patient has had an uneventful course without relapse for more than 3 years.
Figure 2.
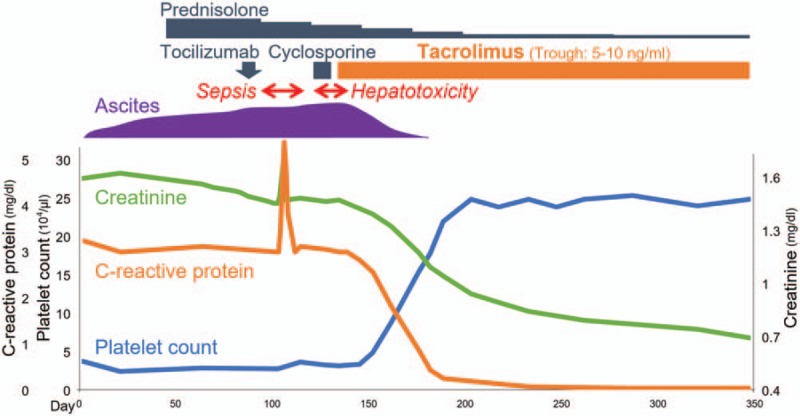
Clinical course of case 1.
2.2. Case 2
A 17-year-old Japanese man without medical history was admitted to our hospital with a 4-week history of abdominal pain, nausea, and fever of 38.8°C. Physical examination revealed swollen axillary lymph nodes (<1 cm in diameter) and abdominal tenderness. Laboratory studies revealed anemia (hemoglobin, 6.7 g/dL); thrombocytopenia (68,000/μL); reduced IgG (810 mg/dL); elevated levels of ALP (870 U/L), sIL-2R (1250 U/mL), and CRP (23.0 mg/dL); and renal dysfunction (serum creatinine 1.6 mg/dL) with microhematuria. Test results for autoantibodies, including ANA and ANCA; and viruses, including HHV-8 and HIV, were negative. Electrocardiogram and echocardiography were normal. CT showed bilateral pleural effusion, ascites, and mild hepatosplenomegaly. Serum IL-6 and VEGF levels (70 and 730 pg/mL, respectively) and IL-6 and VEGF levels in the pleural effusion (370 and 88 pg/mL) and ascitic fluid (460 and 100 pg/mL) were elevated. Axillary lymph node biopsy revealed expanded mantle zones and proliferated high endothelial venules in atrophic germinal centers and interfollicular zones with small number of plasma cells. Bone marrow biopsy revealed normoplasia of megakaryocytes and reticulin fibrosis. The patient was diagnosed with TAFRO syndrome.[3,7]
The CRP level and fever improved spontaneously, but thrombocytopenia, creatinine level, pleural effusion, and ascites deteriorated (Fig. 3). Four weeks after admission, the patient suddenly developed cardiogenic shock without obvious causes; evidence of myocardial infarction, takotsubo cardiomyopathy, or viral myocarditis was not detected. Echocardiography revealed severe and diffuse hypokinesis of both ventricles (left ventricular ejection fraction, LVEF 20%) and moderate pericardial effusion (Fig. 4A). The brain natriuretic peptide (BNP) level was 1230 pg/mL (reference range, <18.4 pg/mL). We initiated methylprednisolone pulse therapy at 1000 mg/day for 3 consecutive days, followed by 60 mg/day (1 mg/kg/day) of oral prednisolone and 4 mg/day (0.07 mg/kg/day) of tacrolimus targeting 5 to 10 ng/mL of trough concentrations, with informed consent. One week after treatment initiation, repeat echocardiography revealed improved wall motion (LVEF 45%) and pericardial effusion. Four weeks later, serial echocardiography showed normal wall motion (LVEF 60%) without pericardial effusion (Fig. 4B), and a decrease in BNP level (8.0 pg/mL). Late gadolinium-enhanced cardiac magnetic resonance imaging showed no myocardial scarring. Thrombocytopenia, renal dysfunction, and anasarca had also improved since tacrolimus treatment initiation, and glucocorticoids were tapered off rapidly. The patient has had an uneventful course without relapse for more than 2 years.
Figure 3.
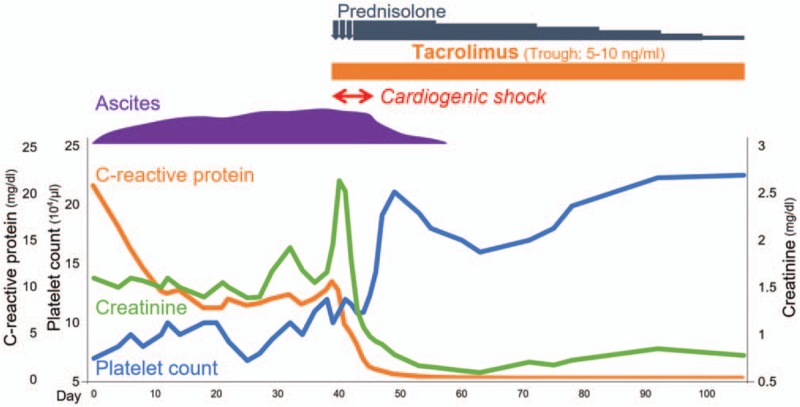
Clinical course of case 2.
Figure 4.
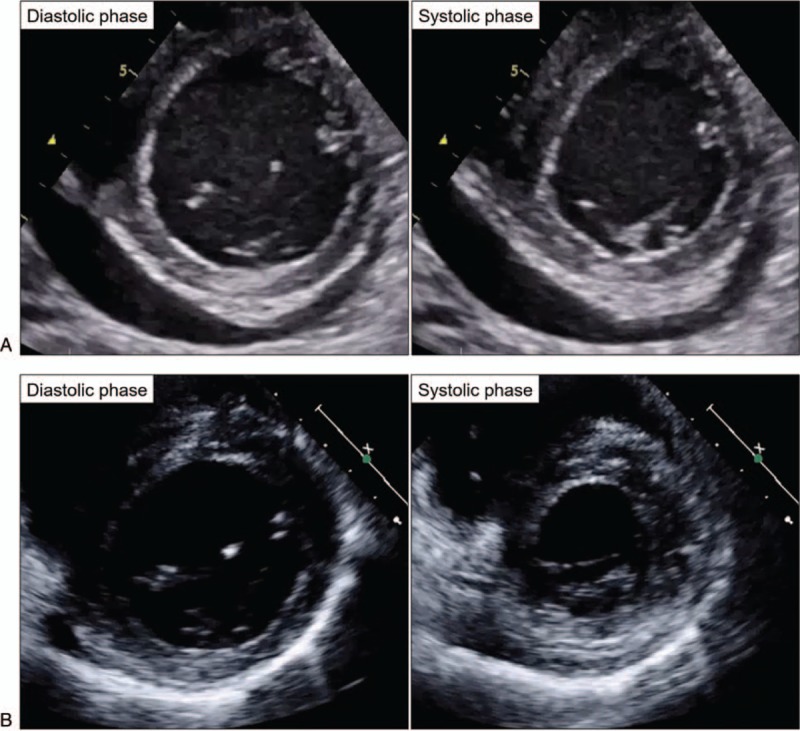
Clinical course of cardiomyopathy (echocardiography). (A) Severe and diffuse hypokinesis (LVEF 20%) and moderate pericardial effusion were detected. (B) Four weeks after treatment initiation, wall motion improved (LVEF 60%), and pericardial effusion disappeared. LVEF, left ventricular ejection fraction.
This case report did not involve any human trials, and ethical review board approval was not necessary. Written informed consent was obtained from both the patients (cases 1 and 2) for the publication of their clinical data.
3. Discussion
This is the first case report of TAFRO syndrome successfully treated with tacrolimus. Although a standard treatment strategy for TAFRO syndrome is not yet established, our cases suggest that tacrolimus is potentially effective and safe as an initial treatment and a glucocorticoid-sparing agent.
First, tacrolimus was shown to be effective in our TAFRO syndrome cases. We reviewed the treatments of previous and current TAFRO syndrome cases to assess treatments and their responses; remission was defined as the absence of abnormalities on physical examinations and laboratory or imaging studies. Patients were treated with glucocorticoids alone or with combination therapy, including IL-6-targeting agents (tocilizumab, siltuximab); calcineurin inhibitors (tacrolimus, cyclosporine A); rituximab; and cytotoxic chemotherapy (Table 1). Treatment effectiveness greatly varied. Our literature review showed that calcineurin inhibitors, including tacrolimus, were effective for TAFRO syndrome, with 71% of patients attaining remission. Although the effectiveness of tacrolimus has not been compared with that of cyclosporine A, the superiority of tacrolimus over cyclosporine A has been extensively reported for kidney or liver transplant rejection, particularly in cases of glucocorticoid-resistant rejection.[10,11] While IL-6-targeting agents were frequently used for TAFRO syndrome, only 45% of patients attained remission.
Table 1.
Responses and adverse events associated with treatments of TAFRO syndrome.
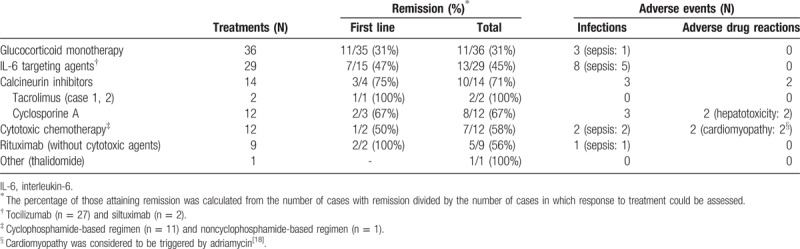
Second, tacrolimus was safe in our TAFRO syndrome cases. Literature review revealed that the frequency of severe infections in patients treated with calcineurin inhibitors was low (Table 1). Hepatotoxicity was an adverse drug reaction of cyclosporine A in some cases, including case 1, which was successfully treated by replacing cyclosporine A with tacrolimus. In fact, hepatotoxicity occurs less frequently with tacrolimus than with cyclosporine A.[12] The frequency of severe infections, including septic shock, was high for treatment with tocilizumab, particularly in combination with high-dose glucocorticoids, as shown in case 1. In fact, in a survey of tocilizumab for rheumatoid arthritis, the most frequent adverse events were severe infections; the significant risk factor was identified as a glucocorticoid dosage of ≥5 mg/day (prednisolone equivalent).[6] In terms of safety, tacrolimus may be useful in treating TAFRO syndrome.
Third, the mechanism of action of tacrolimus and possible pathogenesis of TAFRO syndrome indicate that tacrolimus may be a rational treatment choice. Tacrolimus suppresses Th1 cell responses and impairs transcription of IL-2 and several other proinflammatory cytokines.[13] In accordance with previous studies, we consider that Th1 inflammation may play a role in TAFRO syndrome pathogenesis. Increased IL-2 levels in the ascitic fluid in patients with TAFRO syndrome may suggest the contribution of endogenous IL-2 to disease pathogenesis.[14] Additionally, high serum levels of interferon-γ-induced protein 10 (IP-10) are strongly correlated with TAFRO syndrome but not classical MCD.[15] Overexpression of IP-10 is common in Th1-type inflammatory diseases and is critical for Th1-mediated immune responses.[16] Tacrolimus suppresses Th1-cell responses, which may be one of the underlying mechanisms for the effectiveness of tacrolimus in TAFRO syndrome. Notably, in case 1, treatment with tacrolimus was successful for tocilizumab-resistant TAFRO syndrome; cyclosporine A has also been shown to be effective in tocilizumab-resistant cases,[8,9] suggesting that in addition to IL-6, Th1 inflammation may play a role in TAFRO syndrome pathogenesis.
For case 2, we reported a complete reversal of cardiomyopathy, a rare complication of TAFRO syndrome, using tacrolimus and glucocorticoids. Few cases of TAFRO syndrome with cardiomyopathy have been reported,[17,18] one of which was refractory to tocilizumab, suggesting that some proinflammatory cytokines other than IL-6 may be involved.[17] In fact, in addition to IL-6, Th1 cytokines, such as IL-2 and tumor necrosis factor-α, have direct negative inotropic effect in a concentration-dependent, reversible manner, even after short-term exposure.[19] Tacrolimus may therefore be effective, although the exact mechanism of cardiomyopathy has been poorly understood.
Our 2 cases suggested that tacrolimus is potentially effective and safe for TAFRO syndrome. However, with only 2 cases, it is early to conclude that tacrolimus is truly effective for TAFRO syndrome. The optimal treatment and precise pathogenesis of TAFRO syndrome remain unclear, and further research is needed to validate our findings and conclusions.
Author contributions
Validation: Daisuke Waki, Jun Saegusa, Akio Morinobu.
Writing – original draft: Taiichiro Shirai.
Writing – review & editing: Taiichiro Shirai, Akira Onishi.
Footnotes
Abbreviations: ALP = alkaline phosphatase, ANA = antinuclear antibody, ANCA = antineutrophil cytoplasmic antibody, BNP = brain natriuretic peptide, CRP = C-reactive protein, CT = computed tomography, HHV-8 = human herpesvirus 8, HIV = human immunodeficiency virus, IgG = immunoglobulin G, IL-6 = interleukin-6, IP-10 = interferon-γ-induced protein 10, LVEF = left ventricular ejection fraction, MCD = multicentric Castleman disease, sIL-2R = soluble interleukin-2 receptor, TAFRO = thrombocytopenia, anasarca, fever, reticulin fibrosis, renal dysfunction, and organomegaly, VEGF = vascular endothelial growth factor.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Takai K, Nikkuni K, Momoi A, et al. Thrombocytopenia with reticulin fibrosis accompanied by fever, anasarca and hepatosplenomegaly: a clinical report of five cases. J Clin Exp Hematop 2013;53:63–8. [DOI] [PubMed] [Google Scholar]
- [2].Fajgenbaum DC, Van Rhee F, Nabel CS. HHV-8-negative, idiopathic multicentric Castleman disease: Novel insights into biology, pathogenesis, and therapy. Blood 2014;123:2924–33. [DOI] [PubMed] [Google Scholar]
- [3].Iwaki N, Fajgenbaum DC, Nabel CS, et al. Clinicopathologic analysis of TAFRO syndrome demonstrates a distinct subtype of HHV-8-negative multicentric Castleman disease. Am J Hematol 2016;91:220–6. [DOI] [PubMed] [Google Scholar]
- [4].Liu AY, Nabel CS, Finkelman BS, et al. Idiopathic multicentric Castleman's disease: a systematic literature review. Lancet Haematol 2016;3:163–75. [DOI] [PubMed] [Google Scholar]
- [5].Masaki Y, Nakajima A, Iwao H, et al. Japanese variant of multicentric Castleman's disease associated with serositis and thrombocytopenia – A report of two cases: Is TAFRO syndrome (Castleman-Kojima disease) a distinct clinicopathological entity? J Clin Exp Hematop 2013;53:79–85. [DOI] [PubMed] [Google Scholar]
- [6].Koike T, Harigai M, Inokuma S, et al. Postmarketing surveillance of tocilizumab for rheumatoid arthritis in Japan: interim analysis of 3881 patients. Ann Rheum Dis 2011;70:2148–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Masaki Y, Kawabata H, Takai K, et al. Proposed diagnostic criteria, disease severity classification and treatment strategy for TAFRO syndrome, 2015 version. Int J Hematol 2016;103:686–92. [DOI] [PubMed] [Google Scholar]
- [8].Yamaga Y, Tokuyama K, Kato T, et al. Successful treatment with cyclosporin a in tocilizumab-resistant TAFRO syndrome. Intern Med 2016;55:185–90. [DOI] [PubMed] [Google Scholar]
- [9].Konishi Y, Takahashi S, Nishi K, et al. Successful treatment of TAFRO syndrome, a variant of multicentric Castleman's disease, with cyclosporine A: possible pathogenetic contribution of interleukin-2. Tohoku J Exp Med 2015;236:289–95. [DOI] [PubMed] [Google Scholar]
- [10].Webster AC, Woodroffe RC, Taylor RS, et al. Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: meta-analysis and meta-regression of randomised trial data. BMJ 2005;331:810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Haddad E, Mcalister V, Renouf E, et al. Cyclosporin versus tacrolimus for liver transplanted patients. Cochrane Database Syst Rev 2006;18:CD005161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Grady JGO, Hardy P, Burroughs AK, et al. Randomized controlled trial of tacrolimus versus microemulsified cyclosporin (TMC) in liver transplantation: poststudy surveillance to 3 years. Am J Transpl 2007;7:137–41. [DOI] [PubMed] [Google Scholar]
- [13].Med JJE, Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today 1992;13:1433–9. [DOI] [PubMed] [Google Scholar]
- [14].Kubokawa I, Yachie A, Hayakawa A, et al. The first report of adolescent TAFRO syndrome, a unique clinicopathologic variant of multicentric Castleman's disease. BMC Pediatr 2014;14:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Iwaki N, Gion Y, Kondo E, et al. Elevated serum interferon γ-induced protein 10 kDa is associated with TAFRO syndrome. Sci Rep 2017;7:42316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dufour JH, Dziejman M, Liu MT, et al. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol 2002;168:3195–204. [DOI] [PubMed] [Google Scholar]
- [17].Sumie H, Koichiro O, Hideaki T, et al. Successful treatment by rituximab in a patient with TAFRO syndrome with cardiomyopathy. Nihon Rinsho Meneki Gakkai Kaishi 2016;39:64–71. [DOI] [PubMed] [Google Scholar]
- [18].Yasuda S, Tanaka K, Ichikawa A, et al. Aggressive TAFRO syndrome with reversible cardiomyopathy successfully treated with combination chemotherapy. Int J Hematol 2016;104:512–8. [DOI] [PubMed] [Google Scholar]
- [19].Finkel MS, Oddis CV, Jacob TD, et al. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science 1992;257:387–9. [DOI] [PubMed] [Google Scholar]


