Supplemental Digital Content is available in the text
Keywords: diabetes mellitus, hand strength, insulin
Abstract
Diabetes mellitus (DM) is a significant chronic disease, and health burden from DM is increasing. Recently, studies on the relationship between handgrip strength, which is a measuring tool for muscle strength, and type 2 DM were published. However, the results have been conflicting. In addition, few studies that used data from adults in Korea have been conducted. Thus, this study aimed to identify the association between handgrip strength as well as type 2 DM and insulin resistance in adults using data from the Korea National Health and Nutrition Examination Survey (KNHANES) 2014 to 2015. Inflammation is a condition affecting the muscle strength of individuals with type 2 DM; therefore, its mediating effects were also examined.
We included 8208 participants aged between 19 and 80 years who had undergone a handgrip test and had received information about type 2 DM. General linear and binary logistic regression models were used to examine the association between handgrip strength and type 2 DM variables. In addition, mediation analysis was conducted to estimate the role of inflammation in the relationship between handgrip strength and type 2 DM.
After adjusting for age, sex, education, alcohol consumption, lifetime smoking, obesity, and aerobic physical activity, handgrip strength was inversely associated with fasting glucose, HbA1c, and fasting insulin levels as well as the homeostasis model assessment of insulin resistance (HOMA-IR) score. Multivariable logistic regression analyses showed that handgrip strength was significantly inversely associated with type 2 DM and insulin resistance. The high-sensitivity C-reactive protein (hs-CRP), an inflammation-related biomarker, mediated approximately 10% of the association between handgrip strength and type 2 DM.
Using large, well-defined, nationally representative cross-sectional data on adults in Korea, we found that handgrip strength, which is an indicator of muscle strength, was associated with type 2 DM.
1. Introduction
Diabetes mellitus (DM) is a chronic disease with a prevalence rate that has increased from 4.7% in 1980 to 8.5% in 2014, and its prevalence is increasing most rapidly in middle- and low-income countries.[1] Numerous studies have shown that DM is an important cause of vision loss,[2] chronic kidney disease,[3] lower limb amputation,[4] and other long-term consequences that have a significant effect on the quality of life of an individual.[5] The significant increase in the prevalence indicates a substantial economic burden on health-care systems.[6] In the USA, DM has been associated with high health-care cost, with a significant increase over the past 2 decades.[7] Therefore, early screening and promotion of healthy aging among higher-risk populations are essential to reduce and prevent DM as well as decrease the DM-associated healthcare burden.
Handgrip strength is a simple and cost-effective tool utilized to measure muscular strength, and it has been useful for the diagnosis of sarcopenia in epidemiologic studies.[8] Some studies have reported that early mid-life handgrip strength is associated with functional limitation and disability 25 years later. Thus, handgrip strength has been considered a predictor of healthy aging.[9]
Previous studies on the relationship between handgrip strength and DM have been controversial. Some studies have shown a significant inverse association between handgrip strength and DM.[10–15] However, Leong et al[16] have found no significant association between handgrip strength and DM. Furthermore, a recent Mendelian randomization study has shown no association between handgrip strength and type 2 DM.[17]
The prevalence rates of DM in the USA and Korea (9.1% vs 9.5%, respectively) were similar. However, there are fundamental differences in some of the risk factors in both countries, such as a higher obesity prevalence in the USA than in Korea (35% vs 6.3%, respectively).[18] van der Kooi et al[15] have shown that handgrip strength differed between ethnic groups (Dutch, South Asian Surinamese, African Surinamese, Ghanaian, Turkish, and Moroccan). The Dutch had the highest handgrip strength, whereas the South Asian Surinamese had the lowest handgrip strength. Ntuk et al[19] have also shown that the handgrip strength of South Asian men and women was approximately 5 to 6 kg lower than that of other ethnic groups. Therefore, the attributable risk for DM that is associated with low grip strength was substantially higher in the South Asian population than in the Western population. To date, few studies on the association between handgrip strength and type 2 DM in adults in Korea have been conducted.
Thus, this study aimed to investigate the association between handgrip strength and type 2 DM in adults in Korea using nationwide data. Furthermore, we assessed the mediation effect of inflammation in the relationships between handgrip strength and type 2 DM because some studies have suggested that inflammation-mediated muscular strength induced impaired fasting glucose.
2. Methods
2.1. Study population
Data were collected from the Korea National and Health Examination Survey (KNHANES) VI (2013–2015), which is a nationwide cross-sectional survey conducted annually to evaluate the health and nutrition status of the Korean populations. Data from KNHANES VI contain detailed information on the demographic characteristics, health behavior, and medical history of the participants.
We used data between 2014 and 2015 to measure handgrip strength. The following were excluded from the study: participants who were below 19 years (n = 3009), those with missing information on fasting glucose level, those who did not answer the questionnaire on type 2 DM diagnosis and medications (n = 2666), and those with missing data on right- or left-hand grip strength (n = 1047).
Finally, 8208 participants (2014: 3900 participants and 2015: 4308 participants) were included in the present study (Fig. 1).
Figure 1.
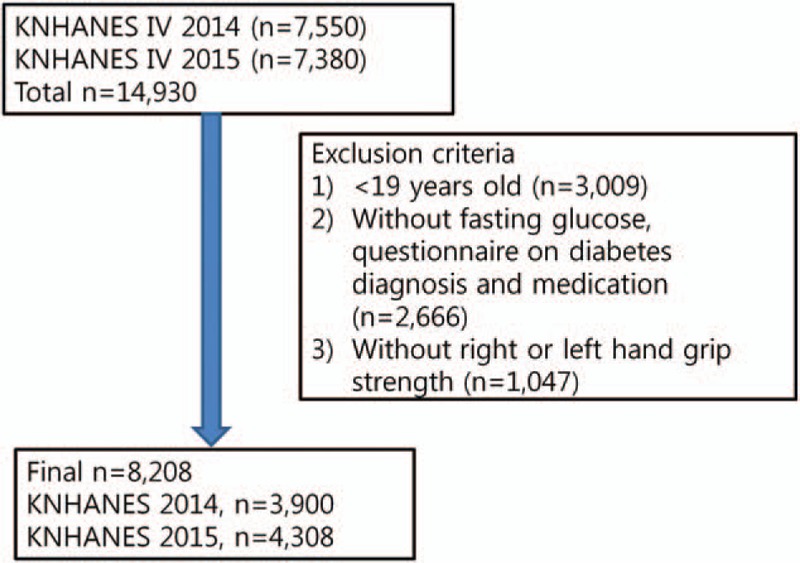
Flow diagram showing the number of participants who were excluded and the number of data that were analyzed.
2.2. Ethics statement
The KNHANES VI was conducted by the Korea Center for Disease Control and Prevention (KCDC). All survey protocols were approved by the institutional review board of the KCDC (approval numbers: 2013-07CON-03-4C, 2013-12EXP-03-5C, and 2015-01-02-6C).
A written informed consent was obtained from all participants, and this study was conducted in accordance with the Declaration of Helsinki. Original data are publicly available for free in the KNHANES website (http://knhanes.cdc.go.kr) for purposes, such as academic research. The details of the KNHANES data could be found elsewhere.[20]
2.3. Measurement of handgrip strength
The handgrip strength of each hand was measured 3 times using a digital grip strength dynamometer (TKK 5401; Takei Scientific Instruments Co, Ltd, Tokyo, Japan). Trained medical technicians instructed the participants who are in sitting position to hold the dynamometer with the distal interphalangeal finger joints of the hand at 90° to the handle and to squeeze the handle as firmly as they could. After the participants had slowly stood up, the handgrip strength was measured during expiration. Study participants conducted 3 attempts per hand, with 1-minute rest period between each attempt to reduce the effect of fatigue due to repetition. The measurements of handgrip strength were presented as an average of the 3 measurements with either hand.[8] Hand grip strength was normalized to body weight.
2.4. Measurement of glucose, HbA1c, insulin, and high-sensitivity C-reactive protein (hs-CRP) levels
All blood samples were collected in fasting state and analyzed within 24 hours after sampling. Plasma glucose level was measured using hexokinase UV with a Hitachi 7600 Automatic Analyzer (Hitachi, Tokyo, Japan). HbA1c values were analyzed using Tosoh G8 high-performance liquid chromatography (Tosoh, Tokyo, Japan). Serum insulin level was analyzed using 1470 WIZARD gamma counter immunoradiometric assay (PerkinElmer, Turku, Finland). Insulin level was measured in 2015 only. Hs-CRP level was measured using Cobas immunoturbidimetry (Roche, Berlin, Germany) in 2015 only.
2.5. Homeostasis model assessment of insulin resistance (HOMA-IR)
HOMA-IR was calculated using the following formula: HOMA-IR = [fasting glucose (mg/dL) × fasting insulin (mIU/L)]/40.[21] The cut-off value of HOMA-IR was 2.5.[22] Fasting insulin level was measured in 2015 only. Therefore, data on the HOMA-IR score was only available from KNHANES VI 2015.
2.6. Assessment of covariates
A health questionnaire was used to obtain information on age (years), sex (male and female), and educational status (≤ elementary school, middle school, high school, and ≥ university). Trained health technicians measured the body weight (kg) and height (cm) of all participants according to standardized procedures. Body mass index (BMI) was calculated as body weight in kg divided by height in m2.
We categorized the participants according to weight based on the World Health Organization criteria[23]: normal weight (BMI < 25 kg/m2), overweight (25 kg/m2 ≤BMI < 30 kg/m2), and obesity (BMI ≥30 kg/m2). Participants were divided into 2 groups: those who had never consumed alcohol in their lifetime and those who consumed alcohol. The participants were also divided into 2 groups in terms of lifetime smoking status: those who had smoked <5 packets of cigarettes and those who had smoked ≥5 packets of cigarettes. Participants who engaged in aerobic physical activity were defined as those who engage in physical activity for least 2 hours and 30 minutes per week, medium- or high-intensity physical activity for 1 hour and 15 minutes, or both medium- and high-intensity physical activity (1 minute of high-intensity workout = 2 minutes of medium-intensity workout). Further details of the measurements from KNHANES VI can be found in their website (http://www.knhanes.cdc.go.kr).
2.7. Statistical analysis
Differences in the demographic and anthropometric characteristics according to type 2 DM were compared using the Student t test or χ2 test, respectively. We assessed the association between handgrip strength and fasting glucose and HbA1c levels using generalized additive models (GAM).
To confirm the relationships between handgrip strength as well as fasting glucose, HbA1c, and insulin levels and HOMA-IR score, a multivariate linear regression analysis was performed after adjusting several variables. Models were initially run after adjusting for age and sex (Model 1) and then repeated after adding lifetime smoking and alcohol consumption, education, obesity, and aerobic physical activity as additional covariates (Model 2).
The study population was stratified into 2 groups depending on the presence of type 2 DM (fasting glucose ≥126 or use of DM medications or insulin injections or medical diagnosis of DM) or insulin resistance (HOMA-IR score ≥2.5). Multivariate binary logistic regression models were used to examine the associations between handgrip strength relative to body weight (per 1 kg/kg body weight difference) as well as type 2 DM and insulin resistance.
We used the medeff module in Stata[24] to assess whether hs-CRP level mediates the association between handgrip strength as well as fasting glucose and HbA1c levels. The medeff command estimates mediation effects, thus providing the percentage of the indirect effect accounted for by the factors that were assessed.
A P value <.05 was considered statistically significant. Continuous and categorical variables were expressed as mean ± standard deviation and n (%), respectively. Analyses were performed using STATA version 13 (Stata Corp, College Station, TX). GAM was performed using the R program version 3.3.3 (The Comprehensive R Archive Network: http://cran.r-project.org) for Windows.
3. Results
The mean age of the participants (n = 8208) was 45.12 (range: 19–80) years, and among them, 4541 (49%) were women. In total, 909 (9%) participants presented with type 2 DM. Based on the data from KNHANES 2015 (n = 4306), 1023 (23%) participants were insulin resistant. The mean right-hand grip strength was 32.64 (range: 5.8–70.57) kg, and the mean left-hand grip strength was 31.10 (range: 5.6–65.67) kg. The normalized grip strengths of the right and left hands were 0.50 (range: 0.11–1.01) kg and 0.48 (range: 0.09–0.94) kg, respectively.
The means of the handgrip strength levels were as follows: 40.51 (95% CI: 40.15–40.87) and 24.39 (95% CI: 24.17–24.61) for the right hand as well as 38.83 (95% CI: 38.48–39.18) and 23.00 (95% CI: 22.79–23.22) for left hand in men and women, respectively. When the average grip strength of the right and left hands was defined, the mean grip strengths were 39.67 (95% CI: 39.32–40.02) and 23.70 (95% CI: 23.48–23.91) for men and women aged between 19 and 80 years, respectively. The mean normalized grip strengths were 0.56 (95% CI: 0.56–0.57) and 0.42 (95% CI: 0.41–0.42) for men and women aged between 19 and 80 years, respectively.
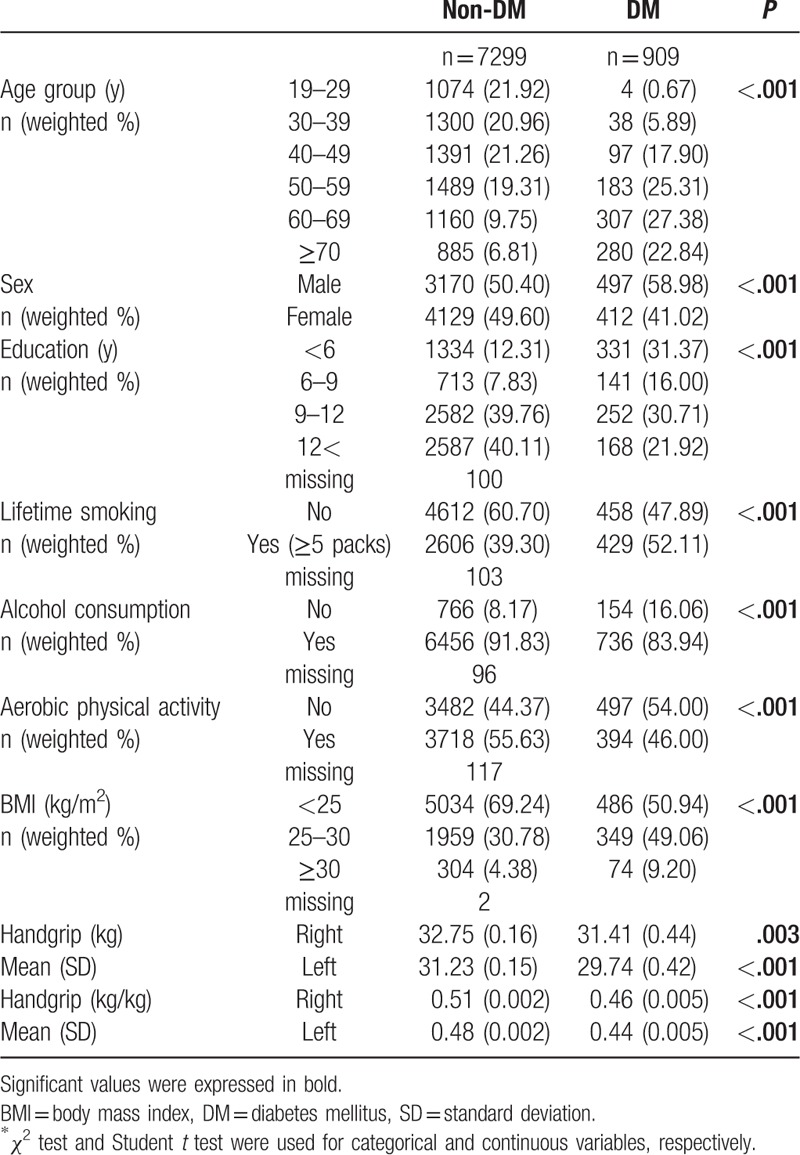
As shown in Table 1, statistically significant differences were observed between the type 2 DM and non-type 2 DM groups in terms of age, sex, education, lifetime smoking and alcohol consumption, aerobic physical activity, and BMI. Handgrip strength and normalized handgrip strength were higher in the non-type 2 DM group than in the type 2 DM group.
Table 1.
General characteristics of the study population (n = 8208).
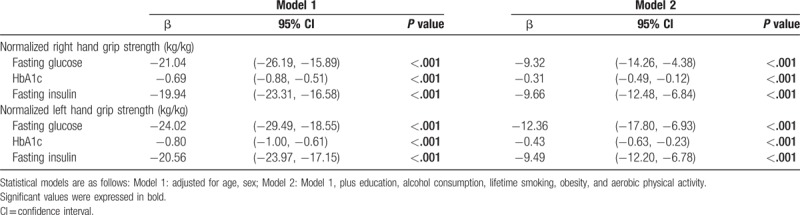
There was an inverse linear relationship between handgrip strength as well as fasting glucose and HbA1c levels using GAM (Supplemental Figs. S1 and S2).
After adjusting for age, sex, education, physical activity, lifetime smoking and alcohol consumption, obesity, and aerobic physical activity, handgrip strength was inversely associated with fasting glucose, HbA1c, and fasting insulin levels and HOMA-IR score (P < .001) (Table 2).
Table 2.
Association between handgrip strength and fasting glucose, HbA1c, and fasting insulin using multivariate linear regression analysis.
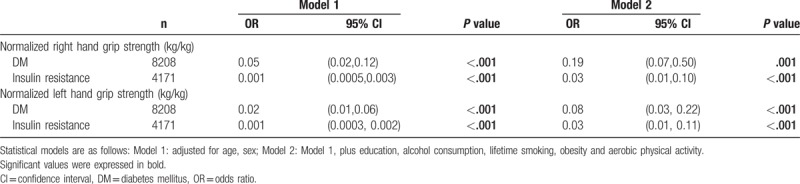
In the multivariate logistic regression analysis, handgrip strength was inversely associated with type 2 DM and insulin resistance (HOMA-IR score ≥2.5) (P < .001) (Table 3).
Table 3.
Association between handgrip strength and risk of diabetes and insulin resistance using multivariate binary logistic regression.
The mediation effect rate of hs-CRP in the relationship between handgrip strength as well as fasting glucose and HbA1c levels ranged from 8% to 11% (P < .05).
In the sensitivity analysis, we conducted a multiple regression analysis that excluded sex. The association between handgrip strength as well as fasting glucose, HbA1c, and fasting insulin levels and HOMA-IR score was significant in both men and women (all P value < .05, except the association between the left-hand grip strength and fasting glucose in women) (Supplemental Table 1). The results did not change after excluding sex.
4. Discussion
In this nationwide survey on adults in Korea, muscular strength, measured via a handgrip test, was inversely associated with fasting glucose, HbA1c, and fasting insulin levels and HOMA-IR score. In addition, handgrip strength was inversely associated with type 2 DM and insulin resistance. Since handgrip strength is a noninvasive, low-cost measurement, it may be a useful marker for identifying individuals at risk of type 2 DM in clinical or public health practice settings. hs-CRP mediated the relationship between handgrip strength and DM.
Our findings are consistent with those of previous study. Ntuk et al[19] have shown that a lower grip strength was associated with a higher prevalence of DM in African, South Asian, and European ethnic groups aged between 40 and 69 years, which includes 418,656 adults in the UK Biobank study. Peterson et al[13] have shown that every 0.05 kg decrease in the normalized handgrip strength was independently associated with a 1.49 (95% CI: 1.42–1.56) and 1.17 (95% CI: 1.11–1.23) odds for DM in American (n = 4544) and Chinese adults (n = 6030), respectively. In older Mexican Americans (>65 years, n = 1903), muscle weakness was associated with DM (hazard ratio: 1.05; 95% CI: 1.02–1.09).[12] van der Kooi et al[15] have shown that handgrip strength had an inverse association with type 2 DM (OR: 0.95; 95% CI: 0.92–0.97) in 12,594 individuals (Dutch, 2086; South Asian Surinamese, 2216; African Surinamese, 2084; Ghanaian, 1786; Turkish, 2223; and Moroccan, 2199). Li et al[10] have shown that a low handgrip strength was a risk factor for type 2 DM in men (n = 1632) in Australia. Loprinzi and Loenneke[11] have shown that handgrip strength was associated with the type 2 DM in adults (men: OR 0.86 and 95% CI: 0.79–0.94 in men; OR: 0.82 and 95% CI: 0.69–0.97 in women) in the USA. Hamasaki et al[25] have reported that handgrip strength was inversely associated with plasma glucose and serum C-peptide levels after adjusting for age, sex, and BMI. However, it could be used as a prognostic indicator of type 2 DM in 1282 patients in Japan. In 959 adolescents in the USA, handgrip strength was inversely associated with fasting insulin level, HOMA-IR score, and 2-hour glucose level.[26] However, Leong et al[16] have recently reported that the inverse association before adjustment, which was not recorded after adjustment, suggested that confounders might account for the association between handgrip strength and DM in 139,691 participants in 17 countries in previous studies.
The underlying mechanisms for the observed associations between the handgrip strength of individuals with type 2 DM and insulin resistance are not yet fully elucidated. The loss of muscle mass and strength leads to a decreased surface area for glucose transport and to the exacerbation of insulin resistance.[27] Inflammation, which is an important factor of insulin resistance, is related to low muscular strength.[28] A higher level of tumor necrosis factor-alpha (TNF-α) was associated with the decline in muscle strength[29] and elevated levels of inflammatory markers, particularly CRP and interleukin-6 (IL-6), which can predict the development of type 2 DM.[30] Some studies have shown that reduced muscle strength was a risk factor for type 2 DM. However, this association was not mediated by inflammatory markers, such as IL-6 and TNF-α, in 1632 participants in Australia.[10] Their study was limited to men aged ≥35 years. Thus, it could not be generalized to women.[10] In contrast to previous studies, we found an evidence that hs-CRP mediates the association between muscle strength and type 2 DM, and the indirect effect of hs-CRP accounted for approximately 10% of the total effect. Other mechanisms were associated with low muscle strength, such as fat accumulation in the skeletal muscle combined with low mitochondrial oxidative capacity, resulting in insulin resistance.[31,32] The precise mechanisms for the observed associations must be examined in future studies.
The present study has key strengths. First, this study evaluated the effect of muscle strength on type 2 DM and insulin resistance using a well-defined nationally representative sample of adults in Korea. Second, to the best of our knowledge, this is the first study that conducted a mediation analysis on inflammation to identify the mechanism of the effect of muscle strength on DM. Our findings have important implications. Adults with low muscle strength may have already been susceptible to type 2 DM owing to increased insulin resistance. Exercise training that consists of aerobic exercise, resistance training, or a combination of both or intensive exercise intervention strategies are effective in reducing HbA1c level in patients with type 2 DM.[33,34] A recent report has suggested that the benefit of physical activity on mortality differed according to the level of cardiorespiratory or grip strength.[35]
This study had several limitations. First, it is not possible to make causal inferences because this is a cross-sectional study. Second, handgrip strength reflected only a part of the overall muscular strength. Cardiorespiratory fitness measurements that affect insulin resistance were not performed. Third, KNHANES data on physical activity comprised a self-reported scale. Fourth, this study only included adults in Korea. Therefore, these results may not be generalizable to populations of different ethnicities. Fifth, HOMA-IR is an indirect measurement of insulin resistance, and the use of the gold-standard hyperinsulinemic-euglycemic clamp test could have yielded different results. Finally, the confounders, such as smoking and alcohol consumption, were measured subjectively (self-report) rather than objectively. Our cutoffs could lead to misclassification bias because they do not distinguish between moderate and heavy or current and past consumption for drinking or smoking.
5. Conclusion
Hand muscle strength is associated with type 2 DM and insulin resistance. Inflammation mediated the relationship between muscle strength and type 2 DM. Further studies must be conducted to determine whether interventions that improve muscle strength are effective in preventing insulin resistance and type 2 DM.
Author contributions
Conceptualization: Mee-Ri Lee, Sung Min Jung.
Data curation: Mee-Ri Lee.
Formal analysis: Mee-Ri Lee.
Investigation: Mee-Ri Lee, Hyuk Bang.
Methodology: Mee-Ri Lee, Yong Bae Kim.
Supervision: Sung Min Jung, Hwa Sung Kim, Yong Bae Kim.
Writing – original draft: Mee-Ri Lee.
Writing – review & editing: Mee-Ri Lee.
Supplementary Material
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, DM = diabetes mellitus, GAM = generalized additive models, HOMA-IR = homeostasis model assessment-insulin resistance, hs-CRP = high-sensitivity C-reactive protein, KCDC = Korea Center for Disease Control and Prevention, KNHANES = Korea National Health and Nutrition Examination Survey, OR = odds ratio.
This work was supported by the Soonchunhyang University Research Fund.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Organization WH. Global Report on Diabetes. Geneva, Switzerland: World Health Organization; 2016. [Google Scholar]
- [2].Ting DS, Cheung GC, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol 2016;44:260–77. [DOI] [PubMed] [Google Scholar]
- [3].Zelnick LR, Weiss NS, Kestenbaum BR, et al. Diabetes and CKD in the United States population, 2009-2014. Clin J Am Soc Nephrol 2017;12:1984–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gurney JK, Stanley J, York S, et al. Risk of lower limb amputation in a national prevalent cohort of patients with diabetes. Diabetologia 2018;61:626–35. [DOI] [PubMed] [Google Scholar]
- [5].Trikkalinou A, Papazafiropoulou AK, Melidonis A. Type 2 diabetes and quality of life. World J Diabetes 2017;8:120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Association AD. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1211–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing 2011;40:423–9. [DOI] [PubMed] [Google Scholar]
- [9].Rantanen T, Guralnik JM, Foley D, et al. Midlife hand grip strength as a predictor of old age disability. JAMA 1999;281:558–60. [DOI] [PubMed] [Google Scholar]
- [10].Li JJ, Wittert GA, Vincent A, et al. Muscle grip strength predicts incident type 2 diabetes: population-based cohort study. Metabolism 2016;65:883–92. [DOI] [PubMed] [Google Scholar]
- [11].Loprinzi PD, Loenneke JP. Evidence of a link between grip strength and type 2 diabetes prevalence and severity among a national sample of U.S. Adults. J Phys Act Health 2016;13:558–61. [DOI] [PubMed] [Google Scholar]
- [12].McGrath R, Vincent BM, Al Snih S, et al. The Association between muscle weakness and incident diabetes in older Mexican Americans. J Am Med Dir Assoc 2017;18: 452.e7-L 452.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Peterson MD, Duchowny K, Meng Q, et al. Low normalized grip strength is a biomarker for cardiometabolic disease and physical disabilities among U.S. and Chinese adults. J Gerontol A Biol Sci Med Sci 2017;72:1525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Peterson MD, Zhang P, Choksi P, et al. Muscle weakness thresholds for prediction of diabetes in adults. Sports Med 2016;46:619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].van der Kooi AL, Snijder MB, Peters RJ, et al. The association of handgrip strength and type 2 diabetes mellitus in six ethnic groups: an analysis of the HELIUS study. PLoS One 2015;10:e0137739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Leong DP, Teo KK, Rangarajan S, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet (London, England) 2015;386:266–73. [DOI] [PubMed] [Google Scholar]
- [17].Xu L, Hao YT. Effect of handgrip on coronary artery disease and myocardial infarction: a Mendelian randomization study. Sci Rep 2017;7:954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].WHO. Diabetes country profiles 2016; Available at: http://www.who.int/diabetes/country-profiles/en/#S. Accessed Nov 12, 2017. [Google Scholar]
- [19].Ntuk UE, Celis-Morales CA, Mackay DF, et al. Association between grip strength and diabetes prevalence in black, South-Asian, and white European ethnic groups: a cross-sectional analysis of 418 656 participants in the UK Biobank study. Diabet Med 2017;34:1120–8. [DOI] [PubMed] [Google Scholar]
- [20].Kweon S, Kim Y, Jang MJ, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol 2014;43:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- [22].Yun KJ, Han K, Kim MK, et al. Insulin resistance distribution and cut-off value in Koreans from the 2008-2010 Korean National Health and Nutrition Examination Survey. PLoS One 2016;11:e0154593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet V 363 2004. 157–63. [DOI] [PubMed] [Google Scholar]
- [24].Hicks R, Tingley D. Causal mediation analysis. Stata J 2011;11:605–19. [Google Scholar]
- [25].Hamasaki H, Kawashima Y, Katsuyama H, et al. Association of handgrip strength with hospitalization, cardiovascular events, and mortality in Japanese patients with type 2 diabetes. Sci Rep 2017;7:7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li S, Zhang R, Pan G, et al. Handgrip strength is associated with insulin resistance and glucose metabolism in adolescents: evidence from National Health and Nutrition Examination Survey 2011 to 2014. Pediatr Diabetes 2018;19:375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol 2014;2:819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Agostinis-Sobrinho CA, Moreira C, Abreu S, et al. Muscular fitness and metabolic and inflammatory biomarkers in adolescents: results from LabMed Physical Activity Study. Scand J Med Sci Sports 2017;27:1873–80. [DOI] [PubMed] [Google Scholar]
- [29].Schaap LA, Pluijm SM, Deeg DJ, et al. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci 2009;64:1183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pradhan AD, Manson JE, Rifai N, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001;286:327–34. [DOI] [PubMed] [Google Scholar]
- [31].Phielix E, Meex R, Ouwens DM, et al. High oxidative capacity due to chronic exercise training attenuates lipid-induced insulin resistance. Diabetes 2012;61:2472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bruce CR, Anderson MJ, Carey AL, et al. Muscle oxidative capacity is a better predictor of insulin sensitivity than lipid status. J Clin Endocrinol Metab 2003;88:5444–51. [DOI] [PubMed] [Google Scholar]
- [33].Umpierre D, Ribeiro PA, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA 2011;305:1790–9. [DOI] [PubMed] [Google Scholar]
- [34].Balducci S, Zanuso S, Nicolucci A, et al. Effect of an intensive exercise intervention strategy on modifiable cardiovascular risk factors in subjects with type 2 diabetes mellitus: a randomized controlled trial: the Italian Diabetes and Exercise Study (IDES). Arch Intern Med 2010;170:1794–803. [DOI] [PubMed] [Google Scholar]
- [35].Celis-Morales CA, Lyall DM, Anderson J, et al. The association between physical activity and risk of mortality is modulated by grip strength and cardiorespiratory fitness: evidence from 498 135 UK-Biobank participants. Eur Heart J 2017;38:116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


