Abstract
Background:
CD133 has been identified as a putative cancer stem cell marker in colorectal cancer (CRC). However, the clinicopathological and prognostic significance of CD133 in CRC patients remains controversial. Thus, we conducted a meta-analysis to quantitatively evaluate the above issues.
Methods:
We collected a comprehensive literature search from PubMed, Web of Science, and Embase database up to September 20, 2016 examining CD133 and clinical features of colorectal cancer patients. We used the odds ratio (OR) with 95% confidence interval (CI) to estimate the effects by overall and stratified analysis.
Results:
The overall result of our meta-analysis indicated that CD133 expression was positively correlated with T category, distant metastasis, lymphatic invasion, and vascular invasion. Moreover, patients with higher CD133 expression had a poorer overall survival (OS) (HR=2.01, P < .001) and a lower 5-year OS rate (OR = 3.26, P < .001) than those with lower expression. Disease-free survival (DFS) and 5-year DFS rate were similar with the above results. Though the correlation between CD133 expression with the clinical characteristic was not positive in some ways when we analyzed the different subgroup. The prognostic value of CD133 expression for 5-year OS rate of CRC patients was noticeable in spite of different patients’ region, multiple antibodies used in studies, various cut-off values of CD133 expression, and adjuvant therapy situation of patients.
Conclusion:
CD133 is a useful predictive or prognostic biomarker for CRC in clinical assessment and may serve as a potential therapeutic target for CRC.
Keywords: CD133, colorectal cancer, prognosis
1. Introduction
Colorectal cancer (CRC) is the third most common cancer and the fourth most common cause of cancer-related death worldwide.[1] Although its diagnosis and therapy have been improved gradually, the survival of patients with CRC remains poor, which are mainly affected by drug resistance, local recurrence, and development of metastatic disease.[2] Increasing research studies have shown that cancer stem cells (CSCs) with the principal properties of multipotency and self-renewal may be responsible for neoplasm formation, metastasis, recurrence, and therapeutic resistance.[3–5]
Cancer stem cells were successfully isolated and identified in many hematologic and solid tumors including colorectal cancer.[5] A variety of molecules have been investigated as putative markers of CSCs in CRC. Among the various markers, CD133 is one of the most robust surface marker of CSCs in CRC.[6] It is widely expressed in numerous types of solid tumors, involving CRC.[7] As a 5 transmembrane single-chain glycoprotein, CD133, with a molecular weight of 120 kDa, was first found to be expressed in hematopoietic stem and progenitor cells by Yin et al.[8] Also the exploration of CD133 as a surface marker of colon cancer stem cells is still in progress. In 2007, O’Brien et al[9] found that CD133+ cells in CRC had the ability to initiate tumor growth.
The paradigm of CD133 as a CSCs biomarker has stimulated numerous studies to explore the prognostic power of CD133 expression in CRC patients. However, the prognostic value of CD133 for colorectal cancer remains controversial despite of numerous independent studies. For example, Kashihara et al and other studies demonstrated that high CD133 expression in CRC correlated with poor clinical outcomes.[10–13] Nevertheless, Hong et al and other studies showed that CD133-negative patients exhibited a poor prognosis.[14,15] Therefore, we performed a meta-analysis to elucidate the correlation between CD133 and clinicopathological features of CRC, and determine the value of CD133 as a prognostic marker for CRC.
2. Materials and methods
2.1. Literature search strategy
We collected a comprehensive literature search from PubMed, Web of Science, and Embase database up to March 20, 2016. The following search terms were used, (“colorectal cancer” or “rectal cancer” or “colon cancer”) and (“CD133” or “prominin 1” or “ac133 antigen” or “AC133 antigen”).
2.2. Study selection criteria
Eligible studies were included when the following criteria were met: the expression of CD133 protein on the cancer tissue (via either surgical or biopsy) by immunohistochemistry, rather than serum or any other kinds of detection methods was investigated; the association between CD133 and clinicopathological characteristics or the association of CD133 overexpression on disease free survival (DFS) and overall survival (OS) of CRC was studied; sufficient published data for calculating an odds ratio (OR), hazard ratio (HR), and 95% confidence interval (CI) were reported; (4) a full-text article in English or Chinese was published. When there were multiple articles by the same group based on similar patients and using the same detection methods, only the most important article with the recent or most information was included into the meta-analysis.
2.3. Data extraction
A standard protocol was applied to extract data. For every eligible study, the following data were sought: the first author's name, publication year, original country, situation of patient (whether received the adjuvant therapy, such as chemotherapy or radiotherapy before undergoing surgery), dilution of the used antibody, the choice of cut-off scores for the definition of positive staining, and survival analysis. We mainly clarified the association between CD133 expression and clinicopathological parameters, including T category, N category, distant metastasis, histology, lymphatic invasion, vascular invasion, and tumor size. More importantly, we investigated the association between CD133 expression and OS/ DFS. HR was directly extracted and synthesized from multivariable analysis where available. For those articles that only plotted as Kaplan–Meier curve, the software GetData Graph Digitizer 2.24 (http://getdata-graph-digitizer.com/) was used to digitize and extract the data of the 5-year OS rate and 5-year DFS rate directly. This investigation was approved by the institutional ethics committee, the Affiliated Tumor Hospital of Guangxi Medical University.
2.4. Statistical analysis
The strength of the association between the CD133 expression and clinicopathological features or 5-year OS/DFS rate were assessed by OR with the corresponding 95%CI. Moreover, the estimated HR with the corresponding 95%CI was used to summarize the relationship between CD133 expression and OS/DFS. In the present study, an OR>1 indicated a higher probability of tumor progression and poorer prognosis in CRC with CD133 overexpression, meanwhile a combined HR>1 implies a worse prognosis in the group with high CD133 expression. In the course of data pooling, statistical heterogeneity was performed by using chi-square-based Q-test. The I2 value indicates the degree of heterogeneity. A P-value < .10 and/or I2>50% are considered significant heterogeneity, and then a random-effect model is used.[16] Otherwise, a fixed-effect model is used.[17] Moreover, stratified-analyses were conducted based on patients’ district, antibodies used in studies, cut-off values of CD133 expression and adjuvant therapy situation of patients to explore the potential source of heterogeneity. Sensitivity analyses, by which each study was omitted in each turn to confirm the influence of individual data set to the pooled OR, were implied to evaluate the robustness of the results. Furthermore, we estimated potential publication bias with funnel plot and Egger's linear regression test. The funnel plot is visual symmetrical and the P-value of Egger's test is >0.05, which indicate that there is no statistically significant publication bias. All statistical tests in the meta-analysis were two-tailed and P-value ≤ .05 was considered statistically significant. Statistical analyses were performed with STATA software version 12.0.
3. Results
3.1. Characteristics of eligible literature
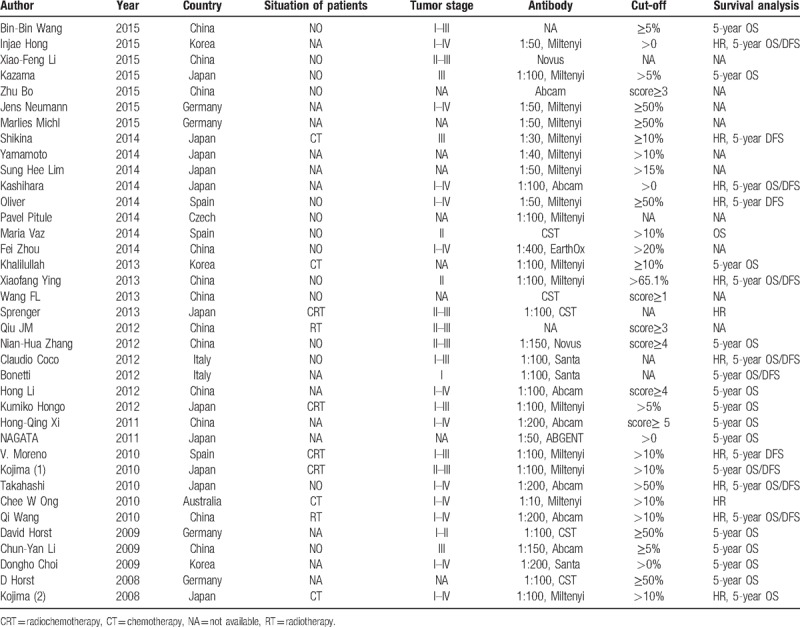
Based on the selection criteria, a total of 37 studies published from 2008 to 2016 were eligible for the meta-analysis.[2,10–15,18–47] A total of 5397 CRC patients from China, Japan, South Korea, Italy, Germany, Australia, Spain, and Czech were enrolled. The study sample sizes ranged from 32 to 523 cases. All specimens were derived from CRC tissues by either biopsy or surgical resection, and were detected by immunohistochemistry (IHC) method. However, besides the antibody source and the dilution ratio, it is worth noting that there was no universally accepted standard about the cut-off value of the low expression or overexpression for CD133.
Around 27 clinical studies assessed CD133 expression and correlated it to tumor clinicopathological characteristics which included tumor size, T category, N category, histological grade, distant metastasis, lymphatic invasion, and vascular invasion. Furthermore, 27 articles were analyzed for the association between CD133 expression and prognosis, which included 5-year OS, 5-year DFS, HR of OS, and HR of DFS. The flow diagram of study selection procedure was shown in Figure 1 and the main characters of the 37 eligible studies were summarized in Table 1.
Figure 1.
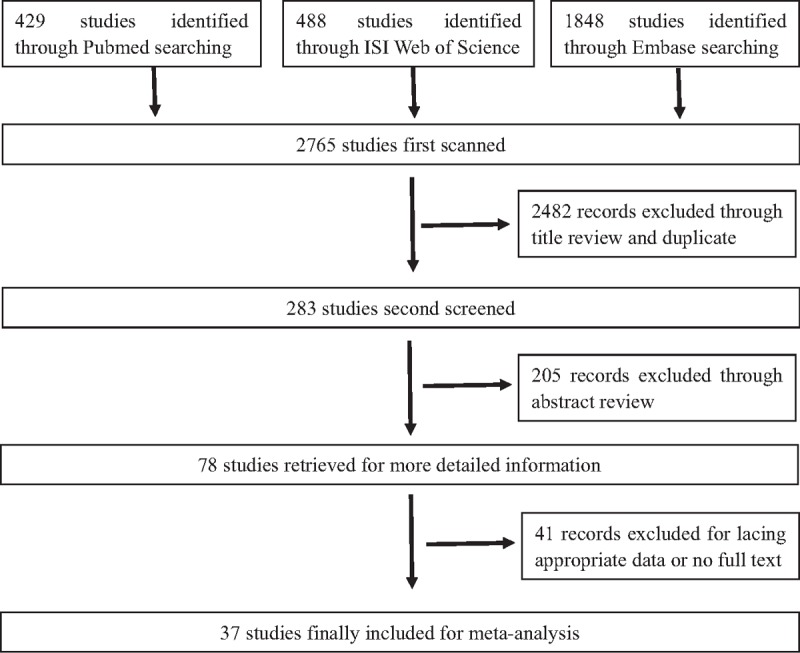
The flowchart of the study selection process.
Table 1.
Main characteristics of the eligible studies.
3.2. Meta-analysis results
3.2.1. Correlation of CD133 expression with clinicopathological characteristics
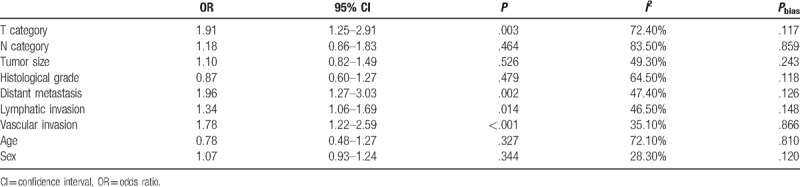
Overall analysis. There were 27 studies that investigated the association between CD133 expression and tumor clinicopathological parameters. The overall analysis showed CD133 expression statistically significant correlated with T category (OR = 1.91, 95%CI = 1.25–2.91, P = .003, Fig. 2A), distant metastasis (OR = 1.96, 95%CI = 1.27–3.03, P = .002, Fig. 2B), lymphatic invasion (OR = 1.34, 95%CI = 1.06–1.69, P = .014, Fig. 2C) and vascular invasion (OR = 1.78, 95%CI = 1.22–2.59, P < .001, Fig. 2D). Specifically, higher CD133 expression means higher T category (T3+T4), greater possibility of distant metastasis, lymphatic invasion, and vascular invasion. However, no clear correlation was found between CD133 expression and histological grade (OR = 0.87, 95%CI = 0.60–1.27, P = .479), N category (OR = 1.18, 95%CI = 0.76–1.83, P = .464), tumor size (OR = 1.10, 95%CI = 0.82–1.49, P = .526), age (OR = 0.78, 95%CI = 0.48–1.27, P = .327), and sex (OR = 1.07, 95%CI = 0.93–1.24, P = .344). The results were summarized in Table 2.
Figure 2.
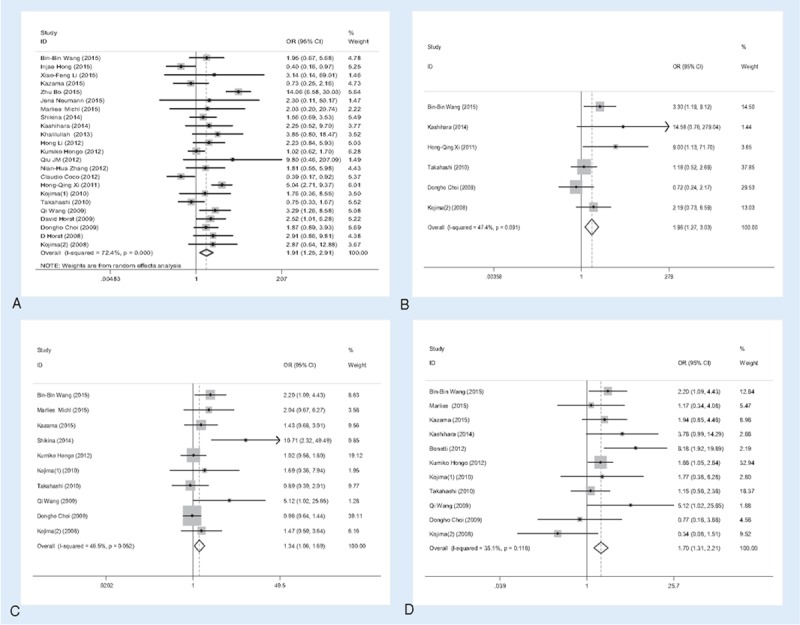
Association of CD133 expression with clinicopathological parameters. High CD133 expression was significantly associated with tumor T category (A), distant metastasis (B), lymphatic invasion (C), and vascular invasion (D).
Table 2.
Overall analysis of CD133 expression association with clinical features.
Subcategory analysis. Because there were large heterogeneity in some groups, we explored the potential source of heterogeneity via stratified analysis based on patients’ district (America–Europe, Asia subgroups), antibodies used in studies (Miltenyi Biotec, Abcam, and others subgroups), cut-off values of CD133 expression (>50%, >5%/10% and others subgroups), adjuvant therapy situation of patients (With, Without, and NA subgroups). We discussed the main clinicopathological parameters in the followed subgroup analysis, which included T category, N category, histological grade, lymphatic invasion, and vascular invasion. It is worth mentioning that the degree of heterogeneity was apparently reduced by the stratified analysis. The results were summarized in Table 3.
Table 3.
Main stratified analysis results of meta-analysis of CD133 expression.
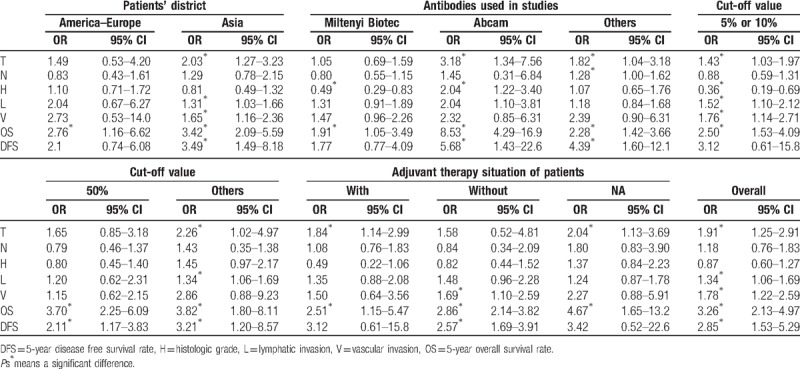
In the subgroup analysis about the patients’ district, we found that CD133 expression was positively correlated with T category, lymphatic invasion and vascular invasion in the Asia subgroup, which was consistent with the results derived from the overall analysis. However, no above clinicopathological parameters were positively correlated with CD133 expression in the America–Europe subgroup. Besides that, N category and Histologic type was not correlated with CD133 expression regardless patients’ district.
For subcategory analysis about the antibodies, results were different among various subgroups. T category and histologic grade were positively correlated with CD133 expression in the Abcam group. However, in the Miltenyi Biotec group, CD133 expression was negatively correlated with histological grade and not correlated with the tumor T category and lymphatic invasion, which was not consistent with overall analysis. In the other antibody group, only T category and N category were positively related with CD133 expression.
In the subgroup analysis based on the cut-off value, for the subgroup studies with cut-off values >5%/10% and other subgroup, the results were nearly consistent with the results derived from overall analysis. In the cut-off values >50% subgroup, we found that there were no relationship between CD133 expression and clinicopathological parameters. N category was not correlated with CD133 expression regardless of cut-off value.
In the stratified analyses by the adjuvant therapy situation of patients, CD133 expression was positively correlated with the T category With adjuvant therapy situation of patients and NA subgroup, which was in accord with overall analysis. In the Without adjuvant therapy situation of patients’ subgroup, only vascular invasion was related to CD133 expression.
3.2.2. Impact of CD133 expression on survival for colorectal cancer patients
The meta-analysis was performed on 27 studies investigating the association of CD133 expression and prognosis of CRC patients. The prognosis was evaluated by the indicators included 5-year OS, 5-year DFS, HR of OS, and DFS.
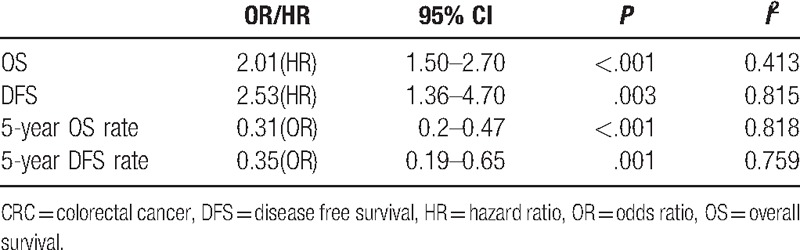
Overall analysis. The data for this analysis indicated that the prognosis of colonrectal cancer patients with CD133+ was poorer than that of the CD133− patients regardless of the indicators used. CD133 expression was highly correlated with low 5-year OS rate (OR = 3.26, 95% CI = 2.13–4.97, P < .001) and low 5-year DFS rate (OR = 2.85, 95% CI = 1.53–5.29, P = .001). Furthermore, the patients with higher CD133 expression presented poorer OS and DFS, and the pooled HRs were significant at 2.01 (95%CI = 1.50–2.70, P < .001) and 2.53 (95%CI = 1.36–4.70, P = .003), respectively. This indicated that CD133 was an independent prognostic factor in colon cancer patients. The results were shown in Figure 3 and Table 4.
Figure 3.

Meta-analysis of the association between CD133 expression and prognosis indicator. (A) 5-year OS; (B) 5-year DFS; (C) OS; (D) DFS. DFS = 5-year disease free survival rate, OS = 5-year overall survival rate.
Table 4.
Overall analysis of CD133 expression association with prognosis indicator of CRC.
Subcategory analysis. On account of large heterogeneity in the 5-year OS rate (PH < .001, I2 = 81.8%) and 5-year DFS rate (PH = .001, I2 = 75.9%) analysis, we performed a subcategory analysis as described above. Higher CD133 expression was related to poorer 5-year OS regardless of subgroup. In the subgroup analysis about the patients’ district, the pooled OR (OR = 3.42, 95%CI = 2.09–5.59, P < .001) in the Asia district was higher than the pooled OR (OR = 2.76, 95%CI = 1.16–6.62, P = .022) in the America–Europe district. Meanwhile, the 5-year OS in the Abcam (OR = 8.53, 95%CI = 4.29–16.95, P < .001) was higher than that in the Miltenyi Biotec (OR = 1.91, 95%CI = 1.05–3.49, P = .034), and in others (OR = 2.28, 95%CI = 1.42–3.66, P = .001) antibody group. In the cut-off values subgroup, we found that the 5-year OS in the others (OR = 3.82, 95%CI = 1.80–8.11, P < .001) was higher than that in the >50% and the 5% or 10% group. Besides, the pooled OR with (OR = 2.51, 95%CI = 1.15–5.47, P = .024) was lower than that in the NA and Without adjuvant therapy group. However, higher CD133 expression related to poorer 5-year DFS rate was not found in the America–Europe, Miltenyi Biotec, cut-off >5%/10%, with and NA subgroups.
3.2.3. Sensitivity analysis and Publication bias
Sensitivity analysis was performed through the sequential omission of individual studies. Moreover, no single study could essentially change the results, demonstrating that the results of our meta-analysis were statistically stable. The shapes of funnel plots and Egger's linear regression test were used to evaluate the publication bias of the eligible literatures. In general, the funnel plots did not show obvious evidence of asymmetry and the P-value of Egger's test was > 0.05, indicating there was no publication bias in the meta-analysis of CD133 and clinicopathological features (Figs. 4 and 5). The detailed results for P-value of Egger's test were summarized in Table 2.
Figure 4.
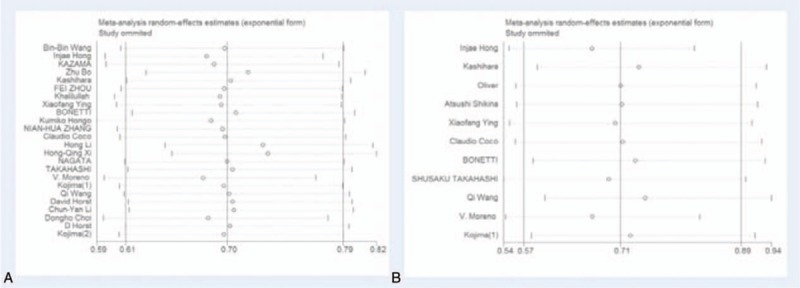
The plot of sensitivity analysis for evaluating the association between CD133 expression and 5-year OS/DFS. (A) 5-year OS; (B) 5-year DFS. DFS = 5-year disease free survival rate, OS = 5-year overall survival rate.
Figure 5.
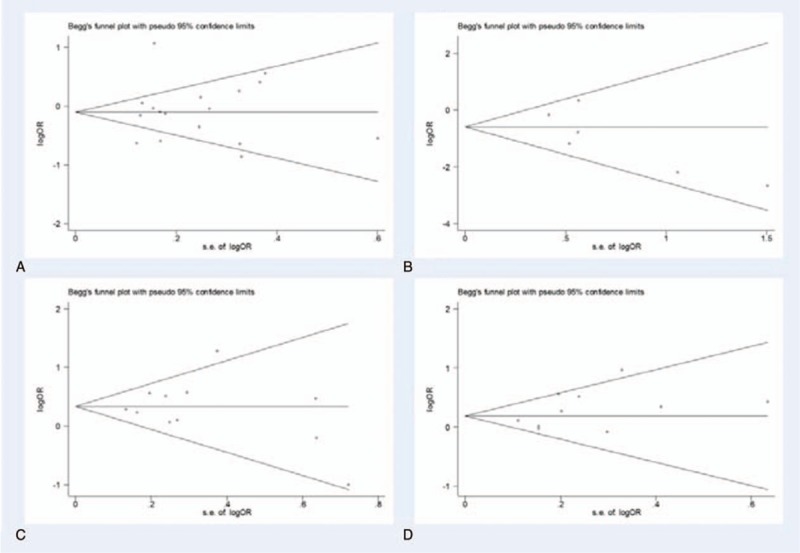
Begg's funnel plot of publication bias for evaluating the CD133 expression association with clinicopathological parameters of CRC. (A) T category; (B) distant metastasis; (C) lymphatic invasion; (D) vascular invasion. CRC = colorectal cancer.
4. Discussion
Lately, some researches have shown that CD133 may be used as a marker for colorectal CSCs.[48] Many researchers have studied the relationship of CD133 expression with clinical features as well as prognostic indicator of CRC. However, there was disputed despite of abundant studies. Our meta-analysis results based on the existing 37 studies revealed that CD133 would be served as a poor predictive indicator in CRC patients. CD133 overexpression was positively associated with T category, distant metastasis, lymphatic invasion, and vascular invasion. Moreover, CD133 expression was correlated with lower 5-year OS/DFS rate and higher HR of OS/DFS in CRC patients.
In this meta-analysis, we firstly investigated the pooled association between CD133 expression and clinicopathological features. The high expression of CD133 was positively correlated with higher T category, distant metastasis, lymphatic invasion, and vascular invasion. Also this almost was in line with the report by Wang et al.[12] Because there were large heterogeneity in some groups, we explored the potential source of heterogeneity via subcategory analysis based on the patients’ district, antibodies used in studies, different cut-off values of CD133 expression, and adjuvant therapy situation of patients. It was interesting to note that the result was different among different subgroups. In the Asian and cut-off >5/10% subgroup, what we found was almost consistent with the results derived from the overall analysis. Nevertheless, in the America–Europe subgroup, there was no correlation between CD133 and clinicopathological features, which may be attributed to the differences in gene and environment among ethnicity. Regarding the Miltenyi Biotec and others subgroup, the results were different from the overall analysis, which may be on account of different dilution rate used in the Miltenyi Biotec subgroup and various antibodies used in the others subgroup. However, there was few correlation between CD133 expression and clinicopathological features in the cut-off >50% and others cut-off subgroup. It may be due to a small number of literatures in the cut-off >50% subgroup and diverse cut-off value used in the others subgroup. Based on the different adjuvant therapy situation of patients’ subcategory analysis, the results were not consistent with the overall analysis, which may be on account of large heterogeneity and no unified adjuvant therapy in these subgroups. In addition, there was no significant correlation between CD133 expression and the tumor size. The small number of included studies might be the reason for this result. The results of our meta-analysis may support the hypothesis that CD133 overexpression might contribute to malignant progression of CRC, which may subsequently leads to a poor prognosis.
We then assessed the relationship between CD133 expression and prognostic significance of CRC. The pooled data indicated that high CD133 expression significantly predicted worse OS, poorer DFS, lower 5-year OS, and 5-year DFS rate. Indeed, accumulating evidence indicated that the CD133 was involved in the initiation and progression of CRC and associated with poor clinical outcomes. By combining the HRs and 95%CIs from primary studies, we showed that elevated CD133 expression was associated with poor OS (HR = 2.01; 95%CI = 1.50–2.70, P < .001) and poor DFS (HR = 2.21; 95%CI = 1.75–2.81, P < .001) in CRC. Meanwhile, we analyzed the relationship between CD133 expression and 5-year OS/DFS. The pooled overall results demonstrated that high CD133 expression significantly predicted low 5-year OS and 5-year DFS of CRC patients. In the following subcategory analysis, the 5-year OS in any subgroup was in accordance with the overall analysis. However, the positive correlation between CD133 expression and the 5-year DFS could not be found in the America–Europe, Miltenyi Biotec, cut-off >5/10%, With, and NA subgroups. It may due to differences in gene and environment among ethnicity, different dilution rate used, diverse adjuvant therapy applied in the above subgroups. It is notable that there is association of CD133 expression with prognostic significance of CRC in our meta-analysis, suggesting that this marker can be developed for the prognostic assessment and clinical targeted therapy.
Several studies have shown that CSC-related factors, including aldehyde dehydrogenase 1 (ALDH1) and leucine-rich repeat containing G protein-coupled receptor 5 (LGR5), are associated with colorectal cancer progression.[49–51] As for the prognostic value of these markers, Horst et al[45] implied that CD133 might be of the most clinical relevance, while the combined evaluation of CD133, CD44, and CD166 might even be more valuable to separate high-risk groups from low-risk colorectal cancer cases. For future studies, co-expression of colorectal CSC markers associated with patient survival may be more serviceable for clinical application in CRC.
Although we executed exhaustive meta-analysis, certain potential limitations existed and some results needed to be elaborated deliberately. Firstly, we only directly extracted HRs and 95% CIs from the original study and we did not calculate HRs and 95% CIs in other ways, which may omit much important information. Secondly, we assessed the 5-year OS/DFS rate from the Kaplan–Meier curves. These estimated data may be less credible than direct data from the original study. Thirdly, in spite of modification for heterogeneity by the application of random-effect model, subcategory analyses and sensitivity analyses, there was large heterogeneity in some subgroups. Fourthly, though we evaluated the publication bias and did not discover obvious bias, it is a remarkable fact that the papers with positive results are prone to publishing. Therefore, the relevancy between CD133 expression and outcome of CRC patients may have exceeded our calculation. Furthermore, studies measuring CD133 gene or mRNA level by RT-PCR was not yet included in this meta-analysis. Finally, except for CD133, this present study did not examines the correlation between other putative CSC markers and the risk of CRC.
In summary, although certain limitations existed, the results of the present study showed that higher CD133 expression was positively correlated with higher T category, lymphatic invasion, and vascular invasion. Moreover, higher CD133 expression was associated with poorer prognosis of CRC. Our results suggest that CD133 expression not only provides a better understanding of the relationship between colon tumorigenesis but may also be a useful predictive or diagnostic biomarker for CRC and beneficial for the novel targeted therapeutic strategies in the future. However, a future larger prospective study may be needed to warrant to test our results.
Author contributions
Data curation: Rongyong Huang, Huaying Ai, Yiping Lu.
Formal analysis: Rongyong Huang, Huaying Ai.
Funding acquisition: Junrong Wu.
Investigation: Rongyong Huang, Huaying Ai.
Writing – original draft: Rongyong Huang, Dan Mo.
Writing – review & editing: Rongyong Huang, Junrong Wu, Yiping Lu.
Footnotes
Abbreviations: CI = confidence interval, CRC = colorectal cancer, CSCs = cancer stem cells, DFS = disease free survival, HR = hazard ratio, OR = odds ratio, OS = overall survival.
This study was supported by Natural Science Foundation of Guangxi Medical University Programs (GXMUYSF201630) and the Project of Improving the Basic Ability of Young Teachers in Universities in Guangxi (No. 2017KY127).
Competing financial interests: The authors declare no competing financial interests.
References
- [1].Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin 2014;64:104–17. [DOI] [PubMed] [Google Scholar]
- [2].Vaz MA, Martinez JC, Devesa JM, et al. Prognostic value of stem cell quantification in stage II colon cancer. PLoS One 2014;9:e88480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vermeulen L, de Sousa e Melo F, Richel DJ, et al. The developing cancer stem-cell model: clinical challenges and opportunities. Lancet Oncol 2012;13:e83–89. [DOI] [PubMed] [Google Scholar]
- [4].Boman BM, Wicha MS. Cancer stem cells: a step toward the cure. J Clin Oncol 2008;26:2795–9. [DOI] [PubMed] [Google Scholar]
- [5].Dick JE. Looking ahead in cancer stem cell research. Nat Biotechnol 2009;27:44–6. [DOI] [PubMed] [Google Scholar]
- [6].Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature 2007;445:111–5. [DOI] [PubMed] [Google Scholar]
- [7].Dalerba P, Dylla SJ, Park IK, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A 2007;104:10158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yin AH, Miraglia S, Zanjani ED, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood 1997;90:5002–12. [PubMed] [Google Scholar]
- [9].O’Brien CA, Pollett A, Gallinger S, et al. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007;445:106–10. [DOI] [PubMed] [Google Scholar]
- [10].Horst D, Kriegl L, Engel J, et al. CD133 expression is an independent prognostic marker for low survival in colorectal cancer. Brit J Cancer 2008;99:1285–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kashihara H, Shimada M, Kurita N, et al. CD133 expression is correlated with poor prognosis in colorectal cancer. Hepatogastroenterology 2014;61:1563–7. [PubMed] [Google Scholar]
- [12].Wang BB, Li ZJ, Zhang FF, et al. Clinical significance of stem cell marker CD133 expression in colorectal cancer. Histol Histopathol 2016;31:299–306. [DOI] [PubMed] [Google Scholar]
- [13].Coco C, Zannoni GF, Caredda E, et al. Increased expression of CD133 and reduced dystroglycan expression are strong predictors of poor outcome in colon cancer patients. J Exp Clin Cancer Res 2012;31:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hong I, Hong SW, Chang YG, et al. Expression of the cancer stem cell markers CD44 and CD133 in colorectal cancer: an immunohistochemical staining analysis. Ann Coloproctol 2015;31:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kojima M, Ishii G, Atsumi N, et al. Immunohistochemical detection of CD133 expression in colorectal cancer: a clinicopathological study. Cancer Sci 2008;99:1578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [17].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [18].Li XF, Guo XG, Yang YY, et al. Effect of CXCR4 and CD133 co-expression on the prognosis of patients with stage II∼III colon cancer. Asian Pac J Cancer Prev 2015;16:1073–6. [DOI] [PubMed] [Google Scholar]
- [19].Kazama S, Kishikawa J, Yasuda K, et al. CD133 expression in lymph node metastases is associated with tumor aggressiveness during lymph node metastasis in colorectal cancer. Anticancer Res 2015;35:6599–605. [PubMed] [Google Scholar]
- [20].Zhu B, Wang D, Zhang Q, et al. Expressions of WWOX and CD133 in colorectal cancer and their clinical significance. J Southern Med Univ 2015;35:1586–90. [PubMed] [Google Scholar]
- [21].Neumann J, Lohrs L, Albertsmeier M, et al. Cancer stem cell markers are associated with distant hematogenous liver metastases but not with peritoneal carcinomatosis in colorectal cancer. Cancer Invest 2015;33:354–60. [DOI] [PubMed] [Google Scholar]
- [22].Michl M, Heinemann V, Jung A, et al. Expression of cancer stem cell markers in metastatic colorectal cancer correlates with liver metastasis, but not with metastasis to the central nervous system. Pathol Res Pract 2015;211:601–9. [DOI] [PubMed] [Google Scholar]
- [23].Shikina A, Shinto E, Hashiguchi Y, et al. Differential clinical benefits of 5-fluorouracil-based adjuvant chemotherapy for patients with stage III colorectal cancer according to CD133 expression status. Jpn J Clin Oncol 2014;44:42–8. [DOI] [PubMed] [Google Scholar]
- [24].Yamamoto S, Tanaka K, Takeda K, et al. Patients with CD133-negative colorectal liver metastasis have a poor prognosis after hepatectomy. Ann Surg Oncol 2014;21:1853–61. [DOI] [PubMed] [Google Scholar]
- [25].Lim SH, Jang J, Park JO, et al. CD133-positive tumor cell content is a predictor of early recurrence in colorectal cancer. J Gastrointest Oncol 2014;5:447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Oliver JA, Ortiz R, Melguizo C, et al. Prognostic impact of MGMT promoter methylation and MGMT and CD133 expression in colorectal adenocarcinoma. BMC Cancer 2014;14:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pitule P, Cedikova M, Daum O, et al. Immunohistochemical detection of cancer stem cell related markers CD44 and CD133 in metastatic colorectal cancer patients. Biomed Res Int 2014;2014:432139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhou F, Mu YD, Liang J, et al. Expression and prognostic value of tumor stem cell markers ALDH1 and CD133 in colorectal carcinoma. Oncol Lett 2014;7:507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mia-Jan K, Jung SY, Kim IY, et al. CD133 expression is not an independent prognostic factor in stage II and III colorectal cancer but may predict the better outcome in patients with adjuvant therapy. BMC Cancer 2013;13:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ying X, Wu J, Meng X, et al. AC133 expression associated with poor prognosis in stage II colorectal cancer. Med Oncol 2013;30:356. [DOI] [PubMed] [Google Scholar]
- [31].Wang FL, Wan DS, Lu ZH, et al. Expression of molecular markers detected by immunohistochemistry and risk of lymph node metastasis in stage T1 and T2 colorecrectal cancers. Chin J Oncol 2013;35:277–81. [DOI] [PubMed] [Google Scholar]
- [32].Sprenger T, Conradi LC, Beissbarth T, et al. Enrichment of CD133-expressing cells in rectal cancers treated with preoperative radiochemotherapy is an independent marker for metastasis and survival. Cancer 2013;119:26–35. [DOI] [PubMed] [Google Scholar]
- [33].Qiu JM, Yang GG, Lu XJ, et al. Association of CD133 expression and sensitivity of rectal cancer to preoperative radiotherapy. Chin J Gastrointest Surg 2012;15:1066–9. [PubMed] [Google Scholar]
- [34].Zhang NH, Li J, Li Y, et al. Co-expression of CXCR4 and CD133 proteins is associated with poor prognosis in stage II-III colon cancer patients. Exp Ther Med 2012;3:973–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Reggiani Bonetti L, Migaldi M, Caredda E, et al. Increased expression of CD133 is a strong predictor of poor outcome in stage I colorectal cancer patients. Scand J Gastroenterol 2012;47:1211–7. [DOI] [PubMed] [Google Scholar]
- [36].Li H, Zhao P, Lu Y, et al. Correlation of aberrant expression of CD133 with FHIT and malignant phenotype of colorectal adenocarcinoma. Int J Colorectal Dis 2012;27:1015–20. [DOI] [PubMed] [Google Scholar]
- [37].Hongo K, Kazama S, Sunami E, et al. Immunohistochemical detection of CD133 is associated with tumor regression grade after chemoradiotherapy in rectal cancer. Med Oncol 2012;29:2849–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Xi HQ, Zhao P. Clinicopathological significance and prognostic value of EphA3 and CD133 expression in colorectal carcinoma. J Clin Pathol 2011;64:498–503. [DOI] [PubMed] [Google Scholar]
- [39].Nagata T, Sakakura C, Komiyama S, et al. Expression of cancer stem cell markers CD133 and CD44 in locoregional recurrence of rectal cancer. Anticancer Res 2011;31:495–500. [PubMed] [Google Scholar]
- [40].Garcia VM, Batlle JF, Casado E, et al. Immunohistochemical analysis of tumour regression grade for rectal cancer after neoadjuvant chemoradiotherapy. Colorectal Dis 2011;13:989–98. [DOI] [PubMed] [Google Scholar]
- [41].Kojima M, Ishii G, Atsumi N, et al. CD133 expression in rectal cancer after preoperative chemoradiotherapy. Cancer Sci 2010;101:906–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Takahashi S, Kamiyama T, Tomaru U, et al. Frequency and pattern of expression of the stem cell marker CD133 have strong prognostic effect on the surgical outcome of colorectal cancer patients. Oncol Rep 2010;24:1201–12. [DOI] [PubMed] [Google Scholar]
- [43].Ong CW, Kim LG, Kong HH, et al. CD133 expression predicts for non-response to chemotherapy in colorectal cancer. Modern Pathol 2010;23:450–7. [DOI] [PubMed] [Google Scholar]
- [44].Wang Q, Chen ZG, Du CZ, et al. Cancer stem cell marker CD133+ tumour cells and clinical outcome in rectal cancer. Histopathology 2009;55:284–93. [DOI] [PubMed] [Google Scholar]
- [45].Horst D, Kriegl L, Engel J, et al. Prognostic significance of the cancer stem cell markers CD133, CD44, and CD166 in colorectal cancer. Cancer Invest 2009;27:844–50. [DOI] [PubMed] [Google Scholar]
- [46].Li CY, Li BX, Liang Y, et al. Higher percentage of CD133+ cells is associated with poor prognosis in colon carcinoma patients with stage IIIB. J Transl Med 2009;7:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Choi D, Lee HW, Hur KY, et al. Cancer stem cell markers CD133 and CD24 correlate with invasiveness and differentiation in colorectal adenocarcinoma. World J Gastroenterol 2009;15:2258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Vaiopoulos AG, Kostakis ID, Koutsilieris M, et al. Colorectal cancer stem cells. Stem Cells 2012;30:363–71. [DOI] [PubMed] [Google Scholar]
- [49].Huang EH, Hynes MJ, Zhang T, et al. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res 2009;69:3382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Barker N, Ridgway RA, van Es JH, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009;457:608–11. [DOI] [PubMed] [Google Scholar]
- [51].Levin TG, Powell AE, Davies PS, et al. Characterization of the intestinal cancer stem cell marker CD166 in the human and mouse gastrointestinal tract. Gastroenterology 2010;139: 2072-2082 e2075. [DOI] [PMC free article] [PubMed] [Google Scholar]


