Abstract
Background:
The presence of antibodies against phospholipase A2 receptor (PLA2R-Abs) in serum at diagnosis is reported to be related to the rate of spontaneous remission in patients with idiopathic membranous nephropathy (IMN); however, there is still lack of enough samples to illustrate this problem. Here, we conducted a comprehensive meta-analysis to investigate the prognostic value of PLA2R-Abs on spontaneous remission for IMN patients in the absence of immunosuppressive therapy.
Methods:
A systematic search of the PubMed, EMBASE, and Cochrane Central databases was performed for relevant original articles published until October 2017. All studies focus on spontaneous remission rates in IMN patients associated with PLA2R-Abs, the endpoint of interest is spontaneous remission rate. Risk ratio (RR) and corresponding 95% confidence intervals (CI) were carried out using a fixed or random effects model. The data were analyzed by Review Manager 5.3 software.
Results:
A total of 5 articles involving 190 patients were included in this meta-analysis. There were significant differences between the 2 groups in spontaneous remission rate. The seropositive of PLA2R-Abs measured at the time of diagnosis was negatively correlated with the likelihood of spontaneous remission (RR = 0.69; 95% CI, 0.56–0.87; P = .001).
Conclusion:
In PLA2R-Abs seronegative patients, the spontaneous remission rate is higher than that of PLA2R-Abs seropositive patients during symptomatic treatment. Therefore, in order to avoid malignant events caused by immunosuppressive therapy, maybe it is more beneficial to adopt conservative treatment for PLA2R-Abs seronegative patients at initial treatment.
Keywords: IMN, meta-analysis, PLA2R-Abs, spontaneous remission
1. Introduction
Membranous nephropathy (MN) is the most common case of the nephrotic syndrome in adults in addition to the focal segmental glomerulosclerosis (FSGS),[1,2] about 80% of cases are idiopathic membranous nephropathy (IMN) and about 20% of cases are secondary membranous nephropathy (SMN).[3,4] Patients with MN who did not find the reason for the disease were called IMN. Although immunosuppressive therapy is now confirmed effective for the management of patients with IMN,[5] it is important to note that some patients experience severe events after immunosuppressive therapy.[6] Therefore, it is necessary to give conservative treatment to 30% to 35% of patients who may have spontaneous remission to avoid malignant events caused by immunosuppressive therapy.[7]
M type phospholipase A2 receptor (PLA2R) is a specific antigen in IMN and PLA2R autoantibodies (PLA2R-Abs) present in about 70% patients.[8–10] Although the diagnosis value of PLA2R-Abs in IMN is now accepted,[11–13] the effect of prognosis is uncertain. Several trails had put forward the view that the presence of serum PLA2R-Abs at diagnosis is associated with the prognosis and disease activity of the IMN, showing that the existence of PLA2R-Abs at diagnosis may predict the spontaneous remission.[14–17]
In 2009, Beck Jr[1] first found that the PLA2R was present in IMN patients and most patients have PLA2R-Abs in their serum. Then Hofstra and Timmermans put forward that compared with the high antibodies titers at diagnosis, the spontaneous remission rate in low antibodies titers group was higher,[18,19] Overall, the relevance of PLA2R-Abs to spontaneous remission is reported in these articles,[20,21] the number of patients is less and cannot be sufficient to illustrate the problem.
We analyzed and extracted the number of spontaneous remission in each study and undertook the present meta-analysis to assess the relationship between PLA2R-Abs and spontaneous remission for patients with IMN.
2. Materials and methods
This meta-analysis was reported according to the preferred reporting items for systematic reviews and meta-analyses guidelines. All analyses were based on previous published studies; thus, no ethical approval and patient consent are required.
2.1. Search strategy
PubMed, EMBASE, and Cochrane Center Register of Controlled Trials (CENTRAL) were searched to identify eligible studies published prior to October 1, 2017. Search terms included “phospholipase A2 receptor antibody,” “PLA2R-Abs,” and “membranous nephropathy,” “idiopathic membranous nephropathy.” In addition, the references involved in the selected article were also identified in the study and then were searched manually.
2.2. Study selection and selection criteria
Two reviewers (WW and JS) screened titles and abstracts to exclude any studies that were clearly irrelevant, Studies were included in the current meta-analysis if they met the following criteria: any observational studies published as original studies; patients with idiopathic membranous nephropathy diagnosed by renal biopsy and excluded secondary factors; patients without other kidney diseases; outcomes about the spontaneous remission in patients with idiopathic membranous nephropathy. The exclusion criteria were as follows: patients on immunosuppressive drug treatment at the time of renal biopsy or accept immunosuppressive treatment after diagnosis, reviews, conference abstracts, case reports, animal studies, letters, and editorials, study was not written in English, only the abstract of a study was available, studies without original data and studies included overlapping populations. Disagreement was discussed and settled using a third opinion (ZZ).
2.3. Data extraction
These articles were reviewed independently by 2 authors and the data were extracted from all the included records. The data included first author, year of publication, race, proteinuria, recruitment period, diagnosis of IMN, total number of patients, sample size, test methods of PLA2R-Abs, follow-up time, definition of spontaneous remission, seronegative definition of PLA2R-Abs.
2.4. Quality assessment
The methodological quality of the included studies was assessed by Newcastle–Ottawa scales (NOS), The NOS assign points depending on the quality of patient selection (maximum of 4 points), comparability of the study group (maximum of 2 points), and outcome assessment (maximum of 3 points). A study can be awarded a maximum of 1 star for each numbered item within the Selection and outcome categories. A maximum of 2 stars can be given for comparability.
2.5. Data analysis
All data analyses were performed in Review Manager Version 5.3 from the Cochrane Collaboration. The effect measure of the dichotomous variables in the cohort study was expressed in RR with 95% CI, calculated by M-H method (fixed- or random-effect models), Q statistic and I2 statistic were used to evaluate the statistical heterogeneity in the study. If I2 > 50% or P value <.05, it indicates that there is a significant statistical heterogeneity in the included study. Subgroup analysis was performed to explore the potential source of heterogeneity, such factors included race, PLA2R-Abs testing methods (e.g., western blotting, indirect immunofluorescence, and enzyme-linked immunosorbent assay), definition of spontaneous remission, seronegative definition of PLA2R-Abs. In addition, considering the heterogeneity of data may be balanced out, we still conducted a sensitivity analysis. The funnel plot was applied for assessing publication bias in this meta-analysis.
3. Results
3.1. Search results, study characteristics, quality assessment, and publication bias
As shown in Fig. 1, after initial search, a total of 480 articles were searched, including 128 duplicates. After screening titles and/or abstracts, another 319 articles were excluded, including reviews, case reports, meta-analysis, basic research, and studies not mentioned in the spontaneous remission. The remaining 33 articles were retrieved for full-text review, among them, 3 publications, only the abstract and title were written in English, the date in 16 articles is inadequate that no credible data can be presented, in 2 articles, the existence of PLA2R-Abs was not measured at renal biopsy, 7 articles not focused on spontaneous remission were excluded. Finally, only 5 studies including 190 patients were included in this mete-analysis.
Figure 1.
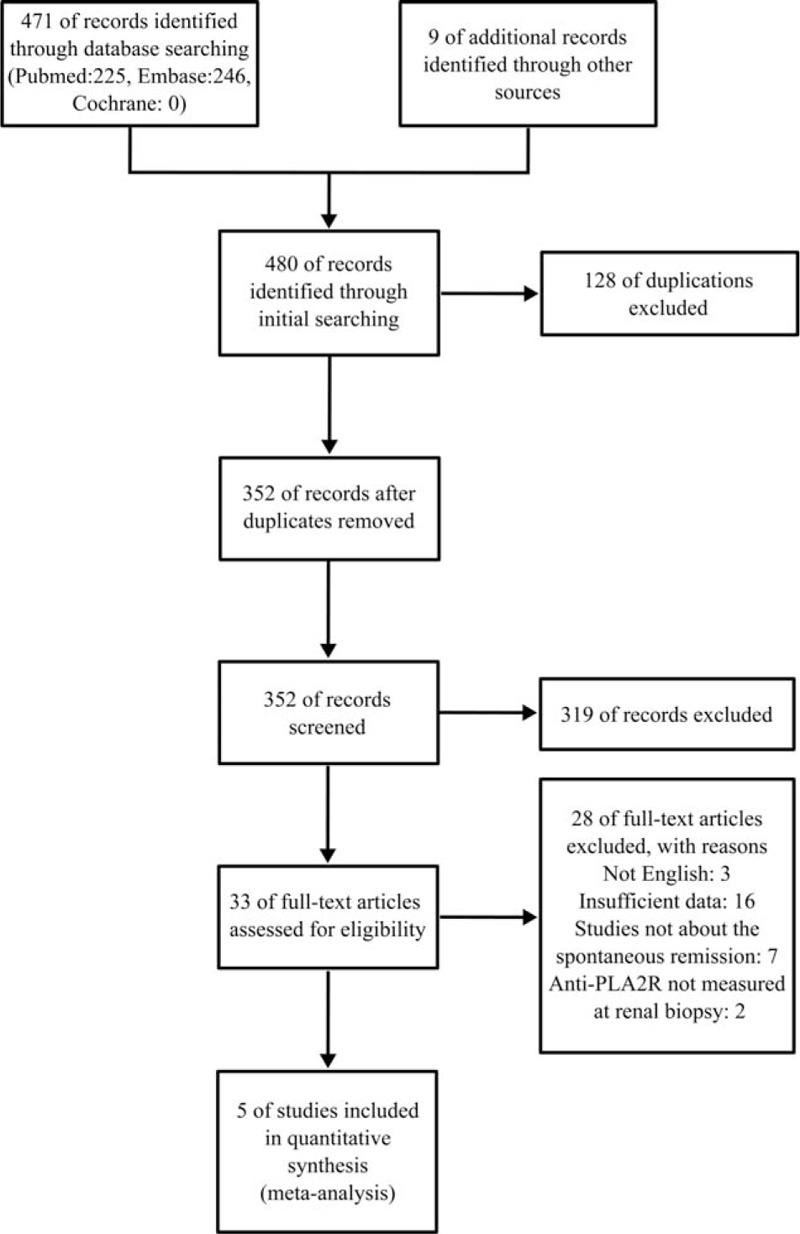
Flowchart for identification of studies.
Relevant characteristics of the eligible studies are summarized in Table 1, all studies were cohort studies and published in recent 5 years, among the 5 articles, 3 were assay by ELISA, 1 was assay by ELISA and IIFC, only 1 was assay by WB. In addition, index of spontaneous remission was expressed by urine protein/creatinine ratio (UPCR) in 3 studies and urine protein/24 hours (24HTP) in 2 studies, the other details of the included studies are described in the table.
Table 1.
Characteristics of included eligible studies.
The quality assessment of all included studies based on NOS is shown in Table 2, all the quality of studies was moderate.
Table 2.
Quality assessment of cohort studies included in the meta-analysis according to the Newcastle−Ottawa scale.

Figure 2 shows a funnel plot of the studies included in this meta-analysis, all studies are evenly distributed around the vertical line, indicating no obvious publication bias. However, publication bias cannot be ruled out, as the reliability of such assessments is particularly weak with the inclusion of a small number of studies.
Figure 2.
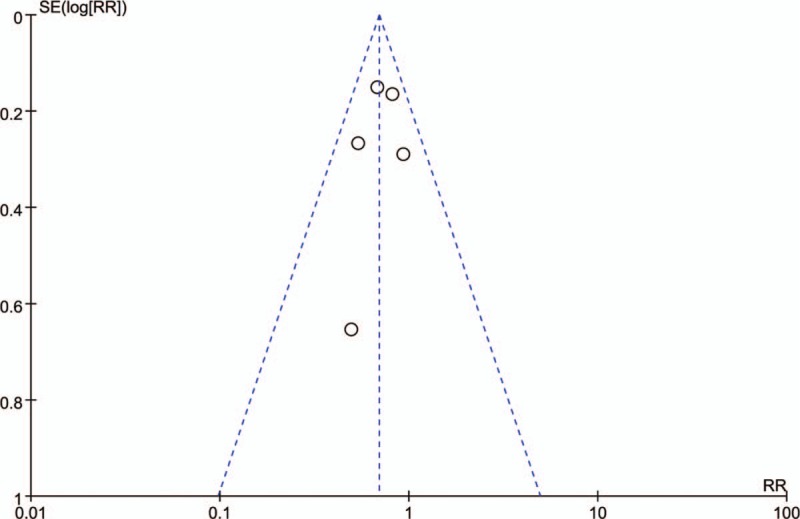
Funnel plot for spontaneous remission rate between PLA2R-Abs (+) and PLA2R-Abs (−).
3.2. Meta-analysis
As shown in Fig. 3, The P value of the Q statistic were .52 and the I2 statistic was 0%, indicating significant homogeneous across these studies. Therefore, we applied a fixed-effect model. In this analysis, spontaneous remission rates were higher in PLA2R seronegative patients compared with seropositive patients (RR: 0.69, 95% CI: 0.56–0.87; P = .001). Subgroup analysis is used to identify factors that may be heterologous sources. The assay of PLA2R-Abs was by WB in 1 study and ELISA in 4 studies, considering that the different assay methods may affect the aggregate result. Therefore, a subgroup analysis was applied by assay methods. In the assay method subgroup analysis, total 190 patients were included (165 patients were assay by ELISA and 25 by WB), PLA2R-Abs seronegative patients had a higher spontaneous remission rate than seropositive patients in ELISA group (RR: 0.65; 95% CI: 0.51–0.83; P = .0006). However, no significant difference of spontaneous remission rate was detected in the WB subgroup (RR: 0.93; 95% CI: 0.53–1.65; P = .81). The results revealed the assay methods might account for the heterogeneity (Fig. 4). Another subgroup analysis was performed based on definition of spontaneous remission, 3 of 5 studies were by UPCR and 2 by 24HTP. Remarkably, the subgroup analysis presented consistent results that there was a statistically significant association between PLA2R-Abs and spontaneous remission that spontaneous remission rate was higher in PLA2R-Abs seronegative patients than seropositive patients. UPCR group: (60.5% [23/38] vs. 44.8% [30/67], RR: 0.64; 95% CI: 0.43–0.95; P = .03); 24HTP group: (94.1% [16/17] vs. 69.1% [47/68], RR: 0.75; 95% CI: 0.60–0.93; P = .009) (Fig. 5).
Figure 3.
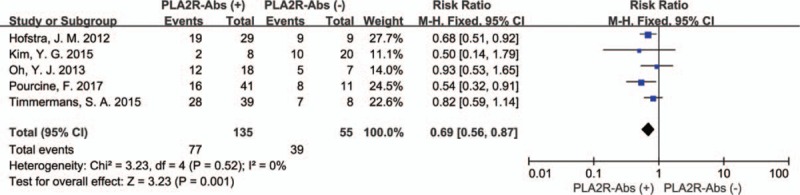
Forest plot for spontaneous remission rate between PLA2R-Abs (+) and PLA2R-Abs (−).
Figure 4.
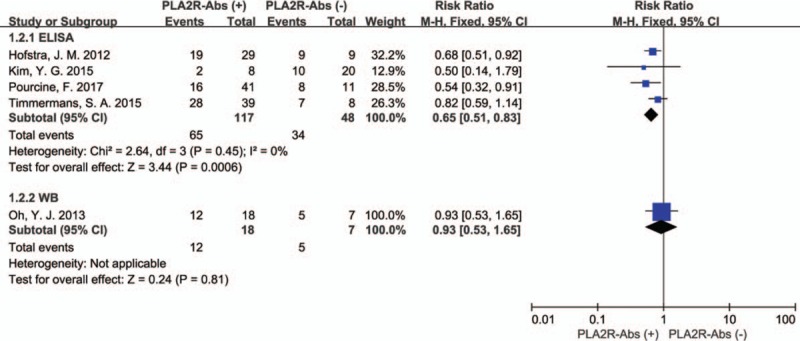
Forest plot for spontaneous remission rate between PLA2R-Abs (+) and PLA2R-Abs (−) in ELISA test and WB test.
Figure 5.
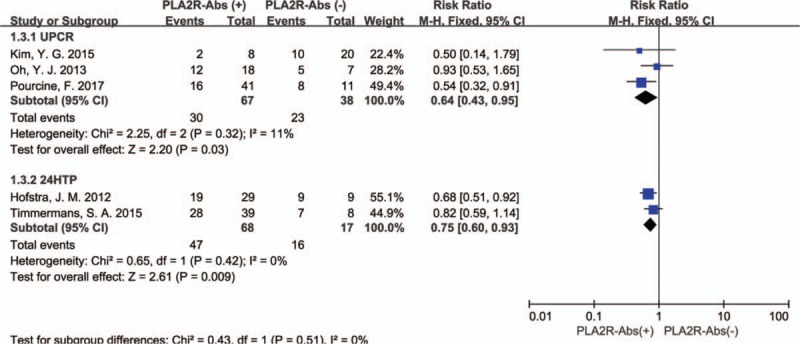
Forest plot for spontaneous remission rate between PLA2R-Abs (+) and PLA2R-Abs (−) in different definition of spontaneous remission.
Sensitivity analysis was used to determine whether modification of the inclusion criteria affected the final results and no study influenced the pooled outcome after removal.
4. Discussion and conclusion
In this study, we analyzed the associations between PLA2R-Abs and spontaneous remission in IMN by a meta-analysis and obtained a powerful conclusion that in IMN patients without immunosuppressive therapy, the PLA2R-Abs measured at renal biopsy is associated with the spontaneous remission. To the best of our knowledge, this is the first meta-analysis providing insights into the influence of PLA2R-Abs in spontaneous remission rates for IMN.
Previous studies described PLA2R-Abs may serve as a new indicator for predicting outcomes in IMN,[22] patients in the low-titer PLA2R-Abs group are more likely to experience spontaneous remission.[23] However, these studies were performed on a limited number of patients and none of them mainly analyzed the link between the spontaneous remission and PLA2R-Abs. In order to assess the effect of the existence of PLA2R-Abs on the spontaneous remission rate, we collected all high-quality studies focusing on the association between spontaneous remission rates and PLA2R-Abs in IMN patients and found significant results.
The present results verify that the PLA2R-Abs is associated with spontaneous remission of the patients with IMN. In this study, compared with PLA2R-Abs seronegative patients, seropositive patients have a lower spontaneous remission rate (57% [77/135] vs 71% [39/55]), (RR: 0.69, 95% CI: 0.56–0.87; P = .001). The results indicating that PLA2R-Abs seronegative patients may have a greater benefit with delayed immunosuppressive treatment. However, subgroup analysis of assay methods showed an inconsistent result, indicating that the detection methods may have an effect on the counting of spontaneous remission rates, which may be caused by differences in the sensitivity of detection methods.
The role of PLA2R-Abs play in the outcomes of IMN is not really certain. Several studies had risen the view that PLA2R-Abs may be the potential pathogenic factor in IMN.[24] Ruggenenti et al found that post-Rituximab treatment, most patients became PLA2R-Abs seronegative over 6 to 9 months followed by remission of proteinuria over 12 to 24 months, which means the PLA2R-Abs may be an independent risk factor for not achieving remission.[25] Besides, evidence for several immunodominant epitopes in PLA2R targeted by PLA2R-Abs was put forward and then it was proved that epitopes in 3 distinct domains CysR, CTLD1, and CTLD7 were independent domains recognized by distinct PLA2R-Abs, suggesting that anti-PLA2R antibodies are of high affinity and could cause an immune response against autoantigen PLA2R.[8,24] As PLA2R-Abs are secreted and enter the blood circulation, they will bind to target receptors on podocytes and are effectively affinity adsorbed out of the circulation. It is possible that PLA2R-Abs are adsorbed by podocytes in this way to initiate MN pathology and proteinuria. Brenchley also mentioned that PLA2R-Abs modulate podocyte cell biology in vitro but nothing is known as to how this affects it in vivo, but how does the PLA2R-Abs produce and the mechanism related to prognosis still need to be explored further.[26]
In view of the only 5 studies included in this meta-analysis, we interpret the above results carefully. Although, based on the results, it seems like the PLA2R-Abs seronegative patients have a high spontaneous remission than seropositive patients with symptomatic treatment, several limitations may exist in our meta-analysis. First, due to the insufficient studies concerning about the association between anti-PLA2R antibodies and spontaneous remission, the power of the 6 individual studies was 25.7%, 33.5%, 7.8%, 64.7%, and 79.7%, respectively. The power of meta-analysis is 56.6%, which is not very high. Therefore, it needs more relevant studies to increase the power of test. In order to make the statistics power more than 80%, we calculated a minimum of 251 samples for the positive group and a minimum of 103 samples for the negative group. Second, the method and cut-off values for assessing PLA2R-Abs and the level of the urine protein at diagnosis are not evaluated due to insufficient data. Third, due to the limited number of studies, more strictly designed studies are urgently needed. Fourth, we only analyzed articles that were published in English. We did not include articles that were published in other languages due to the impracticability of getting accurate medical translation; it may ignore the potential high-quality studies published in other languages. Last, we only considered spontaneous remission rate, other outcomes such as complications and time to remission were not analyzed due to the insufficient data. For a comprehensive evaluation on the association between PLA2R-Abs and spontaneous remission rate, future more and larger prospective studies were needed to solve this problem.
In conclusion, the present meta-analysis demonstrates that IMN patients, whose PLA2R-Abs are seronegative at renal biopsy, have a high spontaneous remission rate with conservative treatment. Therefore, we should detect the PLA2R-Abs at the time of diagnosis and seronegative patients may receive greater benefits from delayed immunosuppressive therapy. Larger sample sizes of populations are required to confirm our findings.
Acknowledgments
The authors thank the members of the research group for useful discussions.
Author contributions
Formal analysis: Shiyi Zhang.
Investigation: Shuai Wang, Xiaoru Hu.
Methodology: Chenyang Tao.
Supervision: Zhanzheng Zhao.
Writing – original draft: Wenli Wu.
Writing – review & editing: Jin Shang.
Footnotes
Abbreviations: 24HTP = urine protein/24h, CENTRAL = Cochrane Center Register of Controlled Trials, CI = confidence intervals, ELISA = enzyme-linked immunosorbent assay, FSGS = focal segmental glomerulosclerosis, IIFC = indirect immunofluorescence, IMN = idiopathic membranous nephropathy, MN = membranous nephropathy, NOS = Newcastle–Ottawa scales, PLA2R = phospholipase A2 receptor, PLA2R-Abs = phospholipase A2 receptor autoantibodies, RR = risk ratio, SMN = secondary membranous nephropathy, UPCR = urine protein/creatinine ratio, WB = western blot.
The authors have no conflicts of interest to disclose.
References
- [1].Beck LH, Jr, Bonegio RGB, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 2009;361:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].El-Husseini A, Saxon D, Jennings S, et al. Idiopathic membranous nephropathy: diagnostic and therapeutic challenges. Am J Nephrol 2016;43:65–70. [DOI] [PubMed] [Google Scholar]
- [3].Couser WG. Primary membranous nephropathy. Clin J Am Soc Nephrol 2017;12:983–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ravindra P, Shankar P, Dharshan R, et al. Profile and clinicopathological predictors of outcomes of patients with membranous nephropathy. Indian J Nephrol 2016;26:S42–3. [Google Scholar]
- [5].Bech AP, Hofstra JM, Brenchley PE, et al. Association of anti-PLA(2)R antibodies with outcomes after immunosuppressive therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 2014;9:1386–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pavenstädt H. Membranous glomerulonephritis. Nephrologe 2016;11:96–105. [Google Scholar]
- [7].Stahl RA, Hoxha E, Helmchen U. [Membranous glomerulonephritis: better therapy with autoantibody monitoring?]. Dtsch Med Wochenschr 2011;136:1733–7. [DOI] [PubMed] [Google Scholar]
- [8].Kao L, Lam V, Waldman M, et al. Identification of the immunodominant epitope region in phospholipase A2 receptor-mediating autoantibody binding in idiopathic membranous nephropathy. J Am Soc Nephrol 2015;26:291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Szymanski J, West K, Cantilena C. Treatment resistant PLA2R negative membranous nephropathy responsive to LDL apheresis. J Clin Apheresis 2017;32:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Prabhu A, Prasad NS, Rangaswamy D, et al. Correlation of anti phospholipid A2 receptor antibodies (Antipla2r) with histopathology and outcomes in idiopathic membranous nephropathy (IMN). Nephrology 2016;21:112. [Google Scholar]
- [11].Seitz-Polski B, Dolla G, Payré C, et al. Epitope spreading of autoantibody response to PLA2R associates with poor prognosis in membranous nephropathy. J Am Soc Nephrol 2016;27:1517–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Van De Logt AE, Hofstra JM, Wetzels JFM. Serum anti-PLA2R antibodies can be initially absent in idiopathic membranous nephropathy: seroconversion after prolonged follow-up. Kidney Int 2015;87:1263–4. [DOI] [PubMed] [Google Scholar]
- [13].Pang L, Zhang AM, Li HX, et al. Serum anti-PLA2R antibody and glomerular PLA2R deposition in Chinese patients with membranous nephropathy: a cross-sectional study. Medicine 2017;96:e7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Radice A, Trezzi B, Maggiore U, et al. Clinical usefulness of autoantibodies to M-type phospholipase A2 receptor (PLA2R) for monitoring disease activity in idiopathic membranous nephropathy (IMN). Autoimmun Rev 2016;15:146–54. [DOI] [PubMed] [Google Scholar]
- [15].Jullien P, Seitz Polski B, Maillard N, et al. Anti-phospholipase A2 receptor antibody levels at diagnosis predicts spontaneous remission of idiopathic membranous nephropathy. Clin Kidney J 2017;10:209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hoxha E, Harendza S, Pinnschmidt H, et al. M-type phospholipase A2 receptor autoantibodies and renal function in patients with primary membranous nephropathy. Clin J Am Soc Nephrol 2014;9:1883–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kim YG, Choi YW, Kim SY, et al. Anti-phospholipase A2 receptor antibody as prognostic indicator in idiopathic membranous nephropathy. Am J Nephrol 2015;42:250–7. [DOI] [PubMed] [Google Scholar]
- [18].Hofstra JM, Debiec H, Short CD, et al. Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J Am Soc Nephrol 2012;23:1735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Timmermans SA, Abdul Hamid MA, Cohen Tervaert JW, et al. Anti-PLA2R antibodies as a prognostic factor in PLA2R-related membranous nephropathy. Am J Nephrol 2015;42:70–7. [DOI] [PubMed] [Google Scholar]
- [20].Oh YJ, Yang SH, Kim DK, et al. Autoantibodies against phospholipase A2 receptor in Korean patients with membranous nephropathy. PLoS One 2013;8:e62151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pourcine F, Dahan K, Mihout F, et al. Prognostic value of PLA2R autoimmunity detected by measurement of anti-PLA2R antibodies combined with detection of PLA2R antigen in membranous nephropathy: a single-centre study over 14 years. PLoS One 2017;12:e0173201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shenoy P. Role of phospholipase A2 receptor antibody in membranous nephropathy-a prospective study from southern India. Nephrology 2016;21:114. [Google Scholar]
- [23].Jin J. Antiphospholipase A2 receptor antiboby titer is associated with idiopathic membranous nephropathy disease activity regardiess of pathological grading. Nephrol Dialysis Transplant 2017;32:iii550. [Google Scholar]
- [24].Seitz-Polski B, Dolla G, Payre C, et al. Epitope spreading of autoantibody response to PLA2R associates with poor prognosis in membranous nephropathy. J Am Soc Nephrol 2016;27:1517–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fresquet M, Jowitt TA, Gummadova J, et al. Identification of a major epitope recognized by PLA2R autoantibodies in primary membranous nephropathy. J Am Soc Nephrol 2015;26:302–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Brenchley PE. Anti-phospholipase A2 receptor antibody and immunosuppression in membranous nephropathy: more evidence for pathogenicity of anti-phospholipase A2 receptor autoantibodies. J Am Soc Nephrol 2015;26:2308–11. [DOI] [PMC free article] [PubMed] [Google Scholar]


