Abstract
This is a retrospective study.
The aim of this study was to illustrate the survival outcomes of patients with classic ependymoma (CE) and identify potential prognostic factors.
CE is the most common category of spinal ependymomas, but few published studies have discussed predictors of the survival outcome.
A Boolean search of the PubMed, Embase, and OVID databases was conducted by 2 investigators independently. The objects were intramedullary grade II ependymoma according to 2007 WHO classification. Univariate Kaplan–Meier analysis and Log-Rank tests were performed to identify variables associated with progression-free survival (PFS) or overall survival (OS). Multivariate Cox regression was performed to assess hazard ratios (HRs) with 95% confidence intervals (95% CIs). Statistical analysis was performed by SPSS version 23.0 (IBM Corp.) with statistical significance defined as P < .05.
A total of 35 studies were identified, including 169 cases of CE. The mean follow-up time across cases was 64.2 ± 51.5 months. Univariate analysis showed that patients who had undergone total resection (TR) had better PFS and OS than those with subtotal resection (STR) and biopsy (P = .002, P = .004, respectively). Within either univariate or multivariate analysis (P = .000, P = .07, respectively), histological type was an independent prognostic factor for PFS of CE [papillary type: HR 0.002, 95% CI (0.000–0.073), P = .001, tanycytic type: HR 0.010, 95% CI (0.000–0.218), P = .003].
It was the first integrative analysis of CE to elucidate the correlation between kinds of factors and prognostic outcomes. Definite histological type and safely TR were foundation of CE's management.
Level of Evidence: 4
Keywords: ependymoma, neoplasm, prognostic factor, retrospective analysis, spinal cord, survival analysis, WHO grade 2
1. Introduction
Ependymomas are the most common primary intramedullary spinal cord tumor in adults, representing 30% to 45% of such lesions.[1–3] The World Health Organization (WHO) categorizes ependymomas into 3 grades, Grade I myxopapillary ependymoma and subependymoma; Grade II ependymoma, which represent classic ependymomas (CEs), including the cellular, clear cell, tanycytic, and papillary variants; and Grade III anaplastic ependymomas.[4] Although CEs can vary in histological classification, they account for 55.7% of spinal cord ependymomas.[5] Gross total resection (TR) of a spinal cord ependymoma may be accomplished in a majority of patients.[6–10] CEs with slowness of progression maintain the risk of late metastasis into the intraspinal or intracranial regions, with relatively poor prognosis.[11]
Demographic features, tumor grade, extension of surgery, and adjuvant radiotherapy (RT) are among the most studied variables in the existing CE literature, though the association of these factors and survival outcomes is quite variable. An integrative study performed by Kukreja et al [12] revealed that completeness of resection plays a crucial role in improving the prognosis of patients with spinal myxopapillary ependymomas (though younger patients have a less favorable outcome). On the contrary, adjuvant RT does not influence progression-free survival (PFS). In contrast, the study by Chen et al [13] suggested that adjuvant radiotherapies are considerable prognostic indicators in primary spinal anaplastic ependymomas, and age in this study was not significantly associated with the result. Various studies have attempted to clarify the prognostic factors of ependymomas,[12–15] but few have focused on CEs individually. Lin et al[16] conducted a population-based study for pediatric Grade II spinal ependymomas. They elucidated that RT and female sex in the subtotal resection (STR) group were factors associated with decreased mortality. Considering that CEs are one of the most prevalent ependymomas,[17] further investigation of the survival outcome of CEs is urgent.
In this study, we reviewed the literature on patients with CE to evaluate the survival outcome and identify potential prognostic indicators.
2. Materials and methods
2.1. Search strategy
A Boolean search of the PubMed, Embase, and OVID databases was conducted in March 2017 by 2 investigators independently. The study language was limited to English. Search terms were (Spinal Cord Neoplasms [MeSH Terms] AND ependymoma[MeSH Terms] AND “humans”[MeSH Terms]). We excluded duplicate results and screened studies by reading the abstract and full text. References of relevant articles, conference abstracts, and books were used as an additional approach to data collection. The final result was coordinated by a third researcher.
2.2. Selection method
Inclusion criteria for original papers were all included studies were case reports and case series reviews. Single case information was presented and the diagnosis was verified by pathological evidence. According to the WHO classification,[4] the CE comprises a histologically heterogeneous group of tumors that include cellular, papillary, clear cell, and tanycytic subtypes. The articles focused on the patient outcomes by follow-up, providing events of recurrence, death, and others factors relevant to the progression of the disease.
Exclusion criteria were patients with other tumors and severe diseases, such as neurofibromatosis, the primary lesion being extramedullary, such as cauda equine and so on, studies did not focus on CEs or where data were not available, the follow-up data were not referenced, and studies performed in the same institution and during the same time frame were also excluded.
2.3. Data extraction
Data collection was comprised of patient characteristics (age, sex, complaints, etc.), location and length of tumor, extent of resection, strategy of adjuvant treatment, recurrence or progression of disease, mortality, time to recurrence or death, and follow-up duration. To analyze the potential prognostic factors of intramedullary CE, we identified the aforementioned factors into groups. The age of the patients was categorized into 2 groups (Age <18 and Age ≥18 years). The extent of resection was divided into 2 subgroups: TR and STR (STR, biopsy included). TR referred the tumors removed in both an en bloc and a piecemeal fashion. The extent of resection was defined on the basis of the author's report or postoperative imaging. The adjuvant treatment fell into mainly 2 groups: RT and surgery alone (SA). The outcome of patients included all cause of death, tumor recurrence, and other complications. Disease progression was defined as tumor-relevant death, recurrence, and repeat surgery.
2.4. Statistical analysis
We depicted the characteristics of patients with intramedullary CE. The primary outcomes of our study were PFS and overall survival (OS), which were analyzed by Kaplan–Meier survival analysis with log-rank tests. Cox proportional hazard model was utilized for multivariate analysis. Hazard ratios (HRs) with 95% confidence intervals (95% CIs) were calculated. The covariates selected were determined by clinical experience and the result of the univariate analysis. Continuous variables were analyzed using the t test, and categorical variables were analyzed by the Pearson Chi-square test. P values <.05 were considered statistically significant. All the statistical operations were conducted using SPSS software (version 23.0; IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp).
3. Results
3.1. Study characteristic
A total of 1249 studies were found according to the search parameters, of which 246 replicated studies and 927 nonrelevant studies were excluded. During full-text review of the remaining 76 studies, 21 studies and 14 references of them[18–52] were deemed to meet the inclusion criteria. Among the 55 excluded studies, 15 were extramedullary ependymoma studies and 40 of the studies did not address CEs. The flowchart of selection is shown in Fig. 1. All included cases provided pathological diagnostic, treatment strategy, and follow-up data. Two studies[23,45] presented a rare subtype of Grade II ependymoma, which is alternatively referred to as giant cell ependymoma (GCE). GCE was first described in 1996,[53] characterized histologically by the presence of pleomorphic giant cells.
Figure 1.
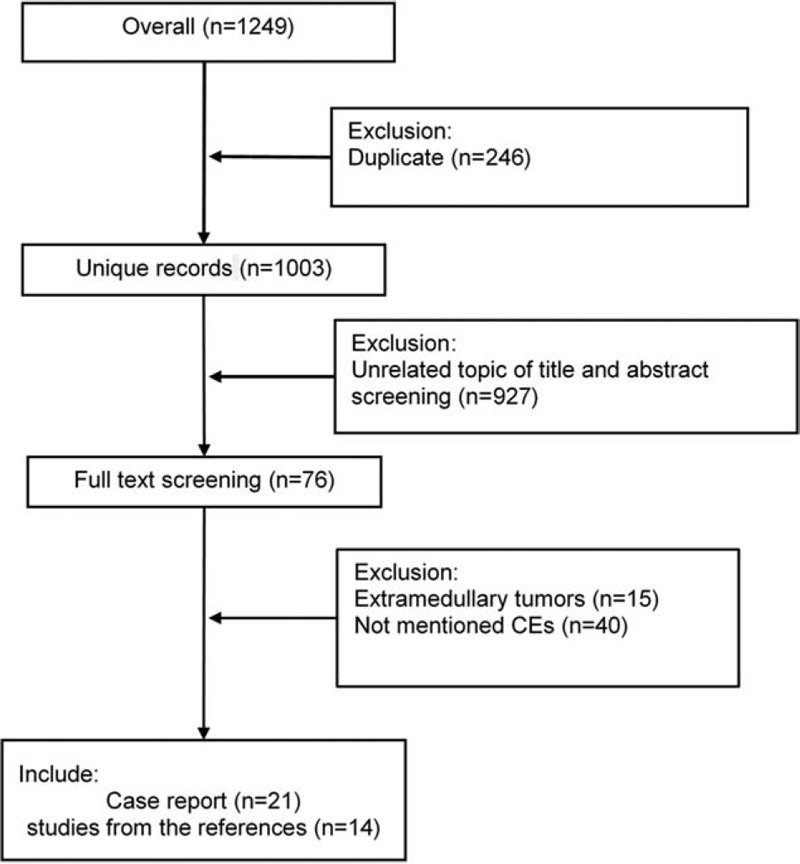
Flow chart of inclusion and exclusion.
3.2. Demographic features
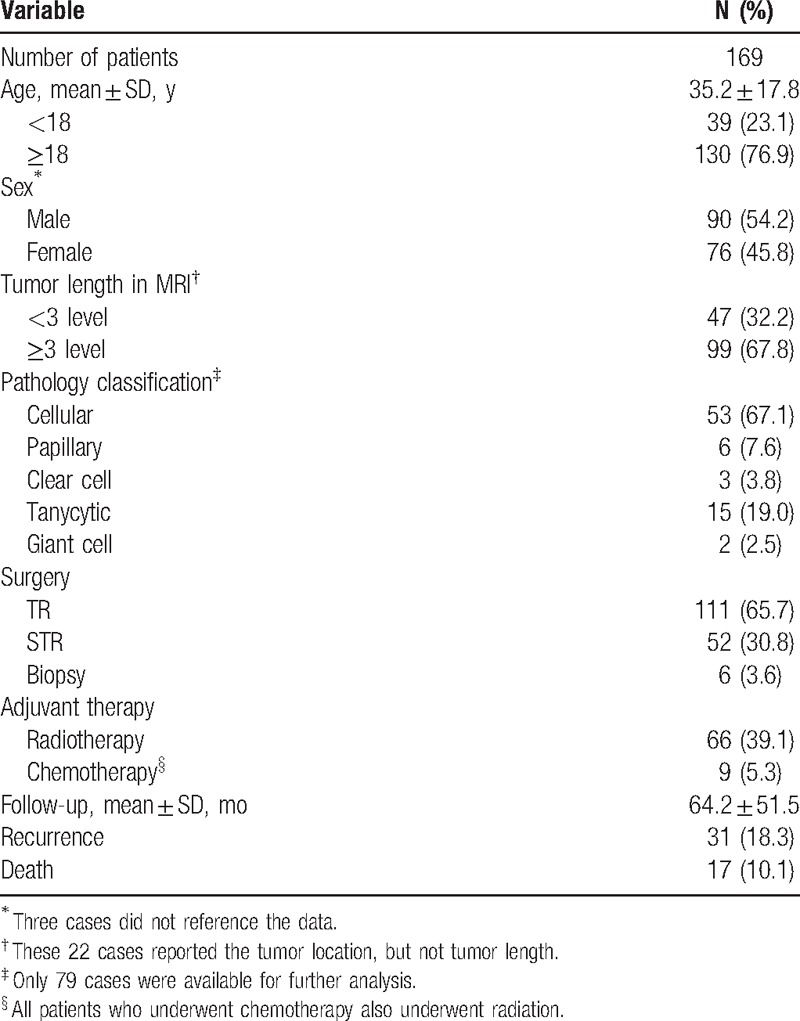
Thirty-five studies including 169 cases were eligible for survival analysis. The primary characteristics of the included patients are summarized in Table 1. The mean age was 35.2 ± 17.8 years. Male to female ratio was 1.2:1. Magnetic resonance imaging (MRI) showed that 67.8% patients had over 3 level lesions. Of the 163 patients who received surgical resection for CEs, 68.1% had undergone TR and 31.9% had undergone STR. The remaining 6 patients underwent biopsy alone. Around two-fifths of the included patients had received RT; however, it accounted 69.0% in people with none TR. Almost two-thirds of the 83 patients who were classified with the certainly histological subtype were deemed to have cellular ependymomas. The giant cell (2 cases), clear cell (3 cases), and papillary (6 cases) ependymomas were rare. During the follow-up (mean 64.2 ± 51.5 months), 10.1% of patients were deceased and 18.3% had suffered tumor recurrence. The recurrence rate at 1 year, 3 years, and 5 years were 4.1%, 8.2%, and 13.5%, respectively. The most common cause of death was tumor recurrence (70.6%), followed by unknown (17.6%) and cardiac disease (11.8%). It is worth noting that only 1 patient with T8-cauda lesions suffered death after TR.
Table 1.
Characteristics of classical ependymoma patients.
3.3. PFS
PFS is a combined end-point that includes progression and death. Since the lower rate of death, it may enhance estimates of treatment effectiveness and improve power. Overall, conditions of 33 patients worsened after the treatment, of which 3 cases were upgraded to extraspinal metastasis. The median time to progression was 147.0 ± 23.4 months for the sample as a whole. Univariate analysis showed that PFS was not affected by patients’ age, sex, or tumor length (Table 2). A log-rank test further revealed a significant difference in the PFS between the TR and STR groups (P = .002). Overall, 9 of 111 patients with TR suffered recurrence or death, with the mean time to PFS being 185.2 ± 14.2 months. On the contrary, progression of the disease was observed in 27 (46.6%) patients with STR, with a mean time to PFS being 129.3 ± 16.0 months (Fig. 2). There was also a significant difference in PFS according to histological type (P = .000). Specifically, patients who were classified as papillary type had a longer progression-free time (mean 194.4 ± 33.2 months) than the other subtypes. When classified by adjuvant therapy, RT patients had a shorter progression-free time than SA, with a mean of 119.6 ± 14.3 months (P = .000, Fig. 3, Table 2). Further analysis stratified by extent of surgery could not change this trend in neither TR nor STR group (P = .020). A multivariate Cox proportional hazards model for PFS was fitted using the following variables: age, sex, pathology subtype, extent of surgery, and adjuvant therapy. The results suggested that pathology type was independently associated with PFS (P = .016, Fig. 4, Table 2). Patients who had cellular, papillary, or tanycytic subtypes had lower risks of progression than those who possessed the clear cell type (HR = 0.027, 95% CI 0.001–0.521, P = .017; HR = 0.002, 95% CI 0.000–0.073, P = .001; HR = 0.010, 95% CI 0.000–0.218, P = .003; HR = 0.142, 95% CI 0.005–3.923, P = .249, respectively).
Table 2.
K-M and Cox regression analysis of PFS in classical ependymoma patients.
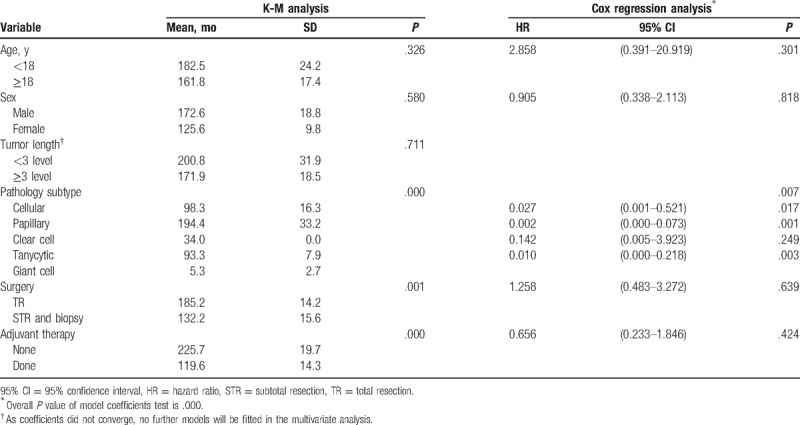
Figure 2.
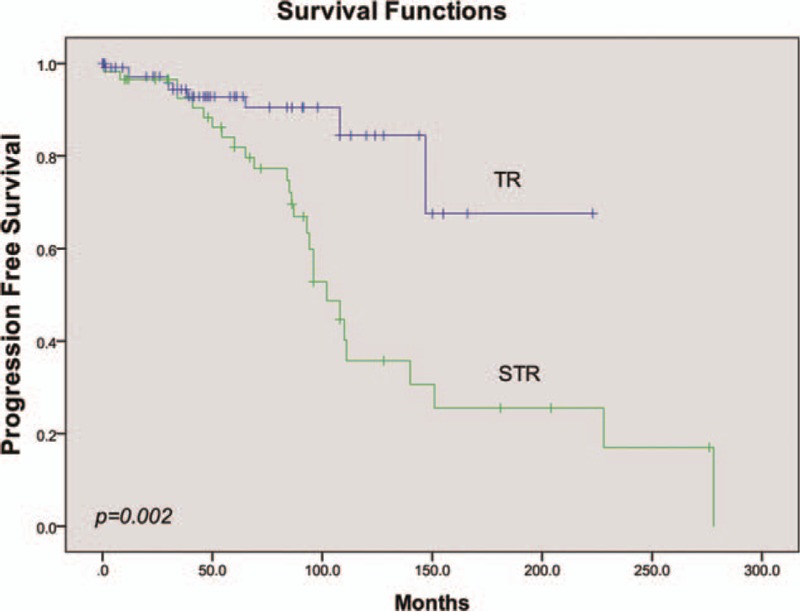
Univariate analysis of PFS stratified by extent of surgery.
Figure 3.
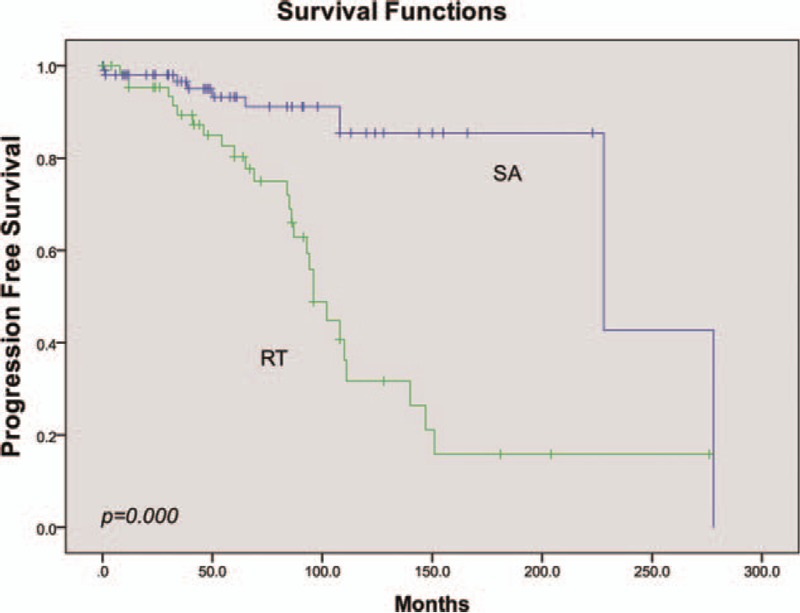
Univariate analysis of PFS stratified by adjuvant therapy.
Figure 4.
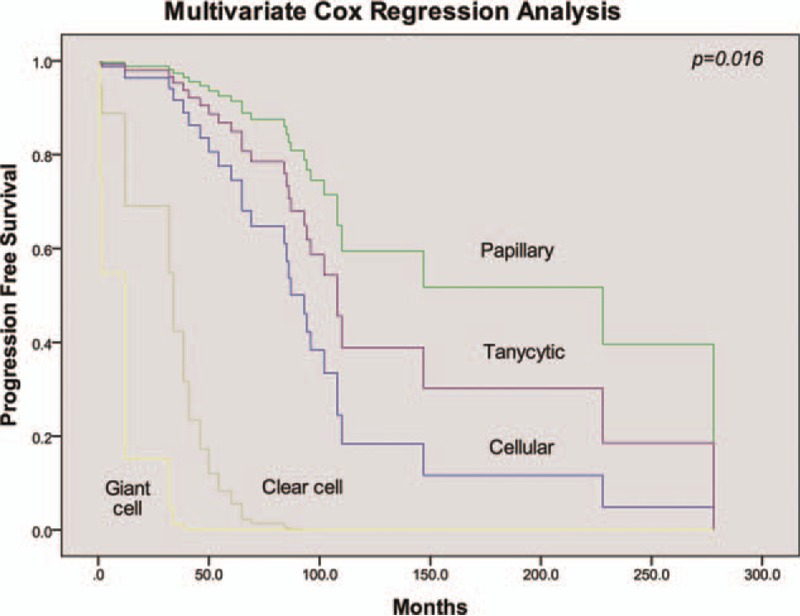
Multivariate analysis of PFS stratified by histological subtype.
3.4. OS
Traditionally, improvement in OS is the gold standard for definitively demonstrating clinical benefit of the cancer therapy in a clinical trial. In our analysis, a total of 17 cases (8.7%) were deceased, mainly due to tumor metastasis (15 cases). The mean survival time was 229.7 ± 14.5 months. The 2 remaining cases were pronounced dead due to heart failure and high level of paralysis, respectively. The OS analysis was not performed on histological type, due to the unavailability of papillary and clear cell type data. Similar to the results of the PFS analysis, univariate analysis showed that neither age nor sex had significant effects on OS (Table 3). The 5-year OS rate of the STR group was 13.8%, which was significantly lower than 2.7% observed in TR group (mean time to OS 200.0 ± 20.7 vs 215.3 ± 4.4, P = .004, Fig. 5). RT did not improve OS compared with SA (mean time to OS 190.8 ± 23.8 vs 264.6 ± 7.5, respectively, P = .007, Fig. 6). Tumor length, surgery extension, and adjuvant therapy were included in Cox regression model, of which none had a statistical effect on OS (P = .196, Table 3).
Table 3.
K-M and Cox regression analysis of OS in classical ependymoma patients.
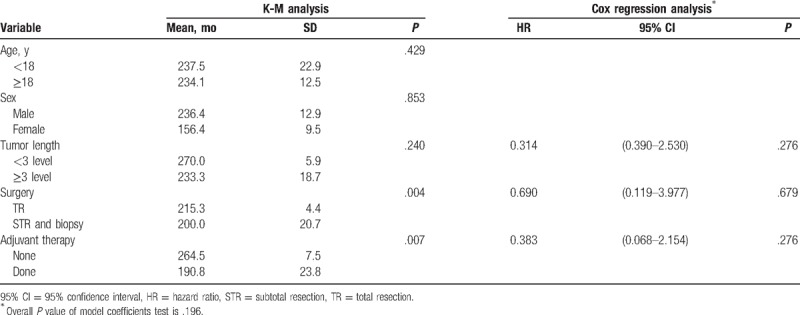
Figure 5.
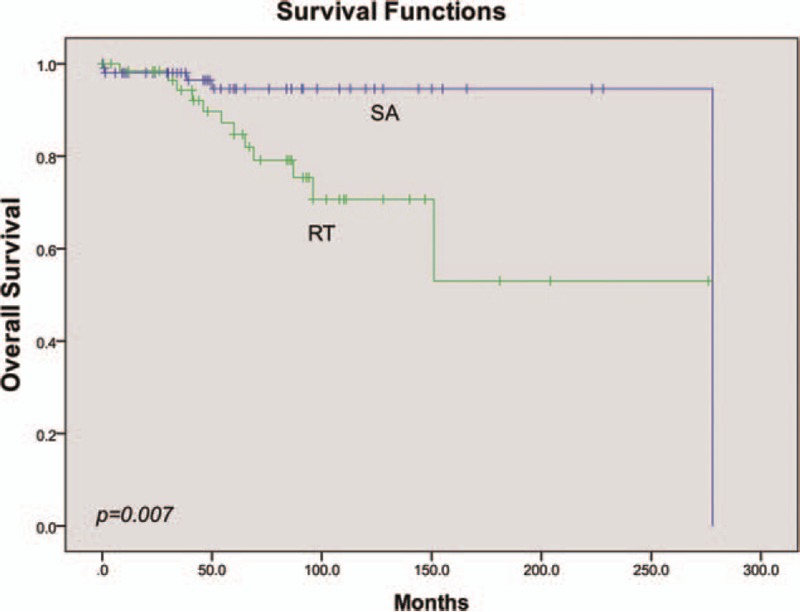
Univariate analysis of OS stratified by adjuvant therapy.
Figure 6.
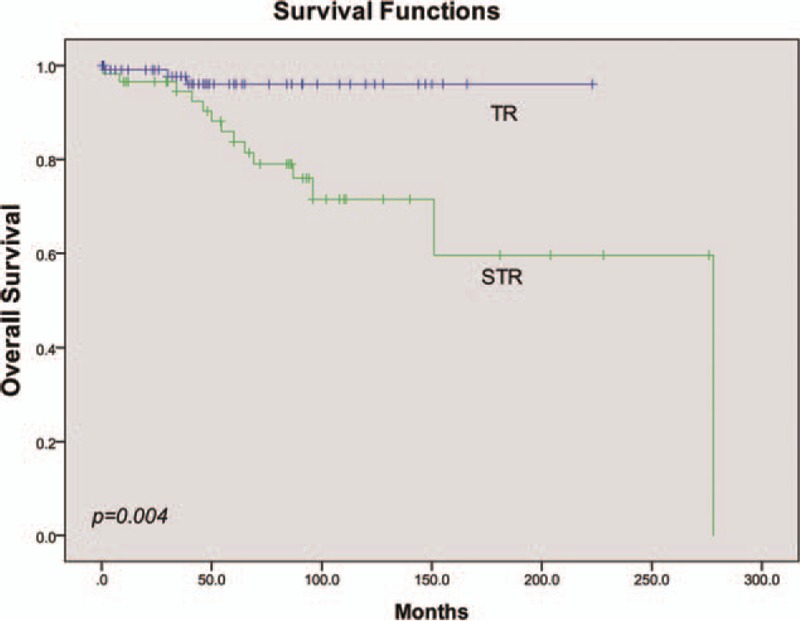
Univariate analysis of OS stratified by extent of surgery.
4. Discussion
Intramedullary tumors are rare, with limited existing information regarding its prognostic factors. Thus, we conducted a comprehensive literature review and integrative analysis to clarify the correlation between potential predictors and outcomes. Here, we performed the first integrative study that specifically evaluated subjects with CEs. A total of 169 cases were included in our investigation, with a mean follow-up time of 64.2 months. We confirmed histological subtype as an independently prognostic indicator of CEs. We also found a substantially better outcome for patients who underwent TR compared with STR or biopsy. Further, we observed that SA was associated with a significant improvement in survival.
Some studies have demonstrated the influence of age on survival outcomes in patients with spinal ependymomas. In a previous analysis of the SEER database, Lin et al[14] demonstrated that younger age was associated with improved long-term survival (P = .01). Alshaya et al[11] similarly performed an autopsy case study and literature review of classic low-grade ependymoma, describing that younger age was the only risk factors for patients with TR of the lesion. Both of the 2 studies had a small sample size. The former was only conducted in 1 database, and the later restricted subjects with metastasis. Conversely, we hold a comprehensive data retrieval in Pubmed, OVID, and EMbase. Our research here suggested neither PFS nor OS was impacted by age, sex, or tumor length.
Although some studies have suggested histological grading as a significant predictor of survival, the prognostic value of pathological evidence in ependymomas has been questioned.[54–56] Waldron et al[57] undertook a review of 59 spinal ependymoma subjects, where they concluded that tumor grade was the major prognostic factor. However, Safaee et al[8] and Vera-Bolanos et al[17] concluded that there was no difference between patients with grades I and II tumors (P = .708, P = .19, respectively). The association between grade III histology and survival, on the contrary, remained significant in a multivariate model (P = .008, P = .003, respectively). In this study, histological subtype was significantly linked to tumor progression both in univariate and multivariate analysis (P = .000, P = .007, respectively). The papillary ependymoma, generally a slow growing tumor, had the longest time to progression (mean 194.4 ± 33.2 months), which implied that other classifications might had higher invasiveness. Hence, a specific subtype of patients with CEs should be confirmed in clinical practice.
In accordance with previous studies,[6–8,10] the extent of surgery was also considered as an indicator in our research. The 5-year mortality in the TR group was 2.7%, while 13.8% patients were deceased in the STR group. In the study by Schick et al[9] of 25 cases, 5 recurrent ependymomas were detected even after STR. Bostrom et al[10] found that complete resection was associated with improvement in the survival of spinal cord ependymomas. In general, most evidence points to the recommendation of maximally safe resection as the first-line of treatment for CEs.
The role of adjuvant radiation therapy in the treatment of CEs has remained controversial.[20,58–60] Wahab et al[61] reviewed 22 cases of spinal ependymomas who received radiation. They discovered that postoperative radiation after STR is safe and prolonged patient survival. On the contrary, a population-based investigation using the SEER database conducted by Amirian et al[62] revealed that RT in adults was detrimental to survival (univariate HR = 2.13, 95% CI 1.72–2.65; multivariate model HR = 1.22, 95% CI 0.97–1.56). Alternatively, the univariate analysis of the study herein showed that CE patients who underwent adjuvant therapy demonstrated improved 5-year mortality. However, the multivariate analysis could not verify this result, leading us to believe that there were other confounding factors affecting the results of the univariate model. Regarding RT, a relationship could not be judged from the result due to the fact that patients who experienced adjuvant radiation may have had a worse baseline compared with the others. Most of those RT patients (61%) had the neoplasm invading surrounding tissue and thus received STR. Seventy-three percent of RT patients had large tumor (≥3 level), which increases the potential risk of recurrence and metastasis of postoperation. Further clinical data are needed to investigate these findings in the future.
Some inherent limitations were inevitable due to the nature of the study. First, all the literature included were retrospective studies simply due to the lack of prospective studies in the literature. Nonrandomized intervention and observational analysis of original papers may have also caused a higher risk of bias. Second, as no individual data were available, integrated studies with large samples were not included in our research leading to a selection bias. Moreover, the deficit of clinical data restricted our interpretation of the results to a certain degree, for example, the standard of neurological function evaluation in each study was different. In addition, the span of time among the original studies was around 40 years, which led to heterogeneity of surgical methods and, subsequently, outcome assessments.
5. Conclusion
Ependymomas are the most common primary intramedullary spinal cord tumor, of which more than half were CE. Lots of reports generally investigated ependymoma based on WHO grade, but few focus on the CE, that is, WHO grade 2, alone. It would be benefited to predict prognostic of patients with CE that making the pathological subtype clear in the clinical work. Specifically, papillary type was associated with prolonged PFS and OS. TR was recommended as the first goal of surgery, while adjuvant therapy could not improve outcomes after neither TR nor STR. Above all, definite histological type and safely TR were foundation of CE's management. More experimental studies with control group should be performed in this issue.
6. Ethics statement
Study participants voluntarily agreed to participate in the study and provided written informed consent before enrollment. The study was approved by the Ethics Committee of Union Hospital Affiliated to Fujian Medical University. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Acknowledgments
We appreciate supports from the colleague of Department of Neurosurgery, Fujian Medical University Union Hospital
Author contributions
Conceptualization: Yinqing Wang, Chunmei Chen.
Data curation: Yinqing Wang, Ranze Cai, Rui Wang, Chunhua Wang.
Formal analysis: Yinqing Wang, Ranze Cai, Rui Wang, Chunhua Wang, Chunmei Chen.
Funding acquisition: Chunmei Chen.
Investigation: Yinqing Wang, Chunmei Chen.
Methodology: Yinqing Wang, Ranze Cai, Rui Wang, Chunhua Wang, Chunmei Chen.
Project administration: Chunmei Chen.
Resources: Chunmei Chen.
Supervision: Chunmei Chen.
Validation: Yinqing Wang, Ranze Cai, Rui Wang, Chunhua Wang.
Footnotes
Abbreviations: CE = classic ependymoma, GCE = giant cell ependymoma, OS = overall survival, PFS = progression-free survival, RT = radiotherapy, SA = surgery alone, STR = subtotal resection, TR = total resection.
Both YW and RC are cofirst authors.
Funding/support: No funds were received in support of this work.
The authors have no conflict of interest regarding the publication of this paper.
References
- [1].Chamberlain MC. Ependymomas. Curr Neurol Neurosci Rep 2003;3:193–9. [DOI] [PubMed] [Google Scholar]
- [2].Duong LM, McCarthy BJ, McLendon RE, et al. Descriptive epidemiology of malignant and nonmalignant primary spinal cord, spinal meninges, and cauda equina tumors, United States, 2004–2007. Cancer 2012;118:4220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gilbert MR, Ruda R, Soffietti R. Ependymomas in adults. Curr Neurol Neurosci Rep 2010;10:240–7. [DOI] [PubMed] [Google Scholar]
- [4].Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Keil VC, Schmitt AJ, Martin SC, et al. Optimising treatment strategies in spinal ependymoma based on 20years of experience at a single centre. J Clin Neurosci 2016;29:52–8. [DOI] [PubMed] [Google Scholar]
- [6].Abdullah KG, Lubelski D, Miller J, et al. Progression free survival and functional outcome after surgical resection of intramedullary ependymomas. J Clin Neurosci 2015;22:1933–7. [DOI] [PubMed] [Google Scholar]
- [7].Epstein FJ, Farmer JP, Freed D. Adult intramedullary spinal cord ependymomas: the result of surgery in 38 patients. J Neurosurg 1993;79:204–9. [DOI] [PubMed] [Google Scholar]
- [8].Safaee M, Oh MC, Kim JM, et al. Histologic grade and extent of resection are associated with survival in pediatric spinal cord ependymomas. Childs Nerv Syst 2013;29:2057–64. [DOI] [PubMed] [Google Scholar]
- [9].Schick U, Marquardt G, Lorenz R. Recurrence of benign spinal neoplasms. Neurosurg Rev 2001;24:20–5. [DOI] [PubMed] [Google Scholar]
- [10].Bostrom A, Kanther NC, Grote A, et al. Management and outcome in adult intramedullary spinal cord tumours: a 20-year single institution experience. BMC Res Notes 2014;7:908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Alshaya W, Mehta V, Wilson BA, et al. Low-grade ependymoma with late metastasis: autopsy case study and literature review. Childs Nerv Syst 2015;31:1565–72. [DOI] [PubMed] [Google Scholar]
- [12].Kukreja S, Ambekar S, Sharma M, et al. Outcome predictors in the management of spinal myxopapillary ependymoma: an integrative survival analysis. World Neurosurg 2015;83:852–9. [DOI] [PubMed] [Google Scholar]
- [13].Chen P, Sui M, Ye J, et al. An integrative analysis of treatment, outcomes and prognostic factors for primary spinal anaplastic ependymomas. J Clin Neurosci 2015;22:976–80. [DOI] [PubMed] [Google Scholar]
- [14].Lin Y, Smith ZA, Wong AP, et al. Predictors of survival in patients with spinal ependymoma. Neurol Res 2015;37:650–5. [DOI] [PubMed] [Google Scholar]
- [15].Oh MC, Kim JM, Kaur G, et al. Prognosis by tumor location in adults with spinal ependymomas. J Neurosurg Spine 2013;18:226–35. [DOI] [PubMed] [Google Scholar]
- [16].Lin Y, Jea A, Melkonian SC, et al. Treatment of pediatric Grade II spinal ependymomas: a population-based study. J Neurosurg Pediatr 2015;15:243–9. [DOI] [PubMed] [Google Scholar]
- [17].Vera-Bolanos E, Aldape K, Yuan Y, et al. Clinical course and progression-free survival of adult intracranial and spinal ependymoma patients. Neuro Oncol 2015;17:440–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Akutsu H, Shibata Y, Okazaki M, et al. Intramedullary clear cell ependymoma in the cervical spinal cord: case report. Neurosurgery 2000;47:1434–7. discussion 7–8. [PubMed] [Google Scholar]
- [19].Celano E, Salehani A, Malcolm JG, et al. Spinal cord ependymoma: a review of the literature and case series of ten patients. J Neurooncol 2016;128:377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chamberlain MC. Salvage chemotherapy for recurrent spinal cord ependymona. Cancer 2002;95:997–1002. [DOI] [PubMed] [Google Scholar]
- [21].Clover LL, Hazuka MB, Kinzie JJ. Spinal cord ependymomas treated with surgery and radiation therapy. A review of 11 cases. Am J Clin Oncol 1993;16:350–3. [DOI] [PubMed] [Google Scholar]
- [22].Dulai MS, Caccamo DV, Briley AL, et al. Intramedullary papillary ependymoma with choroid plexus differentiation and cerebrospinal fluid dissemination to the brain. J Neurosurg Pediatr 2010;5:511–7. [DOI] [PubMed] [Google Scholar]
- [23].Fourney DR, Siadati A, Bruner JM, et al. Giant cell ependymoma of the spinal cord. Case report and review of the literature. J Neurosurg 2004;100:75–9. [DOI] [PubMed] [Google Scholar]
- [24].Hanbali F, Fourney DR, Marmor E, et al. Spinal cord ependymoma: radical surgical resection and outcome. Neurosurgery 2002;51:1162–72. discussion 72–4. [DOI] [PubMed] [Google Scholar]
- [25].Hentschel SJ, McCutcheon IE, Ginsberg L, et al. Exophytic ependymomas of the spinal cord. Acta Neurochir (Wien) 2004;146:1047–50. [DOI] [PubMed] [Google Scholar]
- [26].Ito T, Ozaki Y, Nakagawara J, et al. A case of cervicomedullary junction tanycytic ependymoma associated with marked cyst formation. Brain Tumor Pathol 2005;22:29–33. [DOI] [PubMed] [Google Scholar]
- [27].Kawano N, Yagishita S, Oka H, et al. Spinal tanycytic ependymomas. Acta Neuropathol 2001;101:43–8. [DOI] [PubMed] [Google Scholar]
- [28].Kim DJ, Kim TW, Kim Y, et al. Clear cell ependymoma occurring in the cauda equina. J Korean Neurosurg Soc 2010;48:153–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kim NR, Chung DH, Lee SK, et al. Intramedullary clear cell ependymoma in the thoracic spinal cord: a case with its crush smear and ultrastructural findings. J Korean Med Sci 2007;22(suppl 22):S149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kyoshima K, Akaishi K, Tokushige K, et al. Surgical experience with resection en bloc of intramedullary astrocytomas and ependymomas in the cervical and cervicothoracic region. J Clin Neurosci 2004;11:623–8. [DOI] [PubMed] [Google Scholar]
- [31].Langford LA, Barre GM. Tanycytic ependymoma. Ultrastruct Pathol 1997;21:135–42. [DOI] [PubMed] [Google Scholar]
- [32].Li JY, Lopez JI, Powell SZ, et al. Giant cell ependymoma-report of three cases and review of the literature. Int J Clin Exp Pathol 2012;5:458–62. [PMC free article] [PubMed] [Google Scholar]
- [33].Lin YH, Huang CI, Wong TT, et al. Treatment of spinal cord ependymomas by surgery with or without postoperative radiotherapy. J Neurooncol 2005;71:205–10. [DOI] [PubMed] [Google Scholar]
- [34].Merchant TE, Kiehna EN, Thompson SJ, et al. Pediatric low-grade and ependymal spinal cord tumors. Pediatr Neurosurg 2000;32:30–6. [DOI] [PubMed] [Google Scholar]
- [35].Moon K, Filis AK, Cohen AR. Mobile spinal ependymoma. J Neurosurg Pediatr 2010;5:85–8. [DOI] [PubMed] [Google Scholar]
- [36].Newton HB, Henson J, Walker RW. Extraneural metastases in ependymoma. J Neurooncol 1992;14:135–42. [DOI] [PubMed] [Google Scholar]
- [37].Ohata K, Takami T, Gotou T, et al. Surgical outcome of intramedullary spinal cord ependymoma. Acta Neurochir (Wien) 1999;141:341–6. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- [38].Orozco LD, Tiel RL. Exophytic ependymoma of the thoracic spine. J Clin Neurosci 2011;18:1262–4. [DOI] [PubMed] [Google Scholar]
- [39].Payne NS, 2nd, McDonald JV. Rupture of spinal cord ependymoma. Case report. J Neurosurg 1973;39:662–5. [DOI] [PubMed] [Google Scholar]
- [40].Pencovich N, Bot G, Lidar Z, et al. Spinal ependymoma with regional metastasis at presentation. Acta Neurochir 2014;156:1215–22. [DOI] [PubMed] [Google Scholar]
- [41].Peschel RE, Kapp DS, Cardinale F, et al. Ependymomas of the spinal cord. Int J Radiat Oncol Biol Phys 1983;9:1093–6. [DOI] [PubMed] [Google Scholar]
- [42].Plotkin SR, O’Donnell CC, Curry WT, et al. Spinal ependymomas in neurofibromatosis Type 2: a retrospective analysis of 55 patients. J Neurosurg Spine 2011;14:543–7. [DOI] [PubMed] [Google Scholar]
- [43].Sato K, Kubota T, Ishida M, et al. Spinal tanycytic ependymoma with hematomyelia: case report. Neurol Med Chir (Tokyo) 2005;45:168–71. [DOI] [PubMed] [Google Scholar]
- [44].Scott M. Infiltrating ependymomas of the cauda equina. Treatment by conservative surgery plus radiotherapy. J Neurosurg 1974;41:446–8. [DOI] [PubMed] [Google Scholar]
- [45].Shamji MF, Benoit BG, Perry A, et al. Giant cell ependymoma of the thoracic spine: pathology case report. Neurosurgery 2009;64:E566–7. discussion E7. [DOI] [PubMed] [Google Scholar]
- [46].Shaw EG, Evans RG, Scheithauer BW, et al. Radiotherapeutic management of adult intraspinal ependymomas. Int J Radiat Oncol Biol Phys 1986;12:323–7. [DOI] [PubMed] [Google Scholar]
- [47].Shintaku M, Nagata N, Itoh H. Tanycytic ependymoma of the spinal cord with anaplastic cytological features. Brain Tumor Pathol 2009;26:7–10. [DOI] [PubMed] [Google Scholar]
- [48].Spaar FW, Blech M, Ahyai A. DNA-flow fluorescence–cytometry of ependymomas. Report on ten surgically removed tumours. Acta Neuropathol 1986;69:153–60. [DOI] [PubMed] [Google Scholar]
- [49].Stephen JH, Sievert AJ, Madsen PJ, et al. Spinal cord ependymomas and myxopapillary ependymomas in the first 2 decades of life: a clinicopathological and immunohistochemical characterization of 19 cases. J Neurosurg Pediatr 2012;9:646–53. [DOI] [PubMed] [Google Scholar]
- [50].Ueki K, Sasaki T, Ishida T, et al. Spinal tanycytic ependymoma associated with neurofibromatosis type 2–case report. Neurol Med Chir (Tokyo) 2001;41:513–6. [DOI] [PubMed] [Google Scholar]
- [51].Wen BC, Hussey DH, Hitchon PW, et al. The role of radiation therapy in the management of ependymomas of the spinal cord. Int J Radiat Oncol Biol Phys 1991;20:781–6. [DOI] [PubMed] [Google Scholar]
- [52].Yang T, Wu L, Yang C, et al. Clinical features and long-term outcomes of intraspinal ependymomas in pediatric patients. Childs Nerv Syst 2014;30:2073–81. [DOI] [PubMed] [Google Scholar]
- [53].Zec N, De Girolami U, Schofield DE, et al. Giant cell ependymoma of the filum terminale. A report of two cases. Am J Surg Pathol 1996;20:1091–101. [DOI] [PubMed] [Google Scholar]
- [54].Godfraind C. Classification and controversies in pathology of ependymomas. Childs Nerv Syst 2009;25:1185–93. [DOI] [PubMed] [Google Scholar]
- [55].Korshunov A, Golanov A, Sycheva R, et al. The histologic grade is a main prognostic factor for patients with intracranial ependymomas treated in the microneurosurgical era: an analysis of 258 patients. Cancer 2004;100:1230–7. [DOI] [PubMed] [Google Scholar]
- [56].Raghunathan A, Wani K, Armstrong TS, et al. Histological predictors of outcome in ependymoma are dependent on anatomic site within the central nervous system. Brain Pathol 2013;23:584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Waldron JN, Laperriere NJ, Jaakkimainen L, et al. Spinal cord ependymomas: a retrospective analysis of 59 cases. Int J Radiat Oncol Biol Phys 1993;27:223–9. [DOI] [PubMed] [Google Scholar]
- [58].Lee SH, Chung CK, Kim CH, et al. Long-term outcomes of surgical resection with or without adjuvant radiation therapy for treatment of spinal ependymoma: a retrospective multicenter study by the Korea Spinal Oncology Research Group. Neurooncology 2013;15:921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sgouros S, Malluci CL, Jackowski A. Spinal ependymomas: the value of postoperative radiotherapy for residual disease control. Br J Neurosurg 1996;10:559–66. [DOI] [PubMed] [Google Scholar]
- [60].Benesch M, Weber-Mzell D, Gerber NU, et al. Ependymoma of the spinal cord in children and adolescents: a retrospective series from the HIT database. J Neurosurg Pediatr 2010;6:137–44. [DOI] [PubMed] [Google Scholar]
- [61].Wahab SH, Simpson JR, Michalski JM, et al. Long term outcome with post-operative radiation therapy for spinal canal ependymoma. J Neurooncol 2007;83:85–9. [DOI] [PubMed] [Google Scholar]
- [62].Amirian ES, Armstrong TS, Aldape KD, et al. Predictors of survival among pediatric and adult ependymoma cases: a study using Surveillance, Epidemiology, and End Results data from 1973 to 2007. Neuroepidemiology 2012;39:116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]


