Abstract
Background:
Myoclonus is an undesirable phenomenon that occurs after induction of general anesthesia using etomidate. Opioids such as sufentanil are considered effective pretreatment drugs for myoclonus inhibition, although high doses are required. Transcutaneous acupoint electrical stimulation (TAES), a noninvasive technique involving electrical stimulation of the skin at the acupuncture points, exhibits analgesic effects, promotes anesthetic effects, decreases the dose of anesthetic drugs, and increases endogenous opioid peptide levels. In the present study, we investigated the effects of TAES combined with low-dose sufentanil pretreatment on the incidence and severity of etomidate-induced myoclonus in patients undergoing elective hysteroscopy.
Methods:
In a double-blind manner, 172 patients (American Society of Anesthesiologists class I–II; age, 20–55 years) scheduled to undergo elective hysteroscopy were randomized into the following groups (n = 43 each): control (false TAES followed by saline injection after 30 min), TAES (TAES followed by saline injection after 30 minutes), sufentanil [false TAES followed by low-dose sufentanil (0.1 μg/kg) injection after 30 minutes], and sufentanil plus TAES (TAES followed by low-dose sufentanil injection after 30 minutes). In all groups, general anesthesia was induced by etomidate 0.3 mg/kg after sufentanil or saline injection. The incidence and severity of myoclonus were assessed for 2 minutes after etomidate administration. The visual analogue scale (VAS) scores for pain at 1 hour after surgery were recorded. The heart rate (HR), mean arterial pressure (MAP), and peripheral capillary oxygen saturation (SPO2) were recorded before premedication, after etomidate injection, after uterus expansion, and after recovery from anesthesia.
Results:
The incidence of myoclonus was highest in the control group (88.3%), followed by TAES (74.4%), sufentanil (60.4%), and TAES plus sufentanil (48.8%) groups. Thus, the incidence was significantly higher in the control and TAES groups than in the sufentanil and TAES plus sufentanil groups. Grade 3 myoclonus occurred in 30.2%, 9.3%, 11.6%, and 9.3% patients in the control, TAES, sufentanil, and TAES plus sufentanil groups, respectively, with significant differences between the control group and the other 3 groups. Furthermore, the postoperative VAS scores for pain were significantly lower in the TAES, sufentanil, and TAES plus sufentanil groups compared with those in the control group. There were no significant differences in any other parameters among groups.
Conclusion:
Our results suggest that TAES combined with low-dose opioids such as sufentanil can decrease the incidence and severity of etomidate-induced myoclonus.
Keywords: etomidate, hysteroscopy, myoclonus, sufentanil, transcutaneous acupoint electrical stimulation
1. Introduction
Since the introduction of etomidate as an agent for the induction of general anesthesia in 1973,[1] its use has gained popularity because of a favorable cardiovascular profile,[2–4] minimal respiratory side effects,[5] and rapid onset of action and clearance from the body.[6] Etomidate is a common choice of anesthetic drug for procedures that cause short-term pain, particularly in hemodynamically compromised patients.[7] However, pain on injection and myoclonus are 2 well-known and undesirable side effects, although the former is now minimized by a new lipid formulation of etomidate, known as (etomidate-lipuro).[8] On the other hand, etomidate-induced myoclonus remains a serious clinical problem, with an incidence of 50% to 80% in patients who do not receive premedication.[9,10] Unfortunately, the underlying mechanism for this side effect remains unknown. Myoclonus is a clinically significant side effect that can result in regurgitation and aspiration in patients undergoing emergency surgery without fasting.[9] It is also detrimental for patients with open globe injuries or limited cardiovascular reserves, because it can increase the intraocular pressure and restrict cardiac function.[11] Several pretreatment drugs for the inhibition of myoclonus have been investigated, including benzodiazepines,[12,13] opioids,[14–18] and muscle relaxants,[19,20] and all have the ability to prevent this side effect to some extent. Of these, opioids are the most effective, albeit at high doses, which can cause undesirable adverse effects such as sedation, cough, apnea, respiratory depression, and chest wall rigidity.[17,21,22] Sufentanil is considered an effective clinical opioid analgesic for inhibiting the myoclonus induced by etomidate.[23] For minor surgeries such as elective hysteroscopy, where the average time at site is always 20 to 30 min, benzodiazepines and muscle relaxants cause a significant delay in the recovery from anesthesia. Therefore, the ideal pretreatment drug for preventing myoclonic movements without significant side effects has not yet been identified.
Acupoint electrical stimulation (AES), a classical procedure under Traditional Chinese Medicine, combined with pretreatment drugs is currently an exciting area of research. Transcutaneous AES (TAES) is an improved treatment method used in the practice of acupuncture. Thus, acupuncture and AES are methodologically the same. Transcutaneous AES (TAES) is a noninvasive, safe, and simple technique involving electrical stimulation of skin over acupuncture points. Transcutaneous AES exhibits analgesic effects, promotes anesthetic effects, and reduces the dose of anesthetic drugs.[24,25] Furthermore, it increases the levels of endogenous opioid peptides, such as enkephalin, beta-endorphin, and dynorphin, which produce the same effects as those with opioids.[26]
The acupoint hegu (L14) is located in the web between the thumb and first finger on the back of the hand, at the mid-metacarpal level. The acupoint waiguan (SJ5) is located on the posterior forearm, between the radius and ulna, on the radial side of the extensor digitorum communis tendons, 2 inches proximal from the wrist. Hegu (L14), the most widely treated acupoint, inhibits involuntary contraction of the diaphragm and is used in the treatment of hiccups. Hegu (L14) is also involved in mood stabilization and is helpful in alleviating preoperative stress in patients. Further, this acupoint has an analgesic effect that is useful in treating headache and toothache. In contrast, the main use of waiguan (SJ5) is in the treatment of headache, shoulder pain, intercostal pain, and tremors of the feet. We chose hegu (L14) and waiguan (SJ5) as the 2 acupoints for pretreatment of etomidate-induced myoclonus in this study.
We investigated the effects of TAES combined with low-dose sufentanil pretreatment on the incidence and severity of myoclonus induced by etomidate in patients undergoing elective hysteroscopy. Transcutaneous AES is commonly used alone as a complimentary treatment for the management of pain, particularly acute postoperative pain[27] and chronic pain.[28] We hypothesized that in combination with intravenous sufentanil, TAES would decrease myoclonus following administration of induction doses of etomidate.
2. Methods
This study was approved by the Ethics Committee of Wenzhou Medical University (clinical trial number: ChiCTR-INR-16009084; Registry URL: YJLCYJ-2016-168). This manuscript adheres to the applicable Equator guidelines. Patients scheduled for elective hysteroscopy (American Society of Anesthesiologists class I–II; age, 20–55 years) were included. All patients provided written informed consent for participation in the study. The exclusion criteria were as follows: presence of incisions, scars, or skin infection at the hegu and waiguan acupoints; history of allergy to drugs used in the study; history of spinal surgery; sinus bradycardia; severe neurological diseases; severe respiratory diseases; severe cardiovascular diseases; and any other diseases that required exclusion. None of the patients received premedication.
On arrival in the operating room, standard monitors, including those for electrocardiography, heart rate, noninvasive blood pressure (NIBP), and peripheral capillary oxygen saturation (SpO2), were attached to the patient, and to ensure patient safety, monitoring was continued until the end of the operation. A 22-gauge cannula was inserted into the dorsum of the patient's hand for drug administration. Oxygen was supplied with a non-rebreathing mask and reservoir system with an oxygen flow of 3 L/min.
All patients were randomized in a double-blinded manner to 1 of 4 pretreatment groups (n = 43 each): control, TAES, sufentanil, and sufentanil plus TAES.
2.1. Control group
The patients received false TAES (2/100 Hz; dilatational waves), bilaterally, at the hegu and waiguan acupoints. The selected intensity was the least value at which the patient could feel the stimulation. Intravenous saline (2 mL) was administered 30 minutes after false TAES, following which anesthesia was induced with 0.3 mg/kg etomidate (En Pharmaceutical Co Ltd, Jiangsu).
2.2. TAES group
The patients received TAES (2/100 Hz; dilatational waves) bilaterally, at the hegu and waiguan acupoints for 30 minutes. The selected intensity was the patient's maximum tolerated current value minus 1 mA. Intravenous saline (2 mL) was administered 30 minutes after TAES, following which anesthesia was induced with 0.3 mg/kg etomidate.
2.3. Sufentanil group
The patients received false TAES, as described for the control group. Intravenous sufentanil (EuroCept B.V) 0.1 μg/kg (diluted to 2 mL) was administered 30 minutes after false TAES, following which anesthesia was induced with 0.3 mg/kg etomidate.
2.4. Sufentanil plus TAES group
The patients received TAES (2/100 Hz; dilatational waves) bilaterally at the hegu and waiguan acupoints for 30 minutes. The selected intensity was the patient's maximum tolerated current value minus 1 mA. Intravenous sufentanil 0.1 μg/kg (diluted to 2 mL) was administered 30 minutes after TAES, following which anesthesia was induced with 0.3 mg/kg etomidate.
The incidence and severity of myoclonus were assessed for 2 minutes after etomidate administration. A physician blinded to the treatment assessed myoclonus. The clinical grades of myoclonus were assigned as follows: 0, no myoclonus; 1, mild myoclonus (short movements of a body part, such as the wrist or a finger); 2, moderate myoclonus (mild movements of 2 different muscles, such as those in the face and leg); and 3, severe myoclonus (intense clonic movements of 2 or more muscle groups, e.g., fast abduction of a limb).[10] No other drugs were used between the pretreatment drug and at the end of induction.
After the 2-minutes assessment period, propofol (Astrazeneca Pharmaceutical Co Ltd, London, England) 0.1 mg/(kg·min) and remifentanil (Yichang Pharmaceutical Co Ltd) 0.1 μg/(kg·min) were administered to maintain comfortable anesthesia until 3 minutes before the completion of surgery. In case the patient moved or frowned during surgery, propofol was added at 0.5 to 1 mg/kg. If respiratory depression developed (breathing rate <8/min or oxygen saturation <90%), artificial positive pressure ventilation was adopted. If the systolic blood pressure was <90 mm Hg or the diastolic blood pressure was <60 mm Hg, ephedrine 5 to 10 mg was administered. If the heart rate (HR) was <50 beats/min, atropine 0.5 mg was administered.
The visual analogue scale (VAS) score for pain at 1 hour after surgery was recorded for all patients. In addition, HR, mean arterial pressure (MAP), and SPO2 were recorded before premedication (T1), after etomidate injection (T2), after uterus expansion (T3), and after recovery from anesthesia (T4). Finally, all related adverse reactions, including pain on injection, respiratory depression, intraoperative movements, and postoperative nausea and vomiting, among others, were documented for all patients.
2.5. Statistical analysis
We used a randomization table generated by a computer to allocate the patients into 4 groups (n = 43 per group)—the control group, TAES group, sufentanil group, and sufentanil plus TAES group—to detect differences in the incidence and severity of myoclonus, which were determined by an independent staff member. After obtaining consent from the patients and their families, every patient received TAES (or false TAES) before the surgery. The anesthetist involved in the surgery, who was blinded to the purpose of this study, prepared the same anesthetic based on the patient's weight. Another member, who was blinded to the patient's group, evaluated the clinical parameters of the patients during and after the surgery to collect data.
All statistical analyses were performed with SPSS 13 software (Statistical Product and Service Solutions, SPSS Company). Continuous values are expressed as means ± standard deviations. Intergroup differences were analyzed using one-way analysis of variance (ANOVA). Count data were compared using χ2 tests, and hierarchical data were compared using nonparametric Kruskal–Wallis tests. If differences were observed, Nemenyi tests were used. A P value <.05 was considered statistically significant. The datasets generated and analyzed during the current study are available from the corresponding author on request.
3. Results
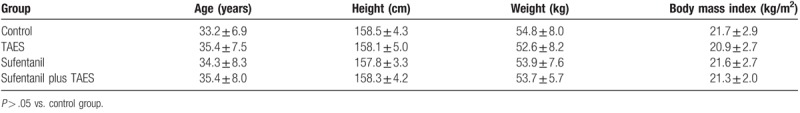
There were no significant differences in patient characteristics among groups (Table 1, P > .05).
Table 1.
Characteristics of control patients and patients who received transcutaneous acupoint electrical stimulation (TAES) and/or low-dose sufentanil pretreatment before etomidate injection.
The incidence of myoclonus was the highest in the control group (88.3%), followed by the TAES (74.4%), sufentanil (60.4%), and sufentanil plus TAES (48.8%) groups. Moreover, the incidence of grade 3 myoclonus was 30.2% in the control group, 9.3% in the TAES group, 11.6% in the sufentanil group, and 9.3% in the TAES plus sufentanil group. Although the incidence of myoclonus was not significantly different between the control and TAES groups, the incidence of severe myoclonus was significantly lower in the latter than in the former (P < .05). In addition, the incidence and severity of myoclonus were significantly lower in the sufentanil (P < .05) and sufentanil plus TAES (P < .01) groups than in the control group (Table 2).
Table 2.
Incidence and severity of myoclonus after etomidate injection in control patients and patients who received transcutaneous acupoint electrical stimulation (TAES) and/or low-dose sufentanil pretreatment before etomidate injection.

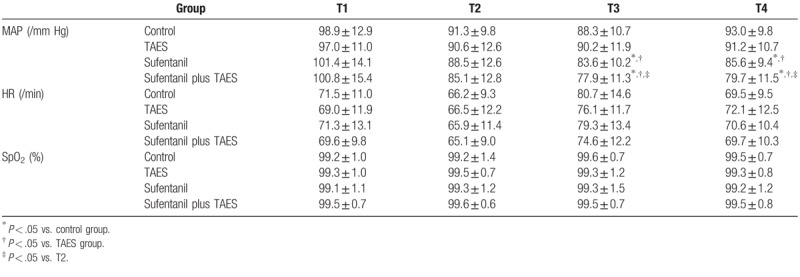
The VAS score for pain at 1 h after surgery was significantly lower in the TAES and sufentanil plus TAES (P < .05) groups than in the control group. The incidence of adverse effects was low and similar among groups (Table 3). HR and SPO2 values were not significantly different among the 4 time points (P > .05). However, MAP values at T3 and T4 were significantly lower in the sufentanil and sufentanil plus TAES groups than in the control group (P < .05). In the sufentanil group and sufentanil plus TAES group, MAP at T3 and T4 was significantly lower than that in the TAES group (P < .05). In the sufentanil plus TAES group, MAP was significantly lower at T3 and T4 than at T2 (P < .05; Table 4).
Table 3.
Time to emergence from anesthesia, visual analogue scale (VAS) score for pain at 1 h after surgery, and incidence of adverse reactions in control patients and patients who received transcutaneous acupoint electrical stimulation (TAES) and/or low-dose sufentanil pretreatment before etomidate injection.

Table 4.
Mean arterial pressure (MAP), heart rate (HR), and peripheral capillary oxygen saturation (SPO2) before premedication (T1), after etomidate injection (T2), after uterus expansion (T3), and after recovery from anesthesia (T4) in control patients and patients who received transcutaneous acupoint electrical stimulation (TAES) and/or low-dose sufentanil pretreatment before etomidate injection.
4. Discussion
We investigated the effects of TAES combined with low-dose sufentanil pretreatment on the incidence and severity of myoclonus induced by etomidate in patients undergoing elective hysteroscopy. The results showed that TAES and sufentanil exerted synergistic effects and significantly decreased the incidence and severity of myoclonus induced by etomidate. In fact, TAES alone was effective in decreasing the severity of myoclonus. Most importantly, no adverse effects were observed, with a remarkable decrease in the VAS score for pain in the TAES and TAES plus sufentanil groups. Finally, as suggested in Table 4, even small doses of sufentanil (0.1 μg/kg) could visibly influence the hemodynamics of the patient. Collectively, our findings provide evidence that TAES is a valuable treatment.
Etomidate has been commonly used for hemodynamically unstable patients, short-term anesthesia, and rapid sequence intubation owing to multiple advantages including rapid onset of action, favorable cardiovascular profile, minimal respiratory and intracranial pressure, and nervous system protection.[1–6,29] However, etomidate-induced myoclonus remains a clinically significant side effect with severe consequences such as vitreous prolapse in a patient with open eye injury,[11] ECG lead detachment with a decrease in oxygen saturation,[30] muscle pain, higher energy consumption and serum potassium levels, higher internal gastric pressure, regurgitation and aspiration.
Etomidate interacts with gamma-aminobutyric acid type A (GABAA) receptors, although the precise mechanism underlying myoclonus caused by this drug is unknown. Doenicke et al[9] conducted an electroencephalogram study and reported that myoclonus induced by etomidate is attributed to subcortical disinhibition, similar to that causing restless leg syndrome during normal human sleep, and is not related to epileptic foci. Another study reported that the depression of inhibitory circuits earlier than that of excitatory neuronal circuits after etomidate administration could be the reason,[31] while yet another study reported that the interruption of GABA neurons increases the sensitivity of skeletal muscle-associated pathways, leading to spontaneous nerve transmissions and, consequently, myoclonus.[32]
An ideal drug for the inhibition of etomidate-induced myoclonus is one that should not interfere with the favorable pharmacodynamic profile of etomidate or cause any undesired side effects. Although several drugs have been investigated from this perspective, their results are inconclusive.
Benzodiazepines[12,13,33] can decrease myoclonic movements to some extent, although it results in moderate hemodynamic side effects and prolonged recovery. Myoclonus inhibition by opioids is dose-dependent. Several studies have evaluated the effectiveness of opioids (fentanyl, sufentanil, and remifentanil) for the inhibition of myoclonus[14–18,22] and have reported that high doses are required for complete inhibition. However, these high doses result in significant side effects such as sedation, cough, apnea, respiratory depression, and chest wall rigidity.[21] While rocuronium[19,20] causes muscle relaxation and significantly decreases the frequency of myoclonus, it also results in airway obstruction, regurgitation, and aspiration. During long surgeries, all the above-mentioned disadvantages can be dealt by the anesthetist, whereas they create problems during short surgeries. AES was developed under a special national condition in China during the 1950s to 1970s. The main mechanism of acupuncture can be explained by 3 different pathways: local nerve excitement, pain signal collision, and endorphin secretion. Acupuncture anesthesia was initiated in 1958, and, reflecting the historical background of China after the 1960s, the use of this technique widely spread throughout the country. It extended to other countries after 1971, creating a significant impact and garnering attention from medical academia worldwide.[34]
While conventional acupuncture requires the insertion of needles at acupoints, TAES is a noninvasive, safe, and simple technique involving electrical stimulation of the skin over these acupoints. According to the principles of Traditional Chinese Medicine, the human body comprises several collaterals and over 700 hundred acupoints, with each one serving a different function. Stimulation of the hegu and waiguan acupoints contributes to sedation and analgesia.
While low-dose sufentanil results in fewer side effects, it cannot efficiently inhibit myoclonus. In the present study, we investigated the effects of low-dose sufentanil in combination with TAES and found a marked decrease in the incidence and severity of myoclonus induced by etomidate, which is a clinically significant finding. In addition, we found that the incidences of intraoperative movements and respiratory depression were the lowest in the TAES group, which indicates that TAES can accelerate awakening and strengthen the sedation and analgesic effects without causing respiratory depression. Low-dose (0.1 μg/kg) sufentanil also decreased the incidence and severity of etomidate-induced myoclonus when administered alone. Although the incidence of respiratory depression was high with sufentanil alone and in combination with TAES, the effects were transient and clinically insignificant. The incidence of myoclonus in the control group was 88%, which is higher than that (50%–80%) reported in previous studies.[9,33] This could be attributed to the longer assessment time in our study (2 minutes) compared to that in previous studies (1 minute). The incidence of myoclonus was the highest at 30 to 60 seconds after etomidate injection, but we considered that assessment for 2 minutes was more appropriate, in case myoclonus developed after 1 minute. Thus, extension of the observation period increased the reliability of our study findings.
Our study has a few limitations. The first and the most important one is that all patients were women of child-bearing age. According to previous studies, male patients are more likely to develop myoclonus than female patients, with children aged 5 to 10 years comprising the most susceptible population (90.2%).[35,36] Second, only the incidence and severity of myoclonus was assessed in the present study, and the durations of myoclonic movements were not recorded. Third, because propofol and remifentanil were required to maintain comfortable anesthesia until 3 minutes before the completion of surgery, the precise effects of TAES plus sufentanil on short-term recovery could not be assessed. Further studies are needed to address these limitations.
5. Conclusions
The results of our study indicate that TAES combined with low-dose sufentanil pretreatment significantly decreases the incidence and severity of myoclonus induced by etomidate administered for the induction of anesthesia, with minimal adverse effects. While TAES was found to be an effective treatment for etomidate-induced myoclonus, it was more effective in combination with low-dose sufentanil. This strategy not only decreases the dose of drugs used but also minimizes side effects, thus providing a safer and more comfortable medical model. We believe that TAES has several additional functions and advantages that remain undiscovered, and the introduction of TAES for clinical indications is expected in the future.
Author contributions
YL designed and performed the experiment; wrote the manuscript. HH designed the experiment. JX performed data collection and analysis. WJ performed the experiment.
CJS implemented the experiment. YP implemented the experiment. LW implemented the experiment. YM designed the experiment. QD, WG, JW provided financial and technical support.
Conceptualization: Yunchang Mo, Junlu Wang.
Data curation: Junjie Xie, Wenjun Jin.
Funding acquisition: Qinxue Dai, Wujun Geng.
Investigation: CanJi Shou.
Project administration: Ya Lv, Leilei Wang.
Resources: Yuanyuan Pan.
Supervision: Ya Lv, Haijuan He.
Validation: Yunchang Mo, Junlu Wang.
Writing – original draft: Ya Lv.
Writing – review & editing: Ya Lv.
Footnotes
Abbreviations: AES = acupoint electrical stimulation, GABAA = gamma-aminobutyric acid type A, HR = heart rate, L14 = the acupoint hegu, MAP = mean arterial pressure, NIBP = noninvasive blood pressure, SJ5 = the acupoint waiguan, SPO2 = peripheral capillary oxygen saturation, TAES = transcutaneous acupoint electrical stimulation, VAS = the visual analogue scale.
Disclosure of funding:
The Municipal Science and Technology Program (2017Y0307; Recipient: YL).
The National Natural Science Fund projects of China (81573742; Recipient: JW).
The National Natural Science Fund projects of China (81603685; Recipient: YM).
The National Natural Science Fund projects of China (81774109; Recipient: WG).
The National Natural Science Fund projects of China (81704180; Recipient: QD).
National Ministry of Science and Technology Plan Project 973 (2013CB531903; Recipient: JW).
Zhejiang TCM science and technology project (2017ZA133; Recipient: JW).
The Provincial Natural Science Fund projects (LY15H290006; Recipient: JW).
Major Disease Prevention and Control of Science and Technology Plan (ZX-01-C2016028; Recipient: WG).
Clinical trial number: ChiCTR-INR-16009084.
Registry URL: YJLCYJ-2016-168.
The authors have no conflicts of interest to disclose.
References
- [1].Morgan M, Lumley J, Whitwam JG. Etomidate: a new water-soluble non-barbiturate intravenous induction agent. Lancet 1975;305:955–6. [DOI] [PubMed] [Google Scholar]
- [2].Desai PM, Kane D, Sarkar MS. Cardioversion: What to choose? Etomidate or propofol. Ann Card Anaesth 2015;18:306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bennett JM, Ehrenfeld JM, Markham L, et al. Anesthetic management and outcomes for patients with pulmonary hypertension and intracardiac shunts and Eisenmenger syndrome: a review of institutional experience. J Clin Anesth 2014;26:286–93. [DOI] [PubMed] [Google Scholar]
- [4].Erdoes G, Basciani RM, Eberle B. Etomidate–a review of robust evidence for its use in various clinical scenarios. Acta Anaesthesiol Scand 2014;58:380–9. [DOI] [PubMed] [Google Scholar]
- [5].Li YW, Ma L, Sui B, et al. Etomidate with or without flumazenil anesthesia for stem cell transplantation in autistic children. Drug Metabol Drug Interact 2014;29:47–51. [DOI] [PubMed] [Google Scholar]
- [6].Dela Cruz JE, Sullivan DN, Varboncouer E, et al. Comparison of procedural sedation for the reduction of dislocated total hip arthroplasty. West J Emerg Med 2014;15:76–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ruth WJ, Burton JH, Bock AJ. Intravenous etomidate for procedural sedation in emergency department patients. Acad Emerg Med 2001;8:13–8. [DOI] [PubMed] [Google Scholar]
- [8].Doenicke A, Roizen MF, Nebauer AE, et al. A comparison of two formulations for etomidate, 2-hydroxypropyl-beta-cyclodextrin (HPCD) and propylene glycol. Anesth Analg 1994;79:933–9. [DOI] [PubMed] [Google Scholar]
- [9].Doenicke AW, Roizen MF, Kugler J, et al. Reducing myoclonus after etomidate. Anesthesiology 1999;90:113–9. [DOI] [PubMed] [Google Scholar]
- [10].Holdcroft A, Morgan M, Whitwam JG, et al. Effect of dose and premedication on induction complications with etomidate. Br J Anaesth 1976;48:199–205. [DOI] [PubMed] [Google Scholar]
- [11].Berry JM, Merin RG. Etomidate myoclonus and the open globe. Anesth Analg 1989;69:256–9. [PubMed] [Google Scholar]
- [12].Hüter L, Schreiber T, Gugel M, et al. Low-dose intravenous midazolam reduces etomidate-induced myoclonus: a prospective, randomized study in patients undergoing elective cardioversion. Anesth Analg 2007;105:1298–302. [DOI] [PubMed] [Google Scholar]
- [13].Alipour M, Tabari M, Azad AM. Comparative study evaluating efficacy of sufentanil versus midazolam in preventing myoclonic movements following etomidate. J Anaesthesiol Clin Pharmacol 2016;32:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Stockham RJ1, Stanley TH, Pace NL, et al. Fentanyl pretreatment modifies anaesthetic induction with etomidate. Anaesth Intensive Care 1988;16:171–6. [DOI] [PubMed] [Google Scholar]
- [15].Hwang JY, Kim JH, Oh AY, et al. A comparison of midazolam with remifentanil for the prevention of myoclonic movements following etomidate injection. J Int Med Res 2008;36:17–22. [DOI] [PubMed] [Google Scholar]
- [16].Hueter L, Schwarzkopf K, Simon M, et al. Pretreatment with sufentanil reduces myoclonus after etomidate. Acta Anaesthesiol Scand 2003;47:482–4. [DOI] [PubMed] [Google Scholar]
- [17].Ri HS, Shin SW, Kim TK, et al. The proper effect site concentration of remifentanil for prevention of myoclonus after etomidate injection. Korean J Anesthesiol 2011;61:127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zou L, Yuan H, Wang HY, et al. [Role of target controlled infusion of remifentanil for the prevention of etomidate induced myoclonus during general anesthesia]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2013;35:112–5. [PubMed] [Google Scholar]
- [19].Choi JM, Choi IC, Jeong YB, et al. Pretreatment of rocuronium reduces the frequency and severity of etomidate-induced myoclonus. J Clin Anesth 2008;20:601–4. [DOI] [PubMed] [Google Scholar]
- [20].Nooraei N, Solhpour A, Mohajerani SA. Priming with atracurium efficiently suppresses etomidate-induced myoclonus. Acta Anaesthesiol Taiwan 2013;51:145–8. [DOI] [PubMed] [Google Scholar]
- [21].He L, Ding Y, Chen H, et al. Dezocine pretreatment prevents myoclonus induced by etomidate: a randomized, double-blinded controlled trial. J Anesth 2015;29:143–5. [DOI] [PubMed] [Google Scholar]
- [22].Khalil SN, Lawson KS, Hanis CL, et al. Alfentanil decreases myoclonus caused by etomidate. Middle East J Anaesthesiol 1999;15:185–92. [PubMed] [Google Scholar]
- [23].Isitemiz I, Uzman S, Toptaş M, et al. Prevention of etomidate-induced myoclonus: which is superior: Fentanyl, midazolam, or a combination? A retrospective comparative study. Med Sci Monit 2014;20:262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wen YR, Yeh GC, Shyu BC, et al. A minimal stress model for the assessment of electroacupuncture analgesia in rats under halothane. Eur J Pain 2007;11:733–42. [DOI] [PubMed] [Google Scholar]
- [25].Feng X, Ye T, Wang Z, et al. Transcutaneous acupoint electrical stimulation pain management after surgical abortion: A cohort study. Int J Surg 2016;30:104–8. [DOI] [PubMed] [Google Scholar]
- [26].Lee JH, Choi YH, Choi BT. The anti-inflammatory effects of 2 Hz electroacupuncture with different intensities on acute carrageenan-induced inflammation in the rat paw. Int J Mol Med 2005;16:99–102. [PubMed] [Google Scholar]
- [27].Cho YH, Kim CK, Heo KH, et al. Acupuncture for acute postoperative pain after back surgery: a systematic review and meta-analysis of randomized controlled trials. Pain Pract 2015;15:279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med 2007;147:492–504. [DOI] [PubMed] [Google Scholar]
- [29].Kim TK, Park IS. Comparative study of brain protection effect between thiopental and etomidate using bispectral index during temporary arterial occlusion. J Korean Neurosurg Soc 2011;50:497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Van Keulen SG, Burton JH. Myoclonus associated with etomidate for ED procedural sedation and analgesia. Am J Emerg Med 2003;21:556–8. [DOI] [PubMed] [Google Scholar]
- [31].Kugler J, Doenicke A, Laub M. The EEG after etomidate. Anaesthesiol Resusc 1977;106:31–48. [Google Scholar]
- [32].Gancher S, Laxer KD, Krieger W. Activation of epileptogenic activity by etomidate. Anesthesiology 1984;61:616–8. [DOI] [PubMed] [Google Scholar]
- [33].Schwarzkopf KRG, Hueter L, Simon M, et al. Midazolam pretreatment reduces etomidate-induced myoclonic movements. Anaesth Intensive Care 2003;31:18–20. [DOI] [PubMed] [Google Scholar]
- [34].Jin L, Wu JS, Chen GB, et al. Unforgettable ups and downs of acupuncture anesthesia in China. World Neurosurg 2017;102:623–31. [DOI] [PubMed] [Google Scholar]
- [35].Kelsaka E, Karakaya D, Sarihasan B, et al. Remifentanil pretreatment reduces myoclonus after etomidate. J Clin Anesth 2006;18:83–6. [DOI] [PubMed] [Google Scholar]
- [36].Nyman Y1, von Hofsten K, Ritzmo C, et al. Effect of a small priming dose on myoclonic movements after intravenous anaesthesia induction with Etomidate-Lipuro in children. Br J Anaesth 2011;107:225–8. [DOI] [PubMed] [Google Scholar]


