Abstract
Rationale:
Small cell carcinoma of the ovary (SCCO) is a rare and aggressive extra-pulmonary variant of small cell tumors of uncertain histogenesis. The pathogenesis and optimal treatment of SCCO is unclear. We present a very rare case of a synchronous primary ovarian small cell carcinoma and endometrioid adenocarcinoma of the uterus in a patient after 2 years of tamoxifen treatment for breast cancer. This is the first such report in the English literature.
Patient concerns:
A 46-year-old woman had a history of left breast cancer that was treated with a simple mastectomy and sentinel lymph node biopsy in 2013. The post-operative pathology was invasive ductal carcinoma of the left breast. she had been taking tamoxifen for 2 years. The patient underwent an exploratory laparotomy to reduce the tumor burden, improve bowel compression symptoms, and promote defecation in 2015. The post-operative pathology revealed a rare, simultaneous occurrence of two tumors (endometrial adenocarcinoma and SCCO [pulmonary type]).
Diagnoses:
Primary ovarian small cell carcinoma of pulmonary type with coexisting endometrial carcinoma in a breast cancer patient.
Interventions:
The patient received 3 courses of chemotherapy after operation. The effect was not apparent and the general health status was poor.
Outcomes:
The patient died of progressive disease 7 months post-operatively.
Lessons:
The present case suggests that tamoxifen use might be among many etiologic factors in SCCO development. Despite its rarity, SCCO requires a high degree of attention in clinical work because it is an aggressive tumor that has a poor prognosis.
Keywords: breast cancer, endometrial carcinoma, ovarian small cell carcinoma, pulmonary type, tamoxifen
1. Introduction
Small cell carcinoma (SCC) is a neuroendocrine tumor that most often occurs in the lung, the incidence of it among ovarian neoplasms is less than 1%.[1] Small cell carcinoma of ovarian (SCCO) has extremely aggressive clinical behavior, resulting in an unfavorable prognosis, even when diagnosed in the early stages. SSCO is divided into 2 types: pulmonary type (SCCOPT) and hypercalcemic type (SCCOHT). Less than 300 cases of SCCOHT have been reported in the English literature.[2] The SCCOPT is rarer, with only 20 cases reported to date.[3] Due to a limited understanding of the underlying pathology, management, and outcome of SCCO, there is no consensus regarding optimal treatment. To date, there have been no reports involving coexisting SCCOPT with endometrial carcinoma of a woman with a previous history of breast cancer in the English literature. We present this unique case combined with the previous literature that may provide the relevant information of multiple cancers involving SCCOPT.
2. Case report
The study was approved by the Institutional Ethics Committees of Shandong cancer hospital Affiliated to Shandong University and conducted in accordance with the ethical guidelines of the Declaration of Helsinki. A written informed consent was obtained from the patient for publication of this case report.
A 46-year-old gravida 2 para 2 Chinese woman was admitted to the Shandong Cancer Hospital Affiliated to Shandong University in September 2015 with chief complaints of an abdominal mass, nausea, loss of appetite, and weight loss of 6 months duration. She had a history of left breast cancer (T1N0M0) that was treated with a simple mastectomy and sentinel lymph node biopsy in July 2013. The immunohistochemical examination revealed the following: ER (+), 90%; PR (+), 90%, HER-2 (−); and Ki-67 (+), 10%. Postoperatively she was treated with oral tamoxifen (20 mg daily) continuously for the ER (+) and PR (+) tissue expression, but without endometrial monitoring. In August 2015, the patient complained of abdominal distension, anorexia, and dyschezia. A chest CT scan showed the following: the lungs were clear; no adenopathy was noted; and the left clavicle, left axilla, and mediastinal para-aortic arch had multiple enlarged lymph nodes. Abdominal and pelvic CT scanning identified a huge pelvic tumor and ascites. The tumor, approximately 15.8 × 10 cm in size, revealed a mixed pattern of multicystic and solid parts. The mass displaced the uterine cavity anteriorly and the rectum posteriorly, thus causing rectal outlet obstruction. A CT-enhanced scan showed significant tumor enhancement. The uterine volume was increased, and the endometrium was significantly thickened. Multiple lymph nodes were enlarged in the pelvic cavity and the retroperitoneal space. The liver and spleen, omentum, mesentery, pelvic peritoneum, and local nodular foci were visualized during the contrast CT phase (Fig. 1). The laboratory tests performed on admission showed a mild elevation in the white blood cell count and C-reactive protein level, but the electrolytes were within normal limits. The serum calcium level was 2.15 mmol/L (normal value, 2.03–2.54 mmol/L). The serum concentration of cancer antigen-125 (CA-125) was 3434 U/mL (normal value, <35.0 U/mL), the cancer antigen-724 (CA-724) was >300 U/mL (normal value, <63 U/mL), the cancer embryo antigen (CEA) was 8.85 ng/mL (normal value, <3.4 ng/mL), the ROMA1 was 99.5% (normal value, <11.4%), and the ROMA2 was 99.4% (normal value, < 29.9%). Taken together, the clinical diagnosis was endometrial carcinoma with bilateral ovarian metastases. Because the disease was late stage, the initial decision was to perform palliative surgery to reduce the tumor burden, improve bowel compression symptoms, and promote defecation. The patient underwent an exploratory laparotomy on September 11, 2015, which revealed 5000 mL of sallow, turbid ascites. Ascites cytology found a large cell, but a diagnosis could not be confirmed (Fig. 2A). An irregular, white, solid tumor (18 cm in the longest diameter) of the ovaries bilaterally was observed, with a spontaneous rupture of the tumor. The uterus was congested and estimated to be the size of an 8-week pregnancy. The peritoneum was congested and edematous, and the omentum was thickened and contracted. Palpation revealed enlargement of the para-aortic and peri-pancreatic lymph nodes with vague boundaries. The mesostenium was thickened and stiff with a group of enlarged lymph nodes. The intraoperative assessment was terminal cancer and the abdominal and pelvic lymph nodes could not be removed. Subsequently, debulking surgery, consisting of an abdominal hysterectomy with a bilateral adnexectomy and omentectomy, were performed. The postoperative course was unremarkable, with no major complications.
Figure 1.
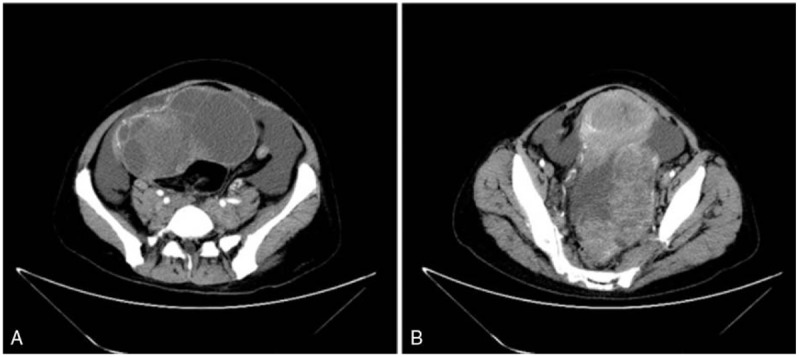
CT enhanced scan shows significant tumor enhancement, and solid and cystic masses in the left and right ovaries with massive ascites.
Figure 2.
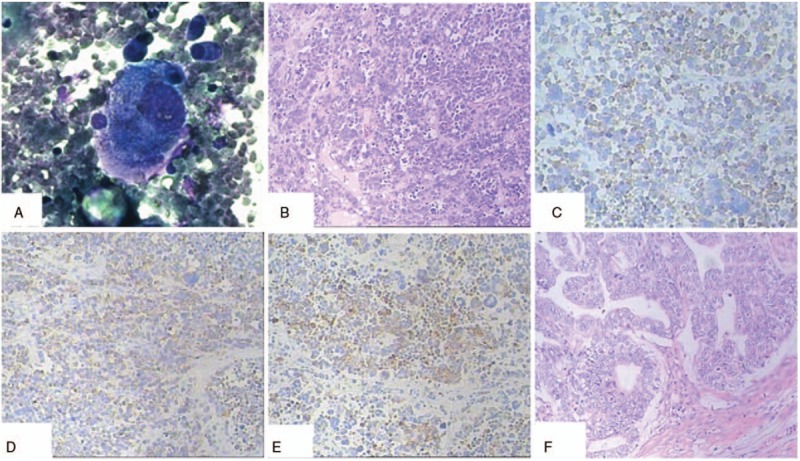
A, Cytologic examination of ascites showed a large cell. B, The tumor was characterized by small cells with scant cytoplasm, powdery chromatin, and mitotic activity. Tumor cells showed variable arrangements in sheets, clusters, and cords (H&E; original magnification, ×100). C, The ovarian small cell carcinoma was positive for synaptophysin (H&E; original magnification, ×100). D, The ovarian small cell carcinoma was positive for chromogranin A (H&E; original magnification, ×100). E, CKpan positivity was noted in the ovarian small cell carcinoma (H&E; original magnification, ×100). F, The uterus and the left adnexal showed well-differentiated endometrioid adenocarcinoma (H&E; original magnification, ×100).
The postoperative pathology revealed a rare, simultaneous occurrence of endometrial adenocarcinoma and SCCOPT. The blood NSE level was 254.3 ng/mL (normal value, < 17 ng/mL) 20 days postoperatively. The patient received 3 courses of chemotherapy, consisting of paclitaxel (180 mg/m2) and carboplatin (AUC 6). In preparing the fourth cycle of chemotherapy, the radiologic assessment showed that the tumor had progressed. Thus, we administered 2 courses of chemotherapy, consisting of paclitaxel (180 mg/m2), carboplatin (AUC 6), and etoposide (100 mg/m2). The effect was not apparent and the general health status was poor. She declined future treatment and returned home. The patient died of progressive disease 7 months postoperatively.
2.1. Pathology
Gross appearance of the surgical specimen. The uterine corpus was soft, measured 12 × 8 × 7 cm, and was gray-red in color. The left ovary measured 13.5 × 14 × 9.5 cm. A section of the left ovary had cystic and solid components; the solid areas were gray and gray-yellow in color. The right ovary measured 16 × 10 × 8.5 cm. A section of the right ovary was gray-white and grey-red in color and had a fine texture.
Microscopic examination. The right ovarian tumor was composed of dense pieces the same size of small cells. The cells had hyperchromatic nuclei with inconspicuous nucleoli and scanty cytoplasm. The nucleus was fusiform or round. There were numerous mitotic figures and apoptotic bodies, and true rosettes and rosette-like structures were scattered throughout the cells (Fig. 2B).
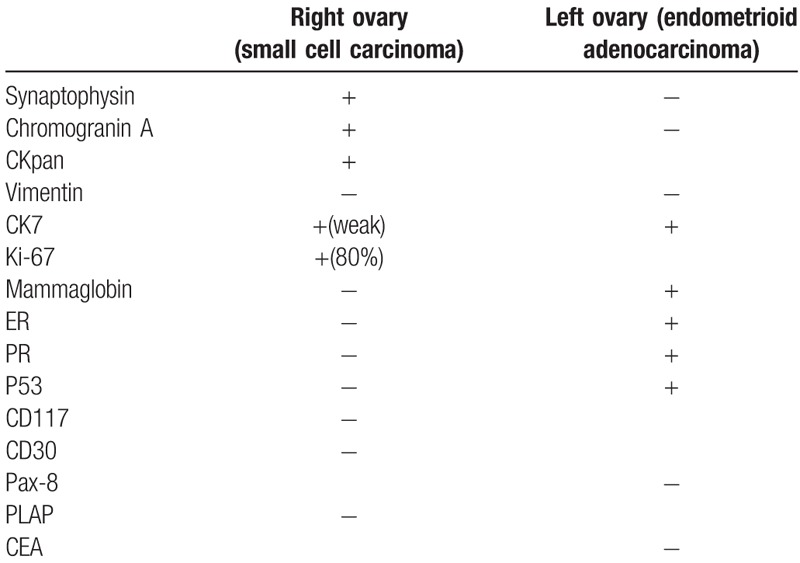
Immunohistochemistry. The immunohistochemical findings are summarized in Table 1. The right ovarian tumor cells were positive for Syn, CgA, and CKpan; CK7 was positive focally (Fig. 2C–E). The other markers examined included mammaglobin, vimentin, ER, PR, CD30, P53, CD117, and PLAP, all of which were negative. Examination of proliferative activity with the monoclonal antibody, MIB-1 (Ki-67 index), showed nuclear positivity in approximately 80% of the tumor cells. The left ovarian tumor cells were positive for Pax-8, CK7, and P53, and partly positive for mammaglobin, ER, and PR. The tumor cells were negative for CEA and vimentin. These findings confirmed that the patient had an epithelial ovarian tumor with neuroendocrine characteristics, that is, a small cell carcinoma.
Table 1.
Immunohistochemical profiles of the carcinomas.
Pathologic diagnosis. The pathologic diagnosis was highly differentiated endometrial carcinoma of the uterus with superficial muscular layer invasion. The tumor invaded the cervical canal and the left adnexa (Fig. 2F). The right fallopian tube was cancer-free. The right ovary had poorly differentiated carcinoma; based on the immunohistochemistry and clinical manifestations, the pathologic diagnosis was ovarian small cell carcinoma (pulmonary type).
3. Discussion
This is the first report in the English literature of a synchronous SCCO and endometrial carcinoma in a woman on tamoxifen therapy for breast cancer. Multiple synchronous primary tumors of the female genital tract are uncommon, accounting for 0.7% of gynecologic malignancies.[4] Simultaneous carcinomas of the ovary and endometrium may cause a diagnostic dilemma and the clinical management may be also a challenge, especially in advanced cases. Consequently, in most cases the diagnosis of synchronous ovarian and endometrial cancers is made postoperatively which was similar to our case.[5]
SCCO is a rare malignancy and presents many challenges for diagnosis, prediction of outcomes, and overall treatment strategies.[6] Dickersin et al[7] first reported 11 cases of SCCOHT in 1982. Eichhorn et al[8] first proposed the concept of SCCOPT in 1992. But SCCOPT and SCCOHT are clinically and histopathologically distinct entities. In general, SCCOHT is more common in clinical practice. It always occurs in adolescents and young women with hypercalcemic paracrine properties. Approximately two-thirds of patients have elevated serum calcium, but only 10% of patients have clinical manifestations of hypercalcemia. The mechanism underlying the secretion of calcium remains controversial, and is generally considered to be produced by the tumor cells[.9] After tumor resection, serum calcium and phosphorus can be recovered in the short term. Therefore, calcium can be used as a reference for clinical diagnosis and treatment.[10] SCCOPT is rare and occurs more often in peri- or postmenopausal women. It has neuroendocrine properties, but is not associated with hypercalcemia. The syndrome of inappropriate antidiuretic hormone secretion may be a clinical feature of SCCOPT.[11] In this case, the patient was peri-menopausal and tests performed before and after surgery did not show any evidence of hypercalcemia.
The pathogenesis of SCCO is still unknown. SCCOHT is possibly familial and heritable.[12] Recent studies have shown SCCOHT to be highly associated with germline or somatic mutations of the SMARCA4 gene.[13] Because SCCOPT is rare, no related pathogenesis has been reported. In our case, SCCOPT occurred after taking tamoxifen for 2 years. Tamoxifen has estrogen agonist activity and the metabolites of tamoxifen may be important in carcinogenesis. Tamoxifen stimulates the ovary when used by premenopausal women, thus raising concern that it might increase the risk of ovarian cancer.[14] The occurrence of ovarian neoplasms in patients with antecedent use of tamoxifen has been documented, and includes carcinosarcoma and granular cell tumor.[15,16] Unfortunately, there are no reports in the literature of SCCO occurring after taking tamoxifen. Based on a review of the literature, a primary neuroendocrine carcinoma of the uterine corpus in a patient after exposure to tamoxifen therapy for breast cancer was reported.[17] Thus, the association between SCCO and tamoxifen is difficult to rule out.
The true origin of SCCO is an ongoing matter of controversy. SCCO, ovarian epithelial tumors, sex cord stromal tumors, and germ cell tumors have similar tissue origins, but there are significant differences between these 3 categories. Young et al[18] considered that SCCO originated from ovarian epithelium based on immunohistochemical staining and electron microscopic observations. Ulbright et al[19] believe that the possibility of germ cell origin is significant because of the early age of onset. However, because SCCO is extremely rare, and the organization is of the undifferentiated type, it is difficult to determine the origin and specific subtype, which is classified as an independent, special entity tumor group at present.[20]
Early detection of SCCO appears to be the key to long-term survival of patients. The clinical manifestation of primary SCCO is lack of specificity. A rapidly growing pelvic mass with no other specific symptoms is the likely pattern. The most common clinical manifestations are abdominal distension, abdominal pain, an abdominal mass, and ascites. Rare cases involve an acute abdomen or vaginal bleeding due to rupture of an aneurysm caused by torsion. Occasionally, some SCCO can secrete hormones and cause paraneoplastic syndromes, but the incidence is lower than small cell carcinomas of the lung.[21] Measurement of tumor markers should be performed, but may not be particularly helpful. Because these markers may be difficult to distinguish these tumors are from common epithelial ovarian malignancies or metastatic small cell carcinomas of the lung. Therefore, the final diagnosis of SCCO is usually unexpected, even after surgery via pathologic examination. When given the pathologic similarity to pulmonary small cell carcinomas, it is important to ensure that newly diagnosed tumors are true primaries of the ovary and not metastases from a pulmonary tumor. Computerized tomography of the thorax should be performed to exclude a primary lung tumor.
The combination of histopathologic features and immunohistochemical expression of tumor markers can provide the definitive diagnosis of a SCCO. The 2 types of SCCO have similar immune phenotypic expression, all of which can be expressed to different degrees and different proportions of CK, EMA, NSE, and CgA.[22] There are some differences between these 2 types. The SCCOHT has a vimentin-positive rate approaching 50%, while the lung type of SCCO is vimentin-negative.[8] The perinuclear dot-like cytokeratin 20 staining was positive in SCCOPT and negative in SCCOHT.[23] On the basis of germline and somatic SMARCA4 mutations which have been described in SCCOHT, the lack of BRG1 expression is a useful tool for diagnosing SCCOHT.[24] These findings may promote the diagnostic distinction between SCCOPT and SCCOHT. In this case, the chest CT scan showed that there was no tumor present in the lungs at the time of the initial diagnosis. The abdominal and pelvic CT scans demonstrated the large volume of the right ovarian tumor, thus we ruled out ovarian metastasis from small cell carcinoma of lung. The immunohistochemical staining in this case revealed a positive reaction for synaptophysin and focally for chromogranin, which suggested neuroendocrine differentiation of the tumor. In addition, Ki-67 was positive in 80% of the tumor cells, which represents a poor prognosis in neuroendocrine carcinomas. CKpan positivity, which represents the epithelial feature of this type of tumor, was also obtained, but vimentin, ER, PR, P53, CD30, CD117, and PLAP staining were negative. Therefore, based on the combination of clinical features and pathologic findings, the diagnosis of SCCOPT was established.
Due to the rarity of SCCO, the majority of cases have been diagnosed at an advanced stage and the effective and optimal treatment regimen has not been established. Surgery, followed by chemotherapy and radiation therapy, is currently the main treatment strategy. Surgery has been shown to be the primary treatment modality in early disease.[25] The basic surgical methods include total hysterectomy and bilateral adnexal resection, retroperitoneal lymph node dissection, pelvic cavity and abdominal aortic lymph node dissection, and peritoneal tumor cytoreductive surgery. Jamy et al[25] conducted a retrospective analysis of a large number of SCCO and concluded that the extent of surgery did not influence outcomes. In this case, the patient with late-stage SCCO was not a candidate for radical surgery. Therefore, a total hysterectomy and bilateral adnexal and omentum resections were performed to reduce the tumor mass and relieve the intestinal obstruction.
Similar to epithelial ovarian cancer, adjuvant chemotherapy is very important for SCCO. Aggressive therapy, including multiagent chemotherapy and possibly radiotherapy, may extend survival.[2] Given the pathologic similarity, the evidence for chemotherapy is generally extrapolated from its use in small cell carcinoma of the lung.[26] In this setting, the combination of a platinum drug and etoposide is generally considered most appropriate. According to recent research, a multiagent chemotherapeutic regimen consisting of vinblastine, cisplatin, cyclophosphamide, bleomycin, doxorubicin, and etoposide (VPCBAE) has a definite therapeutic effect on primary SCCO.[27,28] SCCO may originate from ovarian epithelium, thus some researchers have suggested that paclitaxel and carboplatin therapy, a standard regimen for surface epithelial ovarian carcinoma, might also be effective against SCCO.[29,30] Yaghmour et al[31] proposed immunohistochemistry patterns may help guide the use of chemotherapy in these rare tumors, but still had some limitations. Of note, all of these conclusions are limited to individual case reports. None of these series are large enough to define the appropriate adjuvant chemotherapy for this disease; however, it is worth noting that multi-drug combination chemotherapy may be better in clinical practice.[2] Radiotherapy also plays an important role in the treatment of SCCO. When SCCO is poorly responsive to chemotherapy or in patients with recurrences, radiotherapy plays a significant role in salvage therapy.[32] Harrison et al[33] conducted a comprehensive analysis of 16 SCCO patients; 7 cases had pelvic and abdominal aortic radiotherapy or whole abdominal radiotherapy and only 1 recurrence was reported. Given that the site of recurrence in patients after complete surgery and intensive chemotherapy is the pelvis, Pautier et al[34] supported pelvic radiotherapy. In addition, the radiotherapy effect in patients with early-stage disease is better than patients with advanced-stage disease.[32] Because SCCO recurrences are usually like epithelial ovarian cancer, as well as multiple foci of peritoneal infiltration planting, whole abdominal radiotherapy has the risk of intestinal obstruction, intestinal perforation, intestinal fistulas, and other serious side effects, so radiotherapy should be considered with caution.
The prognosis of SCCO is vague; the most important prognostic factor at present is clinical stage.[34] Patients with localized disease have an OS of 67 months compared to 12 months for regional and 9 months for distant disease.[25] In a study involving small cell carcinoma of the cervix, Liao et al[35] conducted a retrospective analysis of 293 cases of small cell carcinoma of the cervix and demonstrated that FIGO stage, tumor mass, and CgA staining may act as surrogate factors that are prognostic of survival. Thus, the prognosis of SCCO may also be related to these factors. The Ki-67 index and serum NSE level indicated that the prognosis of neuroendocrine carcinoma was poor. In our case, the main result of the current study was that the pretreatment CA-125 level was an independent prognostic factor in women with synchronous endometrial and ovarian cancers; the tumor stage of ovarian cancer also showed a significant prognostic impact.[36] Multiple primary tumors should be treated according to the specific characteristics of every primary tumor. Therefore, after performing palliative surgery, the current patient received 3 cycles of paclitaxel and carboplatin. The blood tumor markers gradually decline. Before the fourth cycle of chemotherapy, the laboratory examination showed that the tumor markers and NSE level increased. Ultrasonography showed bilateral inguinal lymph node enlargement for the first time. All of these findings indicate that the tumor continues to progress. Therefore, we added etoposide, which is the main treatment for small cell lung cancer, to the chemotherapy regimen of paclitaxel and carboplatin for second-line therapy. There was no apparent improvement in the patient. Staging for each type of tumor in our case was late stage and no multidrug combination therapy at the beginning of chemotherapy had an ideal clinical treatment effect.
4. Conclusion
For the first time in the literature, a case of endometrial adenocarcinoma with coexisting SCCO has been reported. SCCO is rarely seen in clinical practice, especially the SCCOPT. Interestingly, the coexistence of these 2 different tumors in a patient with a history of breast cancer and tamoxifen use for 2 years is also a remarkable finding. The present case suggests that tamoxifen use might be among many etiologic factors in SCCO development. Despite its rarity, SCCO requires a high degree of attention in clinical work because it is an aggressive tumor that has a poor prognosis. To facilitate progression toward a more consistent standard of care for this rare gynecologic tumor, additional cases and case series should be reported.
Acknowledgments
We express our thanks to Grants from Science and Technology Development Plan of Jinan, NO. 201401253; Medical and Health Technology Development Program of Shandong province, NO. 2015WS0149 and NO.2015WS0197; Postdoctoral Science Foundation of China, NO. 2017M612317.
Author contributions
Conceptualization: Yanlai Sun.
Data curation: Dejian Ma, Yamei Sun.
Investigation: Jianning Li.
Project administration: Yanlai Sun.
Writing – original draft: Lei Yin.
Writing – review and editing: Yunhai Wei, Yanlai Sun.
Footnotes
Abbreviations: HT = hypercalcemic type, PT = pulmonary type, SCCO = small cell carcinoma of the ovary.
The authors have no conflicts of interest to disclose.
References
- [1].Reed NS, Pautier P, Åvall-Lundqvist E, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for ovarian small cell cancers. Int J Gynecol Cancer 2014;24:S30–4. [DOI] [PubMed] [Google Scholar]
- [2].Callegaro-Filho D, Gershenson DM, Nick AM, et al. Small cell carcinoma of the ovary-hypercalcemic type (SCCOHT): a review of 47 cases. Gynecol Oncol 2016;140:53–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tsolakidis D, Papanikolaou A, Ktenidis K, et al. Primary ovarian small cell carcinoma of pulmonary type with enlarged paraaortic lymph node masses: a case report and review of the literature. Eur J Gynaecol Oncol 2012;33:312–5. [PubMed] [Google Scholar]
- [4].Eisner RF, Nieberg RK, Berek JS. Synchronous primary neoplasms of the female reproductive tract. Gynecol Oncol 1989;33:335–9. [DOI] [PubMed] [Google Scholar]
- [5].Zaino R, Whitney C, Brady MF, et al. Simultaneously detected endometrial and ovarian carcinomas—a prospective clinicopathologic study of 74 cases: a gynecologic oncology group study. Gynecol Oncol 2001;83:355–62. [DOI] [PubMed] [Google Scholar]
- [6].Bahri M, Lahmar R, Ben Salah H, et al. Small cell carcinoma of the ovary. Cancer Radiother 2014;18:198–200. [DOI] [PubMed] [Google Scholar]
- [7].Dickersin GR, Kline IW, Scully RE. Small cell carcinoma of the ovary with hypercalcemia: a report of eleven cases. Cancer 1982;49:188–97. [DOI] [PubMed] [Google Scholar]
- [8].Eichhorn JH, Young RH, Scully RE. Primary ovarian small cell carcinoma of pulmonary type. A clinicopathologic, immunohistologic, and flow cytometric analysis of 11 cases. Am J Surg Pathol 1992;16:926–38. [DOI] [PubMed] [Google Scholar]
- [9].Rana S, Warren BK, Yamada SD. Stage IIIC small cell carcinoma of the ovary: survival with conservative surgery and chemotherapy. Obstet Gynecol 2004;103:1120–3. [DOI] [PubMed] [Google Scholar]
- [10].Romics L, Jr, O’Brien ME, Relihan N. Intracystic papillary carcinoma in a male as a rare presentation of breast cancer: a case report and literature review. J Med Case Rep 2009;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mebis J, De Raeve H, Baekelandt M, et al. Primary ovarian small cell carcinoma of the pulmonary type: a case report and review of the literature. Eur J Gynaecol Oncol 2004;25:239–41. [PubMed] [Google Scholar]
- [12].McDonald JM, Karabakhtsian RG, Pierce HH, et al. Small cell carcinoma of the ovary of hypercalcemic type: a case report. J Pediatr Surg 2012;47:588–92. [DOI] [PubMed] [Google Scholar]
- [13].Kupryjańczyk J, Dansonka-Mieszkowska A, Moes-Sosnowska J, et al. Ovarian small cell carcinoma of hypercalcemic type-evidence of germline origin and SMARCA4 gene inactivation. a pilot study. Pol J Pathol 2013;64:238–46. [DOI] [PubMed] [Google Scholar]
- [14].Spicer DV, Pike MC, Henderson BE. Ovarian cancer and long-term tamoxifen in premenopausal women. Lancet 1991;337:1414. [DOI] [PubMed] [Google Scholar]
- [15].Lavie O, Longacre T, Segev Y, et al. Ovarian carcinosarcomas associated with prolonged use of tamoxifen: case reports. Int J Gynecol Cancer 2009;19:1521–3. [DOI] [PubMed] [Google Scholar]
- [16].Abahssain H, Kairouani M, Gherman R, et al. Granulosa cell tumor of the ovary and antecedent of adjuvant tamoxifen use for breast cancer. World J Surg Oncol 2010;8:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Posligua L, Malpica A, Liu J, et al. Combined large cell neuroendocrine carcinoma and papillary serous carcinoma of the endometrium with pagetoid spread. Arch Pathol Lab Med 2008;132:1821–4. [DOI] [PubMed] [Google Scholar]
- [18].Young RH, Oliva E, Scully RE. Small cell carcinoma of the ovary, hypercalcemic type. A clinicopathological analysis of 150 cases. Am J Surg Pathol 1994;18:1102–16. [DOI] [PubMed] [Google Scholar]
- [19].Ulbright TM, Roth LM, Stehman FB, et al. Poorly differentiated (small cell) carcinoma of the ovary in young women: evidence supporting a germ cell origin. Hum Pathol 1987;18:175–84. [DOI] [PubMed] [Google Scholar]
- [20].Walt H, Hornung R, Fink D, et al. Hypercalcemic-type of small cell carcinoma of the ovary: characterization of a new tumor line. Anticancer Res 2001;21:3253–9. [PubMed] [Google Scholar]
- [21].Crowder S, Tuller E. Small cell carcinoma of the female genital tract. Semin Oncol 2007;34:57–63. [DOI] [PubMed] [Google Scholar]
- [22].McCluggage WG. Ovarian neoplasms composed of small round cells: a review. Adv Anat Pathol 2004;11:288–96. [DOI] [PubMed] [Google Scholar]
- [23].Rund CR, Fischer EG. Perinuclear dot-like cytokeratin 20 staining in small cell neuroendocrine carcinoma of the ovary (pulmonary-type). Appl Immunohistochem Mol Morphol 2006;14:244–8. [DOI] [PubMed] [Google Scholar]
- [24].Karanian-Philippe M, Velasco V, Longy M, et al. SMARCA4 (BRG1) loss of expression is a useful marker for the diagnosis of ovarian small cell carcinoma of the hypercalcemic type (ovarian rhabdoid tumor): a comprehensive analysis of 116 rare gynecologic tumors, 9 soft tissue tumors, and 9 melanomas. Am J Surg Pathol 2015;39:1197–205. [DOI] [PubMed] [Google Scholar]
- [25].Jamy O, Yaghmour G, Hare F, et al. Population-based analysis of the clinical features of primary small cell carcinoma of the ovary. Anticancer Res 2015;35:3091–5. [PubMed] [Google Scholar]
- [26].Cheng S, Evans WK, Stys-Norman D, et al. Chemotherapy for relapsed small cell lung cancer: a systematic review and practice guideline. J Thorac Oncol 2007;2:348–54. [DOI] [PubMed] [Google Scholar]
- [27].Wallbillich JJ, Nick AM, Ramirez PT, et al. Vinblastine, cisplatin, cyclophosphamide, bleomycin, doxorubicin, and etoposide (VPCBAE) in the management of three patients with small-cell carcinoma of the ovary. Gynecol Oncol Case Rep 2012;2:58–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hamilton S, Beattie GJ, Williams AR. Small cell carcinoma of the ovary: a report of three cases and review of the literature. J Obstet Gynaecol 2004;24:169–72. [DOI] [PubMed] [Google Scholar]
- [29].Kurasaki A, Sakurai N, Yamamoto Y, et al. Ovarian pulmonary-type small cell carcinoma: case report and review of the literature. Int J Gynecol Pathol 2013;32:464–70. [DOI] [PubMed] [Google Scholar]
- [30].Suzuki N, Kameyama K, Hirao T, et al. A case of pulmonary type of ovarian small cell carcinoma. J Obstet Gynaecol Res 2007;33:203–6. [DOI] [PubMed] [Google Scholar]
- [31].Yaghmour G, Prouet P, Wiedower E, et al. Genomic alterations in neuroendocrine cancers of the ovary. J Ovarian Res 2016;26:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Callegaro-Filho D, Burke TW, Eifel PJ, et al. Radiotherapy for recurrent small cell carcinoma of the ovary: a case report and review of the literature. Gynecol Oncol Rep 2014;29:23–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Harrison ML, Hoskins P, du Bois A, et al. Small cell of the ovary, hypercalcemic type—analysis of combined experience and recommendation for management. A GCIG study. Gynecol Oncol 2006;100:233–8. [DOI] [PubMed] [Google Scholar]
- [34].Pautier P, Ribrag V, Duvillard P, et al. Results of a prospective dose-intensive regimen in 27 patients with small cell carcinoma of the ovary of the hypercalcemic type. Ann Oncol 2007;18:1985–9. [DOI] [PubMed] [Google Scholar]
- [35].Liao LM, Zhang X, Ren YF, et al. Chromogranin A (CgA) as poor prognostic factor in patients with small cell carcinoma of the cervix: results of a retrospective study of 293 patients. PLoS One 2012;7:e33674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Song T, Seong SJ, Bae DS, et al. Prognostic factors in women with synchronous endometrial and ovarian cancers. Int J Gynecol Cancer 2014;24:520–7. [DOI] [PubMed] [Google Scholar]


