Abstract
Rationale:
Pulmonary renal syndrome (PRS) is a term most commonly used to describe a combination of glomerulonephritis and pulmonary hemorrhage as a manifestation of a multisystem autoimmune disease. It is usually associated with ANCA vasculitis and anti-GBM disease. Diffuse alveolar hemorrhage in a patient with ANCA and anti-GBM negative pauci-immune glomerulonephritis is rare and optimal management is unknown.
Patient concerns:
An 85-year-old man with hypertension, diabetes mellitus, prostate cancer and recently diagnosed pauci-immune necrotizing glomerulonephritis presented to our emergency department with worsening dyspnea and pedal edema for several days. Clinical presentation and radiological studies were suggestive of fluid overload but he developed worsening respiratory failure despite hemodialysis.
Diagnoses:
Bronchoscopy confirmed diffuse alveolar hemorrhage. ANCA and anti-GBM antibodies were negative. The patient was diagnosed with pulmonary renal syndrome – diffuse alveolar hemorrhage in the setting of ANCA and anti-GBM negative pauci-immune glomerulonephritis.
Interventions:
Patient was started on intravenous pulse steroids, cyclophosphamide and received seven sessions of plasmapheresis.
Outcomes:
There was an improvement in patient's respiratory status and repeat bronchoscopy at the end of treatment did not show diffuse alveolar hemorrhage.
Lessons:
Pauci-immune crescentic necrotizing glomerulonephritis is usually associated with the presence of ANCA, however, ANCA may be absent in 10% of these cases. Immunosuppression is the mainstay of treatment for ANCA and anti-GBM associated PRS. This case highlights the importance of immunosuppression and plasmapheresis in patients with ANCA negative vasculitis due to presence of unidentified serum antibodies. If left untreated, these patients can have a fulminant course with high mortality ranging from 25 to 50%.
Keywords: vasculitis, pulmonary hemorrhage, glomerulonephritis, plasmapheresis
1. Introduction
Pulmonary renal syndrome (PRS) is a life threatening condition that is characterized by renal failure with associated respiratory impairment and manifests in the form of rapidly progressive glomerulonephritis with diffuse alveolar hemorrhage (DAH). A variety of immunologic and non- immunologic mechanisms have been implicated in the pathogenesis of PRS. Anti-neutrophil cytoplasmic antibody (ANCA) associated vasculitis and anti-glomerular basement membrane (anti-GBM) disease account for 70% to 90% of these cases. Symptoms are non-specific and a high index of suspicion is required for early diagnosis and prompt treatment considering the fulminant course of this condition. There has been substantial literature published in the past on ANCA associated vasculitis. However, on the other side of this spectrum is ANCA negative vasculitis, a much less understood entity. We report a case of a patient who presented with renal and pulmonary manifestations of ANCA negative vasculitis, and provide a discussion of methods to detect, diagnose and treat this rare condition.
2. Case description
An 85-year-old man presented to our emergency department with progressively worsening shortness of breath and pedal edema for several days. His medical history was significant for hypertension, diabetes mellitus and prostate cancer. He was a physically active, retired laborer with no history of tobacco, alcohol, or drug use. A month prior to his presentation, patient was admitted with acute kidney injury requiring initiation of hemodialysis. A thorough laboratory work up including autoimmune testing and complement levels was unremarkable. He underwent kidney biopsy which showed pauci-immune necrotizing glomerulonephritis with crescents (Fig. 1). He received pulse dose steroids and was discharged on oral steroids, cyclophosphamide, and remained dialysis dependent.
Figure 1.
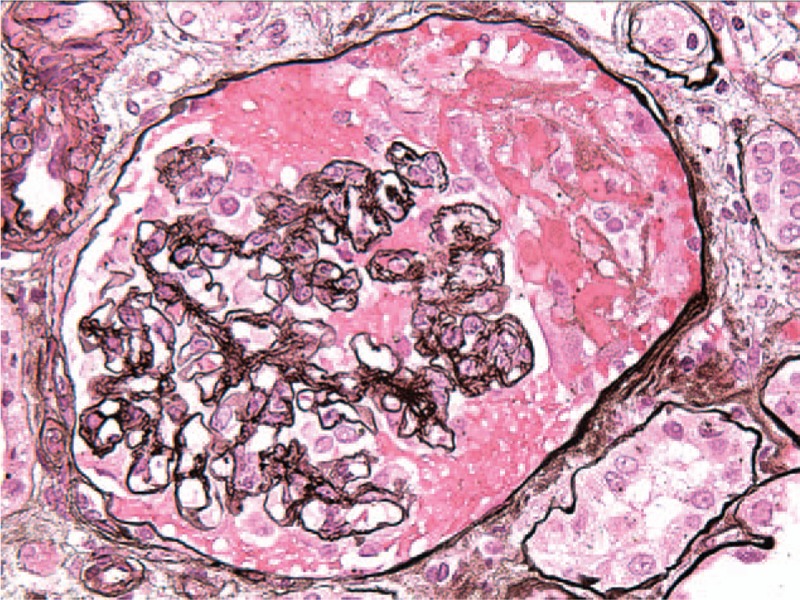
Focal segmental necrotizing and crescentic glomerulonephritis, moderate, predominantly acute, pauci-immune type. The immunofluorescence findings of sparse mesangial staining for complement C3, only focal 2–3+ segmental tuft staining for fibrin/fibrinogen support a diagnosis of pauci-immune necrotizing and crescentic glomerulonephritis.
During the current admission, patient was noted to be hypoxic and in respiratory distress. A quick bedside examination revealed use of accessory muscles of respiration, bilateral crackles on pulmonary auscultation and 3+ pitting edema of his lower extremities. Chest radiograph revealed bilateral infiltrates and pulmonary vascular congestion (Fig. 2). Laboratory studies showed chronic anemia, thrombocytopenia, and renal failure with leukocytosis. Emergent hemodialysis was done and non-invasive positive pressure ventilation was initiated. He developed worsening respiratory failure despite several more hemodialysis sessions for volume removal requiring mechanical ventilation and was transferred to the intensive care unit. Computed tomography (CT) scan of the chest was done which showed diffuse bilateral ground glass opacities in the lungs (Fig. 3). Broad spectrum antibiotics were administered and fiber optic bronchoscopy was performed due to concern for alveolar hemorrhage. An increasing bloody return on sequential lavage of the same segment of the lung was diagnostic of diffuse alveolar hemorrhage (Fig. 4). Repeat anti-neutrophil cytoplasmic antibodies remained negative.
Figure 2.
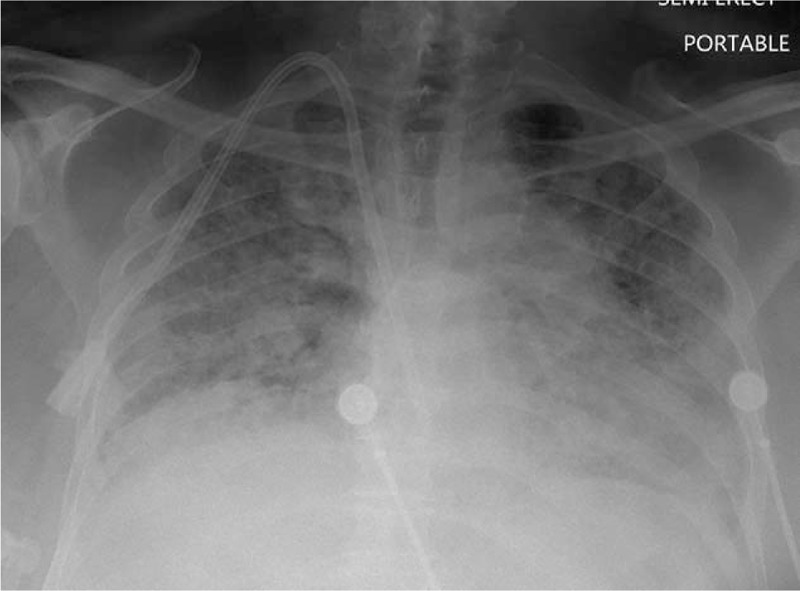
Chest radiograph with diffuse opacities throughout both lungs.
Figure 3.
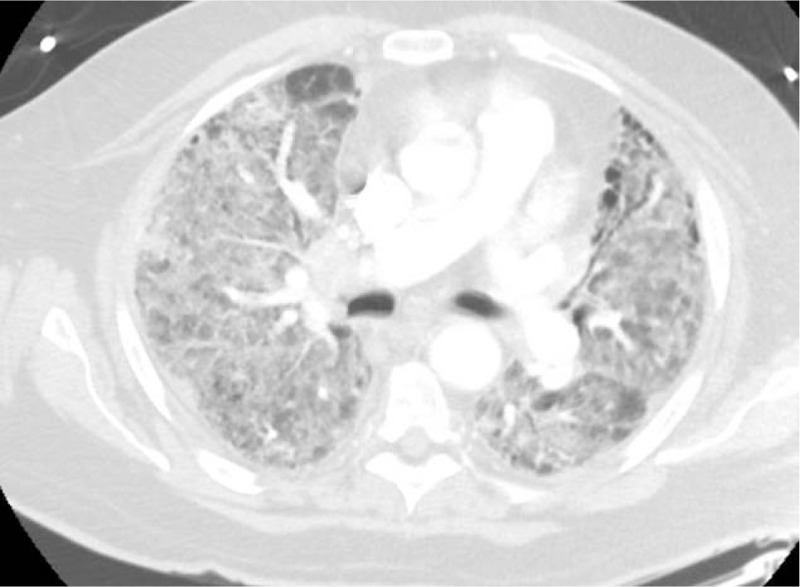
Axial section of CT chest showing diffuse bilateral ground-glass opacities.
Figure 4.
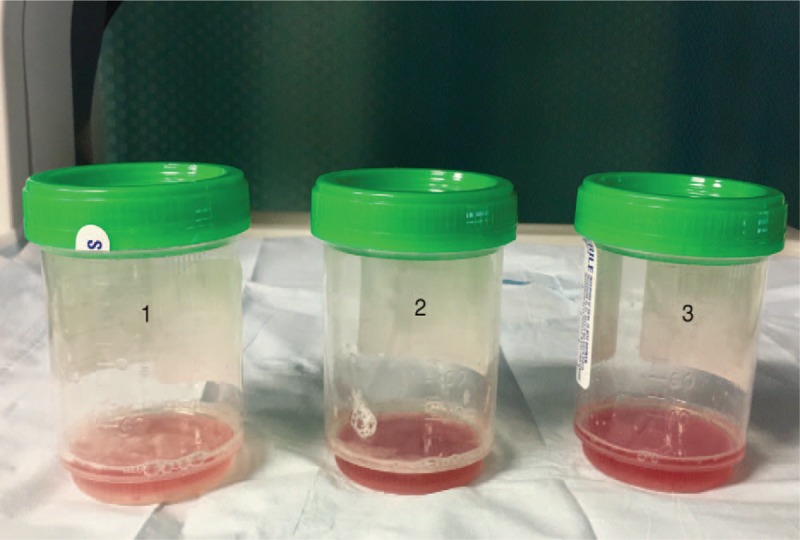
Sequential bronchoalveolar lavage with increase in blood-tinged return characteristic of diffuse alveolar hemorrhage.
Patient was started on intravenous pulse steroids and plasmapheresis was initiated. All cultures including blood, urine, and those of bronchoalveolar lavage remained negative. Patient received a total of seven sessions of plasmapheresis. A repeat bronchoscopy was performed after completion of plasmapheresis and no bleeding was noted. Patient's respiratory status improved and he was successfully weaned off the ventilator. The plan was to continue cyclophosphamide for 6 to 12 months. However, he later developed hypoxic respiratory failure from fluid overload requiring re-intubation and volume removal with hemodialysis. Subsequent hospital course was complicated by development of pneumonia, septic shock and deep vein thrombosis; his family later chose hospice care.
3. Discussion
PRS was first described by Goodpasture in 1919 in a patient with pulmonary hemorrhage, rapidly progressive glomerulonephritis and vasculitis affecting small splenic arteries, arterioles in the gut, pulmonary capillaries, and glomerular capillaries.[1] The pathogenic mechanisms implicated in this syndrome are variable. Studies in the past showed that up to 70% of cases are associated with antibodies to ANCA. Anti-GBM antibodies are positive in 20% of cases.[2] PRS can be an infrequent complication of other autoimmune diseases like systemic lupus erythematosus, cryoglobulinemia, rheumatoid arthritis, systemic sclerosis, mixed connective tissue disease, polymyositis, and dermatomyositis. Certain drugs like hydralazine, allopurinol, and propylthiouracil are known to be associated with potentially reversible drug-associated ANCA positive vasculitis. Thrombotic microangiopathy secondary to infections, malignancy and antiphospholipid syndromes can lead to pulmonary renal syndrome.[3,4]
Small vessel vasculitis that includes ANCA associated vasculitis and immune complex mediated vasculitis is an area of much research in recent times. Its etiology, manifestation and diagnosis still remain vague.[5–7] Pauci-immune crescentic necrotizing glomerulonephritis is defined as rapidly progressive glomerulonephritis with histological presence of focal glomerular necrosis and extra capillary proliferation with little or no glomerular staining for immunoglobulin by immunofluorescence microscopy examination. It is usually associated with the presence of ANCA; however, ANCA may be absent in 10% of these cases.[8,9] Patients with ANCA negative vasculitis present with fever, arthralgia, pulmonary, renal, and neurologic involvement; similar to ANCA positive vasculitis.
Small vessel vasculitis is the underlying pulmonary lesion in most cases of pulmonary renal syndrome. Previous studies demonstrated that in patients with ANCA-negative rapidly progressive glomerulonephritis, pulmonary findings occurred much less commonly than those with ANCA positive vasculitis.[9,10] Eisenberger et al[8] reported pulmonary involvement in 3 out of 20 patients with ANCA negative pauci immune renal vasculitis of which 2 had cavitary nodules. Farah et al[11] reported one case of idiopathic bronchiolitis obliterans organizing pneumonia (BOOP) associated with pauci-immune renal vasculitis that terminated in rapidly progressive glomerulonephritis (RPGN). However, association of diffuse alveolar hemorrhage with ANCA negative glomerulonephritis is rare. The pathogenesis of this association is not clear, neutrophil infiltration was found in glomerular lesions in one study.[12] Wang et al[14] found higher levels of serum neutrophil-gelatinase-associated lipocalin and lactoferrin in patients with ANCA-negative pauci-immune crescentic glomerulonephritis than in patients with ANCA-positive disease. The clinical presentation of pulmonary renal syndrome in ANCA negative vasculitis is non-specific and a high degree of clinical suspicion is essential for a prompt diagnosis. Patients present with cough and dyspnea which may or may not be accompanied by hemoptysis. Hemoptysis may be absent in up to 30% to 35% of patients with DAH and one half of these patients may require mechanical ventilation.[4] Renal involvement presents with hematuria, proteinuria, sometimes leading to end stage renal failure.
Chest radiograph may be normal in 22% cases with DAH.[12] CT chest findings include ground glass opacities, alveolar infiltrates, and consolidations. Radiologic resolution may be seen after 3 and 4 days if the hemorrhage ceases. Laboratory findings include normocytic anemia; urinalysis shows proteinuria, dysmorphic red blood cells (RBCs), and RBC casts. Respiratory cultures should be performed to rule out superimposed pneumonia. Serological studies should be performed to detect antibodies especially ANCA and anti-GBM. Bronchoscopy is required to confirm DAH, blood tinged lavage especially in repeat aliquots, and cytology with hemosiderin-laden macrophages strongly suggests DAH when combined with other clinical and laboratory findings like drop in hemoglobin and bilateral infiltrates on chest radiograph. However, the gold standard diagnostic test is renal or lung biopsy.[13] Studies in the past showed a higher degree of proteinuria and greater prevalence of nephrotic syndrome with more severe glomerular lesions in ANCA negative group when compared to ANCA positive group.[8,9,15]
Immunosuppression is the mainstay of treatment for ANCA associated pulmonary-renal syndrome and Goodpasture's syndrome. High dose intravenous steroids for 3–5 days have been used commonly to achieve remission and are sometimes combined with oral or intravenous cyclophosphamide. Once remission is achieved, steroids are tapered over a 3–5-month period with continuation of cyclophosphamide for 6–12 months [16]. Plasmapheresis may be helpful in the acute setting though there is no proven long term survival benefit [17]. Optimal management of patients with ANCA negative pulmonary renal syndrome has not been established due to rarity of this condition. The role of plasma exchange is not clear in ANCA negative vasculitis, however, it is thought to provide benefit in certain cases owing to the presence of unidentified serum antibodies in these patients[18].
In a retrospective review of 20 patients with DAH who were successfully treated with intravenous methylprednisolone (7 mg/kg/day) and plasmapheresis, 1 patient was ANCA negative and had microscopic polyangiitis on renal biopsy.[19] Sandhu et al[20] reported a case of a 76-year-old woman with similar presentation who was diagnosed with diffuse alveolar hemorrhage based on chest imaging and bronchoscopic findings in the setting of necrotizing crescentic pauci-immune glomerulonephritis. She received intravenous methylprednisone, cyclophosphamide, and 6 cycles of plasmapheresis with resolution of respiratory failure and was successfully extubated. Her serology remained negative for ANCA. Another case was published in 2013 where a 76-year-old Japanese woman presented with worsening cough and developed progressive respiratory failure requiring mechanical ventilation, increasing bilateral infiltrates on chest radiography, and worsening renal failure requiring hemodiafiltration. She was started on IV methylprednisone followed by a prolonged steroid taper with improvement in symptoms and a renal biopsy done later showed rapidly progressive glomerulonephritis with crescent formation.[21] In 2009, Wang et al[22] reported a case of a 66-year-old man from China who presented with fever, acute renal failure, and later developed hemoptysis with imaging suggestive of DAH. This was confirmed with bronchoscopy. A renal biopsy revealed necrotizing small-vessel vasculitis and crescents in the glomeruli. Plasmapheresis was promptly initiated with simultaneous pulse steroid therapy and lead to an improvement in renal failure and resolution of hemoptysis.
If left untreated, these patients can have a fulminant course with high mortality ranging from 25% to 50%.[8,9] Age and serum creatinine on presentation are important prognostic markers with higher mortality in older age group.[8] Patients with negative ANCA were shown to have poorer renal outcome when compared with positive ANCA, however, both groups have similar survival.[8–10,23] Disease relapses, drug toxicity, and infections are the common causes of clinical worsening which should be identified promptly based on clinical judgment.
This case represents a rare association of ANCA and anti-GBM negative pauci immune glomerulonephritis with diffuse alveolar hemorrhage. To our best knowledge, there were 4 published cases of successful treatment with immunosuppression and plasmapheresis in the setting of ANCA negative pulmonary renal syndrome. Another strength of our case is that we confirmed the resolution of DAH after pulse steroids and plasmapheresis therapy with repeat bronchoscopy. One limitation of this case report is that we did not perform a transbronchial biopsy at the time of bronchoscopy; however, such biopsy is rarely used in diagnosis of the cause of DAH [24]
4. Conclusion
We report a case of successful treatment of diffuse alveolar hemorrhage in the setting of ANCA negative pauci-immune glomerulonephritis with steroids, cyclophosphamide, and plasmapheresis. The response to plasmapheresis highlights the possibility of unidentified autoantibodies or T-cell dependent mechanisms in the pathogenesis of this condition. Further studies are needed to confirm this association and formulate a treatment regimen for this highly fatal condition.
Author contributions
Investigation: Vivette D’Agati.
Supervision: Boris Medvedovsky, Kalpana A. Uday.
Writing – original draft: Lakshmi Saladi, Danial Shaikh, Muhammad Saad, Enny Cancio-Rodriguez.
Writing – review & editing: Muhammad Adrish.
Footnotes
Abbreviations: ANCA = anti-neutrophil cytoplasmic antibody, Anti-GBM = anti-glomerular basement membrane, BOOP = bronchiolitis obliterans organizing pneumonia, CT = computed tomography, DAH = diffuse alveolar hemorrhage, PRS = pulmonary renal syndrome, RBC = red blood cell, RPGN = rapidly progressive glomerulonephritis.
The authors declare no conflict of interest.
References
- [1].Goodpasture EW. The significance of certain pulmonary lesions in relation to the aetiology of pneumonia. Am J Med Sci 1919;158:863–70. [DOI] [PubMed] [Google Scholar]
- [2].Gallagher H, Kwan J, Jayne RW. Pulmonary renal syndrome: a 4-year, single center experience. Am J Kidney Dis 2002;38:42–7. [DOI] [PubMed] [Google Scholar]
- [3].West SC, Arulkumaran N, Ind PW, et al. Pulmonary-renal syndrome: a life threatening but treatable condition. Postgrad Med J 2013 1051;89:274–83. [DOI] [PubMed] [Google Scholar]
- [4].Papiris SA, Manali ED, Kalomenidis I, et al. Bench-to-bedside review: pulmonary-renal syndromes—an update for the intensivist. Crit Care 2007;11:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med 1997;337:1512–23. [DOI] [PubMed] [Google Scholar]
- [6].Gonzalez-Gay MA, Garcia-Porrua C. Systemic vasculitis in adults in northwestern Spain, 1988-1997: clinical and epidemiologic aspects. Medicine 1999;78:292–308. [DOI] [PubMed] [Google Scholar]
- [7].Schwarz MI, Brown KK. Small vessel vasculitis of the lung. Thorax 2000;55:502–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Eisenberger U, Fakhouri F, Vanhille P, et al. ANCA-negative pauci-immune renal vasculitis: histology and outcome. Nephrol Dial Transplant 2005;20:1392–9. [DOI] [PubMed] [Google Scholar]
- [9].Chen M, Yu F, Wang SX, et al. Antineutrophil cytoplasmic autoantibody-negative pauci-immune crescentic glomerulonephritis. J Am Soc Nephrol 2007;18:599–605. [DOI] [PubMed] [Google Scholar]
- [10].Hedger N, Stevens J, Drey N, et al. Incidence and outcome of pauci-immune rapidly progressive glomerulonephritis in Wessex, UK: a 10-year retrospective study. Nephrol Dial Transplant 2000;15:1593–9. [DOI] [PubMed] [Google Scholar]
- [11].Farah R, Vaisban E, Geron R, et al. A rare lung manifestation with rapidly progressive glomerulonephritis. Eur J Intern Med 2005;16:56–8. [DOI] [PubMed] [Google Scholar]
- [12].Bowley NB, Steiner RE, Chin WS. The chest x-ray in antiglomerular basement membrane antibody disease (Goodpasture's syndrome). Clin Radiol 1979;30:419–29. [DOI] [PubMed] [Google Scholar]
- [13].Collard HR, Schwarz MI. Diffuse alveolar hemorrhage. Clin Chest Med 2004;25:583–92. [DOI] [PubMed] [Google Scholar]
- [14].Wang F, Chen M, Zhao MH. Neutrophil degranulation in antineutrophil cytoplasmic antibodies-negative pauci-immune crescentic glomerulonephritis. J Nephrol 2009;22:491–6. [PubMed] [Google Scholar]
- [15].Hung PH, Chiu YL, Lin WC, et al. Poor renal outcome of antineutrophil cytoplasmic antibody negative pauci-immune glomerulonephritis in Taiwanese. J Formos Med Assoc 2006;105:804–12. [DOI] [PubMed] [Google Scholar]
- [16].McCabe C, Jones Q, Nikolopoulou A, et al. Pulmonary-renal syndromes: an update for respiratory physicians. Respir Med 2011;105:1413–21. [DOI] [PubMed] [Google Scholar]
- [17].Jayne DR, Gaskin G, Rasmussen N, et al. European Vasculitis Study Group. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol 2007;18:2180–8. [DOI] [PubMed] [Google Scholar]
- [18].Cong M, Chen M, Zhang JJ, et al. Anti-endothelial cell antibodies in antineutrophil cytoplasmic antibodies negative pauci-immune crescentic glomerulonephritis. Nephrology 2007;3:228–34. [DOI] [PubMed] [Google Scholar]
- [19].Klemmer PJ, Chalermskulrat W, Reif MS, et al. Plasmapheresis therapy for diffuse alveolar hemorrhage in patients with small-vessel vasculitis. Am J Kidney Dis 2003;42:1149–53. [DOI] [PubMed] [Google Scholar]
- [20].Sandhu G, Casares P, Farias A, et al. Diffuse alveolar haemorrhage in ANCA-negative pauci-immune crescentic glomerulonephritis. NDT Plus 2010;3:449–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yamaguchi H, Shirakami A, Haku T, et al. Pulmonary-renal syndrome with negative ANCAs and Anti-GBM antibody. Case Rep Nephrol 2013: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang CC, Shiang JC, Tsai MK, et al. Prompt plasmapheresis successfully rescue pulmonary-renal syndrome caused by ANCA-negative microscopic polyangiitis. Clin Rheumatol 2009;28:1457–60. [DOI] [PubMed] [Google Scholar]
- [23].Chen M, Kallenberg CG, Zhao MH. ANCA-negative pauci-immune crescentic glomerulonephritis. Nat Rev Nephrol 2009;5:313–8. [DOI] [PubMed] [Google Scholar]
- [24].Schnabel A, Holl-Ulrich K, Dalhoff K, et al. Efficacy of transbronchial biopsy in pulmonary vaculitides. Eur Respir J 1997;10:2738–43. [DOI] [PubMed] [Google Scholar]


