Abstract
L-citrulline (Cit) increases arginine (Arg), the primary substrate for nitric oxide biosynthesis. We tested the hypothesis that muscle blood flow during exercise would be enhanced by Cit supplementation in older adults. Femoral artery blood flow was measured during calf exercise using Doppler ultrasound, and vascular conductance (FVC) was calculated in 25 older adults (13W, 12M) before and after 14 days of Cit (6 g/day) and placebo (maltodextrin) in a randomized, double-blind, crossover study. Plasma [Arg] and resting blood pressure were also measured before and after each condition. Women and men were analyzed separately due to significant sex-by-condition interactions for the change in exercise blood flow and FVC. Plasma [Arg] was increased by 30% and 35% following Cit (P < 0.01) in women and men, respectively, with no change after placebo. Citrulline lowered diastolic blood pressure in men (75 ± 9 vs. 71 ± 6 mmHg, P = 0.02), but remained unchanged in women. Blood flow and FVC during exercise at higher workloads were increased following Cit in men (flow: 521 ± 134 vs. 584 ± 166 mL/min, P = 0.04; FVC: 5.0 ± 1.5 vs. 5.8 ± 1.7 mL/min/mmHg, P = 0.01) but was not different after placebo. These variables were not altered by Cit in women. Adjusting for baseline diastolic blood pressure removed (P = 0.10) the difference in FBF and FVC following Cit men. These results indicate that L-citrulline has a modest effect of improving muscle blood flow during submaximal exercise in older men.
Keywords: citrulline, blood flow, aging, exercise
Introduction
The term “vascular aging” has often been used to encompass adverse changes in arterial structure and function with advancing age. Endothelial dysfunction is a hallmark characteristic of vascular aging, and is an important contributor to lower peripheral vasodilation (Crecelius et al. 2010) and blood flow responses to exercise in older as compared to younger adults (Poole et al. 2003; Parker et al. 2008). A major deficit to endothelial function is reduced production and bioactivity of nitric oxide (NO), a signaling molecule important for regulating blood flow and metabolism during exercise (Heinonen et al. 2011). A growing area of study is the effectiveness of dietary supplements to enhance NO production, one of which is L-arginine (Arg). Oxidation of endogenous Arg in a reaction catalyzed by NO synthase results in NO production. Oral supplementation with Arg in humans increases plasma levels of Arg and NO biomarkers (Schwedhelm et al. 2008). In addition, accompanying changes in vascular function are seen with Arg supplementation in older adults including improved peripheral flow-mediated vasodilation (Bode-Böger et al. 2003) and reflex cutaneous vasodilation (Holowatz et al. 2006).
L-citrulline (Cit) is a nonessential amino acid that converts to argininosuccinate then Arg in reactions catalyzed by arginosuccinate synthase and lyase enzymes, respectively. Unlike oral Arg supplementation which is subject to pre-systemic elimination by hepatic metabolism (Morris 2004), oral Cit is not taken up by the liver and is transported to the kidneys where most of Cit is catabolized to Arg (van de Poll et al. 2004). As a result, supplementation with Cit increases plasma levels of Arg (Churchward-Venne et al. 2014; Kim et al. 2015), with evidence that oral Cit elevates plasma Arg levels more than an equivalent dose of oral Arg in older adults (Moinard et al. 2016). Supplementation with Cit has been shown to reduce resting blood pressure (Figueroa et al. 2017) and lower peripheral arterial stiffness (Ochiai et al. 2012; Figueroa et al. 2015) in middle-aged to older adults. Few studies have investigated the impact of Cit on blood flow during exercise in older adults. Using an acute dose of Cit (10g), Churchward-Venee et al. (2014) did not observe an additional benefit above an amino acid mixture on femoral blood flow following unilateral knee extension resistance exercise in older men. Similarly, Kim et al. (2015) found no change in forearm reactive hyperemia after acute Cit (3g) despite increasing whole-body NO synthesis rate in older adults. These findings are in contrast to what is observed following chronic oral Cit in young men. Bailey et al. (2015) demonstrated that taking Cit (6 g/day) for 7 days lowers the level of deoxygenated hemoglobin in active muscle during moderate intensity exercise. Bailey et al. (2016) also reported a higher tissue oxygenation index during exercise when providing Cit (11.4 g/day) in the form of watermelon juice for 16 days. Both these reports indicate that muscle perfusion during exercise may be improved following chronic supplementation with Cit, but whether a similar positive influence on exercise blood flow can be found with Cit in older adults has yet to be examined.
To date, little is known regarding the effect of chronic Cit in older adults, a condition that increases muscle oxygenation patterns in young adults (Bailey et al. 2015; Bailey et al. 2016). Thus, the purpose of this study was to test the hypothesis that chronic Cit will enhance muscle blood flow and peripheral dilation during exercise in older adults.
Methods
Ethical Approval
All participants provided written informed consent prior to data collection. The Human Research Protection Program at Texas Tech University provided ethics approval for this study (#504985). This study and its procedures conformed to standards set by the latest version of the Declaration of Helsinki and is registered on ClinicalTrials.gov (identifier NCT03127917).
Participants
Thirteen older women (61–80y) and 12 older men (63–78y) took part in this study after giving informed consent to participate. Participants had no history of cardiovascular disease, pulmonary disease, and were not taking medication for high blood pressure or cholesterol. Three women were on estrogen-related therapy (two taking Evista, one taking Premarin). Removing these women did not change any result so they were included in the analysis. Participants were included in the study if they had a body mass index <30 kg/m2, resting seated brachial blood pressure <160/100 mm Hg, fasting blood glucose <115 mg/dL, and ankle-brachial index values >0.90.
Study design and protocol
This study was a randomized, double-blind, crossover study involving Cit (BioKyowa, Cape Girardeau, MO) and maltodextrin (NOW Foods, Bloomingdale, IL) that was given as a placebo control. Supplements in the form of capsules were taken orally for two weeks with a two week washout period. This duration of supplementation and washout is consistent with recent work that finds Cit supplementation improves muscle oxygenation patterns during exercise (Bailey et al. 2015; Bailey et al. 2016). The dose of Cit was 6 g/day (3g taken twice daily), and the last dose of Cit or placebo was consumed the night before the study visit. This dose of Cit has been shown to significantly increase plasma [Arg] (Schwedhelm et al. 2008; Bailey et al. 2015). The order of supplements were randomized and equally balanced within each sex. All visits were scheduled in the morning, and participants were instructed to arrive after refraining from food, caffeine, vitamins, and other supplements/medications for at least 8 hours. An initial visit was made by participants for the purpose of written informed consent and screening. The remaining four experimental visits were similar in procedures, but surrounded the Cit and placebo conditions (i.e., pre- and post-visits for each condition). First, blood was collected for measurement of plasma levels of Arg, nitrite and nitrate (NOx), and fasting blood glucose. Height and weight was measured using a standard scale followed by applanation tonometry for central arterial stiffness. Lastly, leg vascular function was then assessed at rest and during unilateral calf exercise.
Central arterial stiffness
Participants rested in a supine position for 10 min before two measurements of blood pressure were taken with a 1 min rest period between measurements on the left arm using an automated device (HEM-907XL, Omron Healthcare, Lake Forest, IL). Brachial blood pressure was used to calibrate an aortic pressure waveform derived from a composite of consecutive radial artery pressure waveforms measured at the left wrist using applanation tonometry and a general transfer function (SphygmoCor PVx system, Atcor Medical, Sydney, Australia). Pulse wave analysis of the aortic pressure waveform provided pulse wave transit time. Carotid-femoral pulse wave velocity, an index of central aortic stiffness, was calculated using an online standardization tool that uses blood pressure, carotid-femoral distance, and pulse wave transit time to calculate pulse wave velocity (Londono et al. 2015; abstract).
Exercise blood flow
Participants rested in a semi recumbent position. Diameter and mean blood velocity were measured in the right superficial femoral artery using Doppler ultrasound (Vivid 7, GE Healthcare, Waukesha, WI) with a 5–13 MHz linear transducer probe at rest and during single calf muscle exercise. The participant’s right leg was extended, raised to a horizontal level, and the right foot was attached to a custom pulley system. Participants moved their right foot through dorsiflexion and plantarflexion in a full range of motion while lifting an external load of 0.5 kg and 1.5 kg at a cadence of 40 contractions per minute. Each workload lasted 3 min and the order of workload (light to heavier) was the same for all visits. Blood pressure was measured at rest and every minute during exercise (HEM-907XL, Omron Healthcare, Lake Forest, IL). Blood velocity waveforms were sampled in real time at 1000 Hz through a Doppler audio transformer (Herr et al. 2010) that connected the Doppler to a data acquisition system (Powerlab 8SP, ADInstruments, Colorado Springs, CO). Blood velocity and diameter were analyzed offline from samples recorded at rest and during exercise. Mean blood velocity was derived from averaging across cardiac cycles for 60s at rest and at the end of exercise (i.e., last minute of exercise). Average diameter was measured using an automated edge detection system (Brachial Analyzer, Medical Imaging Applications, Coralville, IA) from 20s video clips recorded at rest and during exercise immediately after blood velocity waveforms were sampled. Blood flow was calculated by multiplying the cross-sectional area (πr2) of the femoral artery with mean blood velocity. Femoral vascular conductance (FVC) was calculated by dividing mean blood flow by mean blood pressure.
Blood Analysis
Fasting blood glucose was measured by finger prick (Accu-Chek Active, Roche Diagnostics, Indianapolis, IN). Blood was collected anaerobically by venipuncture from an antecubital vein into tubes (BD Vacutainer, Franklin Lakes, NJ) containing K2 EDTA (10.8 mg). Immediately upon obtaining the blood sample, tubes were centrifuged at 2,000 rpm for 10 min. Subsequently, the supernatant was collected and immediately frozen at −80°C until analysis for plasma NOx. Plasma concentrations of NOx were assessed by a commercially available colorimetric assay that uses a Griess reagent in a microtiter plate (Cayman Chemical, Ann Arbor, MI). Plasma [Arg] was measured using liquid chromatography (LC)-tandem mass spectrometry (MS) following derivatization procedures described by Mao et al. (2010) and LC MS conditions for analysis described by Zheng et al. (2015).
Statistical analysis
Descriptive statistics were calculated for participant characteristics and an independent sample t-test was used to compare the means between women and men. For crossover data, primary outcome variables examined were pre-to-post changes in resting vascular function and blood flow for each condition (Cit and placebo). Consistent with methods detailed by Senn (2002), we established a general linear model to test the effect of condition on primary outcome variables after adjusting for sequence (Cit then Placebo vs. placebo then Cit) and period (1st two weeks vs. 2nd two weeks) effects of the crossover design. The analyses were stratified by sex based on the results of preliminary analysis showing significant sex-by-condition interaction effect on changes in blood flow. For each sex-specific general linear model, we additionally included resting diastolic blood pressure and body mass index as covariates, as they were significantly different by sex (see Table 1). The normality of primary outcome variables as well as residuals estimated from the models was examined using Kolmogorov-Smironov test to ensure the use of parametric linear model. In addition, since the use of change scores as primary outcome variables in the model limited us to examine the within-condition differences, paired t-tests were used to compare pre-to-post values within each condition. Statistical significance was considered P ≤ 0.05.
Table 1.
Participant characteristics.
| Women (n=13) | Men (n=12) | P value | |
|---|---|---|---|
| Age (yrs) | 70 ± 5 | 71 ± 5 | 0.67 |
| Height (cm) | 163 ± 5 | 176 ± 5 | <0.01 |
| Weight (kg) | 59 ± 6 | 79 ± 9 | <0.01 |
| Body mass index (kg/m2) | 22.0 ± 2.8 | 25.5 ± 2.1 | <0.01 |
| Lowest ankle-brachial index | 1.13 ± 0.07 | 1.19 ± 0.14 | 0.20 |
| Seated systolic BP (mmHg) | 130 ± 13 | 137 ± 16 | 0.23 |
| Seated diastolic BP (mmHg) | 65 ± 8 | 77 ± 9 | <0.01 |
| Seated heart rate (bpm) | 58 ± 3 | 59 ± 11 | 0.75 |
| Fasting blood glucose (mg/dL) | 96 ± 6 | 93 ± 15 | 0.57 |
Values are mean ± SD. BP, blood pressure.
Results
Participant characteristics
Table 1 shows participant characteristics at the first study visit prior to starting Cit or placebo. Men were taller, weighed more, and had a larger body mass index, and higher diastolic blood pressure than women (P < 0.01). No sex difference (P > 0.05) was found for age, ankle-brachial index, resting systolic blood pressure, heart rate, or fasting blood glucose.
Resting vascular function
Table 2 shows the effect of Cit on resting blood pressure and arterial stiffness. The change in diastolic pressure was significantly different between Cit and placebo in women and men (P ≤ 0.05). Diastolic blood pressure decreased following Cit by 5% in men, but was not significantly different (P = 0.23) from pre to post Cit in women. Adjusting for baseline blood pressure removed (P = 0.17) the difference in the change in diastolic blood pressure between Cit and placebo in men. The change in carotid-femoral PWV and plasma [NOx] were not different between Cit and placebo (P > 0.05), however, plasma [Arg] was significantly different between Cit and placebo in women (P ≤ 0.05) and men (P = 0.01). Plasma [Arg] increased by 30% and 35% in women and men, respectively, after Cit (P ≤ 0.01) with no significant change following placebo (P > 0.05). Adjusting for body mass index and baseline blood pressure did not alter the significant (P ≤ 0.05) increase in plasma [Arg] following Cit as compared to placebo in women or men.
Table 2.
Effect of L-citrulline on resting vascular function.
| Placebo | L-citrulline | Adjusted | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Change | Pre | Post | Change | P value† | P value | |
| Women | Model 1, 2 | |||||||
| Seated systolic BP (mmHg) | 129 ± 15 | 130 ± 10 | 1.0 ± 8.0 | 127 ± 12 | 128 ± 15 | 0.1 ± 9.7 | 0.73 | 0.76, 0.89 |
| Seated diastolic BP (mmHg) | 64 ± 7 | 66 ± 6 | 2.0 ± 5.0 | 65 ± 7 | 64 ± 7 | −1.4 ± 4.2 | 0.05 | <0.01,<0.01 |
| Carotid-femoral PWV (m/s) | 8.9 ± 1.5 | 9.1 ± 1.7 | 0.2 ± 1.1 | 9.0 ± 1.5 | 9.0 ± 1.4 | 0.1 ± 0.4 | 0.77 | 0.69, 0.74 |
| Plasma NOx (µmol/L) | 34 ± 11 | 36 ± 15 | 1.2 ± 10.2 | 34 ± 16 | 31 ± 10 | −3.3 ± 13.9 | 0.50 | 0.46, 0.31 |
| Plasma L-arginine (µmol/L) | 96 ± 47 | 105 ± 56 | 9.2 ± 33.2 | 101 ± 36 | 144 ± 44* | 43.3 ± 38.8 | 0.02 | 0.03, 0.05 |
| Men | ||||||||
| Seated systolic BP (mmHg) | 135 ± 11 | 132 ± 11 | −2.7 ± 12.1 | 135 ± 14 | 132 ± 13 | −2.5 ± 5.3 | 0.87 | 0.73, 0.64 |
| Seated diastolic BP (mmHg) | 74 ± 9 | 73 ± 6 | −0.5 ± 7.6 | 75 ± 9 | 71 ± 6* | −3.9 ± 5.3 | 0.04 | 0.05, 0.17 |
| Carotid-femoral PWV (m/s) | 8.8 ± 1.2 | 8.8 ± 1.0 | −0.1 ± 0.4 | 8.7 ± 1.2 | 8.8 ± 1.2 | 0.1 ± 0.7 | 0.26 | 0.31, 0.67 |
| Plasma NOx (µmol/L) | 32 ± 10 | 32 ± 12 | −0.1 ± 5.7 | 33 ± 8 | 33 ± 10 | 0.2 ± 11.0 | 0.87 | 0.95, 0.72 |
| Plasma L-arginine (µmol/L) | 96 ± 54 | 94 ± 37 | −2.3 ± 39.1 | 78 ± 29 | 119 ± 50* | 37.4 ± 31.9 | 0.01 | <0.01, 0.03 |
Values are mean ± SD. BP, blood pressure, PWV, pulse wave velocity; NOx, nitrite and nitrate; NS, P-value greater than 0.05.
, comparison of the change value between conditions after controlling for sequence and period effects in a crossover design.
, difference (P < 0.05) between pre and post values within condition.
Model 1 adjusted for order, period, and baseline body mass index. Model 2 adjusted for model 1 plus baseline seated diastolic BP.
Exercise blood flow
Table 3 shows the effect of Cit on femoral blood flow and vascular conductance during calf muscle exercise. The change in mean blood pressure, blood flow, and FVC was not different (P > 0.05) between Cit and placebo in women at rest or during exercise at either workload. In contrast, men showed lower mean blood pressure from pre to post following Cit at rest (P < 0.01) and during exercise (0.5 kg: P = 0.04; 1.5 kg: P = 0.02), although the change in mean blood pressure following Cit was not significantly different as compared to placebo at any time point (P > 0.05). The change in blood flow following Cit was different (P = 0.01) than placebo only for exercise at the higher workload such that blood flow increased by 11% following Cit (P < 0.05) with no difference after placebo (P = 0.57). The change in FVC was also different (P = 0.01) following Cit as compared to placebo with FVC increasing by 14% with Cit (P = 0.01) with no difference after placebo (P = 0.78). Adjusting for baseline blood pressure, but not body mass index, removed the significant difference between Cit and placebo in blood flow (P = 0.10) and FVC (P = 0.10) during exercise at the higher workload.
Table 3.
Effect of L-citrulline on blood flow during exercise.
| Placebo | L-citrulline | Adjusted | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Change | Pre | Post | Change | P Value† | P value | |
| Women | ||||||||
| Mean Blood Pressure (mmHg) | Model 1, 2 | |||||||
| Rest | 86 ± 8 | 88 ± 6 | 1.9 ± 5.6 | 86 ± 8 | 85 ± 8 | −0.8 ± 5.3 | 0.16 | 0.09, 0.15 |
| 0.5 kg | 95 ± 8 | 96 ± 7 | 1.4 ± 6.6 | 96 ± 10 | 95 ± 9 | −1.1 ± 6.9 | 0.23 | 0.35, 0.46 |
| 1.5 kg | 95 ± 7 | 97 ± 7 | 2.1 ± 6.0 | 97 ± 9 | 96 ± 8 | −0.4 ± 5.3 | 0.22 | 0.24, 0.36 |
| Mean Blood Flow (mL/min) | ||||||||
| Rest | 80 ± 28 | 75 ± 36 | −5.4 ± 38.5 | 84 ± 32 | 73 ± 35 | −10.8 ± 27.5 | 0.58 | 0.12, 0.19 |
| 0.5 kg | 374 ± 80 | 364 ± 90 | −9.8 ± 65.9 | 381 ± 99 | 340 ± 102 | −41.4 ± 96.3 | 0.28 | 0.38, 0.23 |
| 1.5 kg | 452 ± 117 | 454 ± 101 | 2.4 ± 67.6 | 430 ± 71 | 415 ± 98 | −15.5 ± 68.2 | 0.55 | 0.40, 0.25 |
| FVC (mL/min/mmHg) | ||||||||
| Rest | 0.9 ± 0.3 | 0.8 ± 0.3 | −0.08 ± 0.40 | 0.9 ± 0.3 | 0.8 ± 0.4 | −0.11 ± 0.31 | 0.79 | 0.25, 0.40 |
| 0.5 kg | 3.9 ± 0.9 | 3.7 ± 0.9 | −0.18 ± 0.86 | 3.9 ± 1.1 | 3.5 ± 1.0 | −0.40 ± 0.91 | 0.43 | 0.49, 0.20 |
| 1.5 kg | 4.7 ± 1.2 | 4.6 ± 0.9 | −0.10 ± 0.69 | 4.5 ± 1.0 | 4.3 ± 1.0 | −0.17 ± 0.69 | 0.85 | 0.64, 0.30 |
| Men | ||||||||
| Mean Blood Pressure (mmHg) | ||||||||
| Rest | 94 ± 9 | 92 ± 6 | −1.8 ± 7.4 | 95 ± 9 | 91 ± 7* | −3.7 ± 3.8 | 0.17 | 0.13, 0.55 |
| 0.5 kg | 102 ± 10 | 101 ± 10 | −0.2 ± 9.2 | 105 ± 12 | 100 ± 9* | −4.1 ± 6.3 | 0.32 | 0.24, 0.58 |
| 1.5 kg | 102 ± 11 | 102 ± 11 | −0.6 ± 7.9 | 103 ± 11 | 100 ± 10* | −3.4 ± 4.5 | 0.56 | 0.47, 0.70 |
| Mean Blood Flow (mL/min) | ||||||||
| Rest | 95 ± 45 | 94 ± 59 | −0.7 ± 50.9 | 85 ± 33 | 84 ± 29 | −1.8 ± 20.3 | 0.84 | 0.99, 0.94 |
| 0.5 kg | 448 ± 132 | 439 ± 127 | −8.5 ± 70.9 | 465 ± 131 | 463 ± 140 | −1.6 ± 81.3 | 0.55 | 0.70, 0.98 |
| 1.5 kg | 565 ± 121 | 555 ± 160 | −10.5 ± 62.6 | 521 ± 134 | 584 ± 166* | 62.6 ± 98.0 | 0.01 | 0.02, 0.10 |
| FVC (mL/min/mmHg) | ||||||||
| Rest | 1.0 ± 0.4 | 1.0 ± 0.6 | 0.02 ± 0.55 | 0.9 ± 0.3 | 0.9 ± 0.3 | 0.01 ± 0.20 | 0.74 | 0.89, 0.97 |
| 0.5 kg | 4.3 ± 1.0 | 4.3 ± 1.2 | −0.04 ± 0.71 | 4.4 ± 1.2 | 4.6 ± 1.4 | 0.17 ± 0.69 | 0.31 | 0.40, 0.83 |
| 1.5 kg | 5.5 ± 1.0 | 5.4 ± 1.4 | −0.06 ± 0.82 | 5.0 ± 1.5 | 5.8 ± 1.7* | 0.77 ± 0.93 | 0.01 | 0.02, 0.10 |
Values are mean ± SD. FVC, femoral vascular conductance; NS, P-value greater than 0.05.
, comparison of the change value between conditions after controlling for sequence and period effects in a crossover design.
, difference (P < 0.05) between pre and post values within condition.
Model 1 adjusted for order, period, and baseline body mass index. Model 2 adjusted for model 1 plus baseline seated diastolic BP.
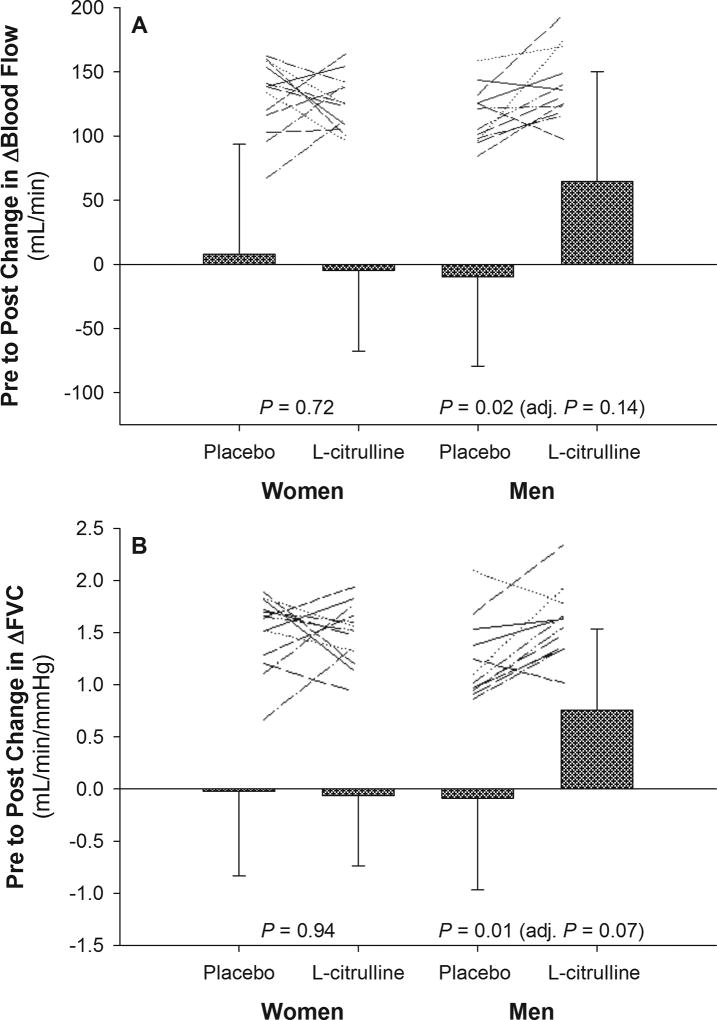
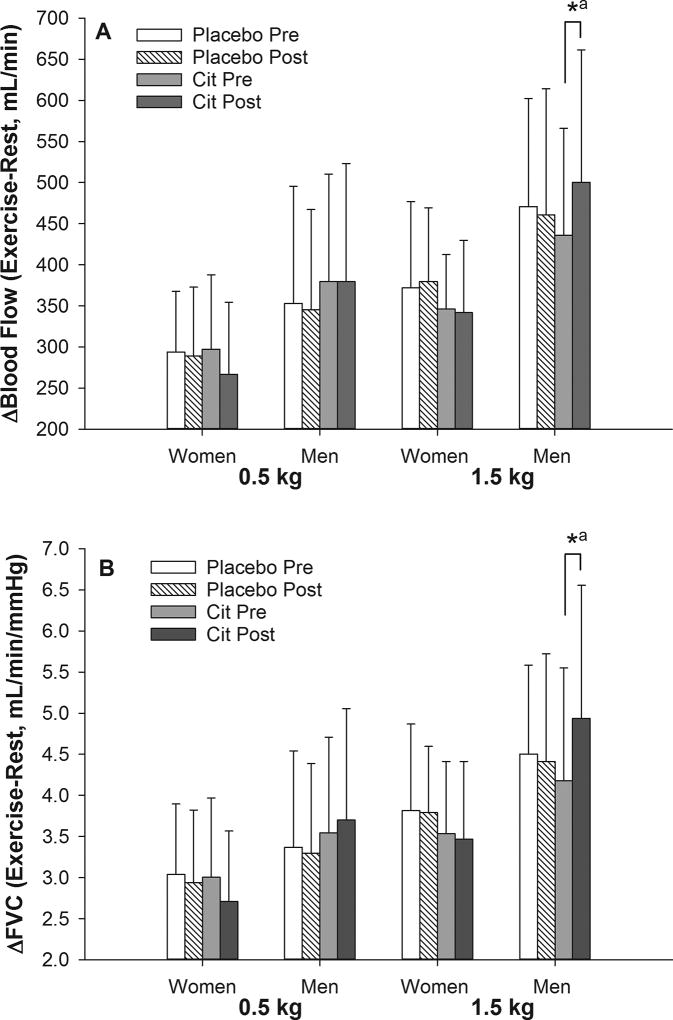
The blood flow and FVC response to exercise was also calculated as an increase from rest (Δ Exercise – Rest). Similar to the other results, the change in Δblood flow (Cit: 64 ± 85 vs. placebo: −10 ± 69 mL/min, P = 0.02; Figure 1A) and ΔFVC (Cit: 0.75 ± 0.77 vs. placebo: −0.09 ± 0.87 mL/min/mmHg, P = 0.01; Figure 1B) was different following Cit as compared to placebo for exercise at 1.5 kg in men. It was found that Δblood flow increased by 13% (P = 0.02; Figure 2A) and ΔFVC by 16% (P < 0.01; Figure 2B) after Cit while both variables were not different following placebo (P > 0.05). Adjusting for baseline blood pressure, but not body mass index (Δblood flow, P =0.03; ΔFVC, P = 0.02), removed the difference between Cit and placebo in Δblood flow (P = 0.14) and ΔFVC (P = 0.07) at 1.5 kg in men. The change in Δblood flow (P = 0.72) and ΔFVC (P = 0.94) was not different between conditions in women for either workload (see Figures 1 and 2 for 1.5 kg; data not shown for 0.5 kg), or for exercise at 0.5 kg for men (data not shown).
Figure 1.
Change in blood flow and femoral vascular conductance (FVC) expressed as an increase from rest to exercise (Δ) following placebo and L-citrulline conditions. Only data for the 1.5 kg workload condition is presented. Data are mean ± SD. Lines represent non-scaled individual responses. P values are from statistical comparisons of the change values between conditions after controlling for sequence and period effects in a crossover design. The P value in parentheses is adjusted for BMI and baseline diastolic pressure in addition to sequence and period effects.
Figure 2.
Femoral blood flow and vascular conductance (FVC) during calf muscle exercise expressed as an increase from rest to exercise (Δ) in older women and men. The change in Δblood flow and ΔFVC was different between L-citrulline (Cit) and placebo only for exercise at the higher workload in men. Data are mean ± SD. *, difference between pre and post values within condition after controlling for sequence and period effects in a crossover design (P < 0.05).a, no longer significant (P > 0.05) after adjusting for BMI and baseline diastolic pressure in addition to sequence and period effects.
Discussion
The aim of this study was to determine if short term (14 days) Cit supplementation enhances peripheral blood flow and dilatory responses to dynamic calf exercise in older adults. This study was the first to assess the impact of Cit on volumetric blood flow during active contractions. The novel finding is that Cit increases the femoral blood flow response to exercise by enhancing vascular conductance in older men. This finding was observed during exercise at the higher workload indicating that the effect may be dependent on intensity. Importantly, the significant augmentation in exercise blood flow by Cit was removed by adjusting for baseline blood pressure suggesting that the improvement in blood flow with Cit was related to older adult’s basal vascular state.
Cit effect on blood pressure
Older men had higher resting diastolic blood pressure than older women, and experienced a modest blood pressure lowering effect following Cit. The change in resting diastolic blood pressure following Cit in men was inversely correlated (r = −0.72, P < 0.001) with baseline diastolic blood pressure suggesting that the reduction in absolute diastolic pressure after Cit supplementation was likely due to having higher basal blood pressure. Hart et al. (2009) has shown diastolic (but not systolic) blood pressure is positively related to resting muscle sympathetic nerve activity (MSNA) in older men. Supplementation with Cit may have reduced sympathetic vasomotor tone by enhancing smooth muscle relaxation. This is plausible considering that past work finds chronic Cit increases urinary excretion of cyclic GMP (Schwedhelm et al. 2008), a byproduct of NO stimulated guanylyl cyclase in smooth muscle that results in vasodilation. Also, evidence exists that acute nitrate supplementation is effective at reducing MSNA at rest (Notay et al. 2017).
Cit effect on plasma biomarkers
Plasma [NOx] was not altered with Cit in the present study. Other studies in middle-aged adults have reported elevated biomarkers of NO production following chronic Cit (Schwedhelm et al. 2008; Ochiai et al. 2012), while some studies in younger adults have not (Bailey et al. 2015; Suzuki et al. 2016). The lack of change in plasma [NOx] in the present paper indicates that Cit was unable to augment systemic NO production, however it is possible that Cit still had an influence on NO bioactivity within tissue. Resting diastolic blood pressure was lowered and blood flow responses to exercise were improved in men; evidence that Cit had a positive vascular effect in men despite no change in plasma [NOx].
The goal of Cit supplementation is to increase Arg levels. Arginine is substrate for NO production, and therefore, may counter low NO bioavailability associated with aging and disease (El Hattab et al. 2012; Kim et al. 2015). Both women and men showed significantly elevated plasma [Arg] following Cit supplementation indicating successful intestinal absorption of Cit and subsequent production of Arg in the kidney. This is an interesting observation considering that participants took their last dose the night before testing (9–12h prior). Moinard et al. (2016) has reported that plasma [Arg] returns to basal levels about 5–8 hours after an acute dose of Cit (10g) in older men. Therefore, our results indicate that Arg accumulates in blood over time with chronic Cit in older adults.
Cit effect on exercise blood flow
The protocol employed in this study involved two workloads performed in succession. The first workload was set at an external resistance of 0.5 kg which was enough weight to provide tautness to the pulley system allowing for a semi-unloaded stage of exercise. At this light intensity there was no influence of Cit on exercise blood flow or FVC in either sex. Since this stage of exercise required low tension development, it can be interpreted that Cit was unable to influence the response of the endothelium to stimuli such as shear stress and mechanical distortion of vessels that largely drive local resistance arteriolar dilation during passive to light exercise (Wray et al. 2005). The second workload was set at an external resistance of 1.5 kg since our pilot work found weight above this amount led to some older adults struggling to complete 3 min of exercise. At this intensity of exercise that involved greater contractile work, and thus metabolism, we found Cit increases both absolute and relative (Δ from rest) blood flow responses to exercise in men but not women. This finding indicates that Cit enhances blood flow when oxygen is at a greater demand and/or when the stimulus for vasodilation is more appreciable. The improved blood flow response to exercise for men may be due to several mechanisms. Mean blood pressure was lower during exercise in men which may have reflected lower sympathetic vasomotor activity allowing for greater conductance for blood flow. However, mean blood pressure was also lower at rest and during the first stage of exercise without accompanying improvements in FVC or blood flow, thus this mechanism alone is unlikely. Secondly, Cit could have improved NO bioavailability which can enhance the accumulation of vasodilators supporting blood flow (Crecelius et al. 2010) and/or NO improved the regulation of mitochondrial respiration by binding to cytochrome c oxidase (Brown 2001), thereby facilitating greater blood flow (and oxygen) distribution to active muscle. This postulation is consistent with Bailey et al. (2015) that found 7 days of Cit supplementation (6 g/day) increases vastus lateralis muscle oxygenation in young men during moderate-intensity cycling indicating improved muscle perfusion.
Our finding that exercise blood flow is elevated with chronic Cit in older men is in contrast to what is reported following acute Cit administration. Post-exercise hyperemia (Churchward-Venne et al. 2014) and peak reactive hyperemia (Kim et al. 2015) are unaltered by acute doses of Cit in older adults that are overweight with normal to elevated basal blood pressures. This indicates that chronic Cit administration, even short term, is necessary to produce a positive benefit to exercise blood flow. The present study, for the first time, shows Cit increases volumetric blood flow during exercise. However, this improvement was dependent on resting blood pressure, indicating that Cit may only benefit older adults with some degree of vascular dysfunction. The observation that Cit did not increase exercise blood flow in older women is likely due to women having healthier vasculature than men as reflected by lower resting diastolic blood pressure and body mass index (Table 1). Another consideration worthy of discussion is Arg transport into the vasculature. While women were not limited in converting Cit to Arg as reflected by elevated plasma [Arg], transportation of Arg to membrane-bound endothelial nitric oxide synthase is accomplished by cationic amino acid transporter-1 (CAT-1) which can be inhibited through several mechanisms including posttranslational modulation by phosphorylated protein kinase Cɑ (PKCɑ) and extracellular signal-regulated kinase (ERK). Older female rats are shown to have higher phosphorylated PKCɑ protein levels than older male rats (Schwartz et al. 2009). Moreover, estrogen is found to increase CAT-1 activity by decreasing phosphorylated ERK (Bentur et al. 2015) and estrogen decreases arginase levels (enzyme that metabolizes arginine) in vascular cells (Hayashi et al. 2006), thus older women with low estrogen levels may have impaired Arg transport and/or intracellular activity than older men. While this study cannot address if this was a contributing factor in our results, we cannot discount the possibility of sex differences in the ability of Cit to increase NO bioavailability in older adults.
Limitations
Several limitations accompany this study. Although we were sufficiently powered to detect a significant increase in Arg following Cit supplementation based on past work (Schwedhelm et al. 2008), no other study has reported changes in bulk blood flow during exercise to perform a power analysis for our main outcome variable. However, the data presented here can be used by future researchers to perform such power analysis. The present sample of older adults had no history of cardiovascular disease, thus our results may not translate to clinical populations. We included older adults with normal to high blood pressure with six men and four women having Stage I hypertension due to elevated systolic blood pressure. While this sample reflects vascular aging since systolic blood pressure significantly increases with advancing age (Franklin et al. 1997), it does not well represent a model of healthy aging. Another limitation is that plasma [NOx] was measured rather than other NO biomarkers that may be more specific to endothelial function (e.g., nitrite) (Lauer et al. 2001). We observed no change in plasma [NOx] in women or men, thus have no evidence that NO production was elevated. Although we attribute improved blood pressure and exercise blood flow after Cit in men to elevated endothelial NO bioavailability, consistent with other researchers (Bailey et al. 2015; Bailey et al. 2016; Figueroa et al. 2017), we appreciate that there may be other pathways that Cit may impart benefits to vascular function. For instance, Cit increases neuronal NO synthase expression (Yabuki et al. 2013), which recent research shows to be an important regulator of blood pressure and vascular resistance in humans (Shabeeh et al. 2017). This central effect may be augmented by Arg-induced elevations in neuronal cyclic AMP levels (Sidorov et al. 1999) that can subsequently trigger adenosine-stimulated NO synthesis (Dubey et al. 1998). The present study cannot address if this central pathway contributed to our results, thus requiring future research to uncover the specific pathway(s) responsible for improved blood flow responses to exercise in men following Cit supplementation.
Conclusions
In this sample of older adults, Cit supplementation increased femoral blood flow and vascular conductance during lower-limb exercise in men. The improvement in exercise blood flow in men was removed when adjusting for baseline blood pressure indicating that the benefit of Cit supplementation is more pronounced in older men with higher resting blood pressure. These results support arginine supplementation using Cit can improve muscle blood flow during exercise in older men, however the 10–15% increase in hemodynamics observed in this study is less than the ~25% age-related impairment in muscle blood flow reported for older men (Poole et al. 2003). While this diminishes the clinical meaningfulness of our results, physiological implications of enhanced blood flow within the magnitude observed after Cit in this study has implications for improving exercise tolerance as demonstrated in older adults with vascular dysfunction (Sullivan et al. 1988).
New Findings.
What is the central question of this study?
Does short-term supplementation with L-citrulline in order to increase L-arginine improve exercise blood flow and peripheral dilation responses to exercise in older adults?
What is the main finding and its importance?
L-citrulline increases femoral blood flow by 11% and vascular conductance by 14% during lower-limb exercise in older men while no changes were observed in older women. This modest improvement in bulk muscle blood flow in older men has implications for altering muscle metabolism that may result in enhanced exercise tolerance in older adults.
Acknowledgments
The authors would like to thank Dr. Masoud Zabet and Ruchika Bhawal at the TTU Center for Biotechnology and Genomics for blood analysis. In addition, we thank Dr. Eunhee Chung for her administrative support during the initial portion of this project.
Funding
This work was supported by an American Heart Association Beginning Grant-in-Aid (award number 15BGIA22710012 to J.U.G.).
Footnotes
Author contributions
This study was conducted in the Department of Kinesiology and Sport Management at Texas Tech University. J.U.G. conceptualized and designed the study. J.U.G., A.R., and J.A. collected, analyzed, and drafted the work providing individual intellectual contributions. Y.K. assisted with statistical analysis, interpretation of results, and critically revised the manuscript for important intellectual content. All authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
References
- Bailey SJ, Blackwell JR, Lord T, Vanhatalo A, Winyard PG, Jones AM. l-Citrulline supplementation improves O2 uptake kinetics and high-intensity exercise performance in humans. J Appl Physiol. 2015;119:385–395. doi: 10.1152/japplphysiol.00192.2014. [DOI] [PubMed] [Google Scholar]
- Bailey SJ, Blackwell JR, Williams E, Vanhatalo A, Wylie LJ, Winyard PG, Jones AM. Two weeks of watermelon juice supplementation improves nitric oxide bioavailability but not endurance exercise performance in humans. Nitric Oxide. 2016;59:10–20. doi: 10.1016/j.niox.2016.06.008. [DOI] [PubMed] [Google Scholar]
- Bentur OS, Schwartz D, Chernichovski T, Ingbir M, Weinstein T, Chernin G, Schwartz IF. Estradiol augments while progesterone inhibits arginine transport in human endothelial cells through modulation of cationic amino acid transporter-1. Am J Physiol Regul Integr Comp Physiol. 2015;309:R421–R427. doi: 10.1152/ajpregu.00532.2014. [DOI] [PubMed] [Google Scholar]
- Bode-Böger SM, Muke J, Surdacki A, Brabant G, Böger RH, Frölich JC. Oral L-arginine improves endothelial function in healthy individuals older than 70 years. Vasc Med. 2003;8:77–81. doi: 10.1191/1358863x03vm474oa. [DOI] [PubMed] [Google Scholar]
- Brown GC. Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochimica Biophysica Acta. 2001;1504:46–57. doi: 10.1016/s0005-2728(00)00238-3. [DOI] [PubMed] [Google Scholar]
- Churchward-Venne TA, Cotie LM, MacDonald MJ, Mitchell CJ, Prior T, Baker SK, Phillips SM. Citrulline does not enhance blood flow, microvascular circulation, or myofibrillar protein synthesis in elderly men at rest or following exercise. Am J Physiol Endocrinol Metab. 2014;307:E71–E83. doi: 10.1152/ajpendo.00096.2014. [DOI] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Nitric oxide, but not vasodilating prostaglandins, contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am J Physiol Heart Circ Physiol. 2010;299:1633–1641. doi: 10.1152/ajpheart.00614.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Jackson EK. Cyclic AMP-adenosine pathway induces nitric oxide synthesis in aortic smooth muscle cells. Hypertension. 1998;31:296–302. doi: 10.1161/01.hyp.31.1.296. [DOI] [PubMed] [Google Scholar]
- El Hattab AW, Hsu JW, Emrick LT, Wong LJ, Craigen WJ, Jahoor F, Scaglia F. Restoration of impaired nitric oxide production in MELAS syndrome with citrulline and arginine supplementation. Mol Genet Metab. 2012;105:607–614. doi: 10.1016/j.ymgme.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa A, Alvarez-Alvarado S, Ormsbee MJ, Madzima TA, Campbell JC, Wong A. Impact of L-citrulline supplementation and whole-body vibration training on arterial stiffness and leg muscle function in obese postmenopausal women with high blood pressure. Exp Gerontol. 2015;63:35–40. doi: 10.1016/j.exger.2015.01.046. [DOI] [PubMed] [Google Scholar]
- Figueroa A, Wong A, Jaime SJ, Gonzales JU. Influence of L-citrulline and watermelon supplementation on vascular function and exercise performance. Curr Opin Clin Nutr Metab Care. 2017;20:92–98. doi: 10.1097/MCO.0000000000000340. [DOI] [PubMed] [Google Scholar]
- Franklin SS, Gustin W, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. Circ. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- Hart EC, Joyner MJ, Wallin BG, Johnson CP, Curry TB, Eisenach JH, Charkoudian N. Age-related differences in the sympathetic-hemodynamic balance in men. Hypertension. 2009;54:127–133. doi: 10.1161/HYPERTENSIONAHA.109.131417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Esaki T, Sumi D, Mukherjee T, Iguchi A, Chaudhuri G. Modulating role of estradiol on arginase II expression in hyperlipidemic rabbits as an atheroprotective mechanism. Proc Natl Acad Sci. 2006;103:10485–10490. doi: 10.1073/pnas.0603918103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen I, Saltin B, Kemppainen J, Sipilä HT, Oikonen V, Nuutila P, Knuuti J, Kalliokoski K, Hellsten Y. Skeletal muscle blood flow and oxygen uptake at rest and during exercise in humans: a pet study with nitric oxide and cyclooxygenase inhibition. Am J Physiol Heart Circ Physiol. 2011;300:H1510–H1517. doi: 10.1152/ajpheart.00996.2010. [DOI] [PubMed] [Google Scholar]
- Herr MD, Hogeman CS, Koch DW, Krishnan A, Momen A, Leuenberger UA. A real-time device for converting Doppler ultrasound audio signals into fluid flow velocity. Am J Physiol Heart Circ Physiol. 2010;298:H1626. doi: 10.1152/ajpheart.00713.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowatz LA, Thompson CS, Kenney WL. L-Arginine supplementation or arginase inhibition augments reflex cutaneous vasodilatation in aged human skin. J Physiol. 2006;15:573–581. doi: 10.1113/jphysiol.2006.108993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IY, Schutzler SE, Schrader A, Spencer HJ, Azhar G, Deutz NEP, Wolfe RR. Acute ingestion of citrulline stimulates nitric oxide synthesis but does not increase blood flow in healthy young and older adults with heart failure. Am J Physiol Endocrin Metab. 2015;309:E915–E924. doi: 10.1152/ajpendo.00339.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, Kelm M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci. 2001;98:12814–12819. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londono F, Bossuyt J, Engelen L, Stehouwer C, Ferreira I, Laurent S, Boutouyrie P, Segers P, Van Bortel L. A simple calculator for the assessment of measurements of carotid-femoral pulse wave velocity and local arterial stiffness relative to the reference values database. J Hypertens. 2015;33:e60. (abstract) [Google Scholar]
- Mao HM, Wei W, Xiong WJ, Lu Y, Chen BG, Liu Z. Simultaneous determination of l-citrulline and l-arginine in plasma by high performance liquid chromatography. Clin Biochem. 2010;43:1141–1147. doi: 10.1016/j.clinbiochem.2010.05.017. [DOI] [PubMed] [Google Scholar]
- Moinard C, Maccario J, Walrand S, Lasserre V, Marc J, Boirie Y, Cynober L. Arginine behaviour after arginine or citrulline administration in older subjects. Br J Nutr. 2016;115:399–404. doi: 10.1017/S0007114515004638. [DOI] [PubMed] [Google Scholar]
- Morris SM. Enzymes of arginine metabolism. J Nutr. 2004;134:2743S–2747S. doi: 10.1093/jn/134.10.2743S. [DOI] [PubMed] [Google Scholar]
- Notay K, Incognito AV, Millar PJ. Acute beetroot juice supplementation on sympathetic nerve activity: A randomized, double-blind, placebo-controlled proof-of-concept study. Am J Physiol Heart Circ Physiol. 2017;313:H59–H65. doi: 10.1152/ajpheart.00163.2017. [DOI] [PubMed] [Google Scholar]
- Ochiai M, Hayashi T, Morita M, Ina K, Maeda M, Watanabe F, Morishita K. Short-term effects of l-citrulline supplementation on arterial stiffness in middle-aged men. Int J Cardiol. 2012;155:257–261. doi: 10.1016/j.ijcard.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Parker BA, Smithmyer SL, Pelberg JA, Mishkin AD, Proctor DN. Sex-specific influence of aging on exercising leg blood flow. J Appl Physiol. 2008;104:655–664. doi: 10.1152/japplphysiol.01150.2007. [DOI] [PubMed] [Google Scholar]
- Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol. 2003;284:H1251–H1259. doi: 10.1152/ajpheart.00790.2002. [DOI] [PubMed] [Google Scholar]
- Schwartz IF, Chernichovski T, Krishtol N, Grupper A, Laron I, Schwartz D. Sexual dimorphism in glomerular arginine transport affects nitric oxide generation in old male rats. Am J Physiol Renal Physiol. 2009;297:F80–F84. doi: 10.1152/ajprenal.00020.2009. [DOI] [PubMed] [Google Scholar]
- Schwedhelm E, Maas R, Freese R, Jung D, Lukacs Z, Jambrecina A, Spickler W, Schulze F, Böger RH. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: impact on nitric oxide metabolism. Br J Clin Pharmacol. 2008;65:51–59. doi: 10.1111/j.1365-2125.2007.02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn SS. Cross-over Trials in Clinical Research. John Wiley & Sons; 2002. [Google Scholar]
- Shabeeh H, Khan S, Jiang B, Brett S, Melikian N, Casadei B, Chowienczyk PJ, Shah AM. Blood pressure in healthy humans is regulated by neuronal NO synthase. Hypertension. 2017;69:970–976. doi: 10.1161/HYPERTENSIONAHA.116.08792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidorov AV, Kazakevich VB, Moroz LL. Nitric oxide selectively enhances cAMP levels and electrical coupling between identified RPaD2/VD1 neurons in the CNS of Lymnaea stagnalis (L.) Acta Biol Hung. 1999;50:229–233. [PubMed] [Google Scholar]
- Sullivan MJ, Higginbotham MB, Cobb FR. Exercise training in patients with severe left ventricular dysfunction. Hemodynamic and metabolic effects. Circ. 1988;78:506. doi: 10.1161/01.cir.78.3.506. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Morita M, Kobayashi Y, Kamimura A. Oral L-citrulline supplementation enhances cycling time trial performance in healthy trained men: Double-blind randomized placebo-controlled 2-way crossover study. J Int Soc Sports Nutr. 2016;13:1–8. doi: 10.1186/s12970-016-0117-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Poll MCG, Soeters PB, Deutz NEP, Fearon KCH, Dejong CHC. Renal metabolism of amino acids: its role in interorgan amino acid exchange. Am J Clin Nutr. 2004;79:185–197. doi: 10.1093/ajcn/79.2.185. [DOI] [PubMed] [Google Scholar]
- Wray DW, Donato AJ, Uberoi A, Merlone JP, Richardson RS. Onset exercise hyperaemia in humans: partitioning the contributors. J Physiol. 2005;565:1053–1060. doi: 10.1113/jphysiol.2005.084327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuki Y, Shioda N, Yamamoto Y, Shigano M, Kumagai K, Morita M, Fukunaga K. Oral l-Citrulline administration improves memory deficits following transient brain ischemia through cerebrovascular protection. Brain Res. 2013;1520:157–167. doi: 10.1016/j.brainres.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Zheng G, Jin W, Fan P, Feng X, Bai Y, Tao T, Yu L. A novel method for detecting amino acids derivatized with phenyl isothiocyanate by high-performance liquid chromatography–electrospray ionization mass spectrometry. Int J Mass Spectrom. 2015;392:1–6. [Google Scholar]