Abstract
Objective
Little is known about developmental outcomes in neonatal abstinence syndrome (NAS). We hypothesized that children treated for NAS would score lower than the normative sample on the Bayley Scales of Infant Development, 3rd edition.
Study design
We performed a retrospective cohort study of 87 infants treated for NAS and evaluated at 2 years of age.
Results
Children treated for NAS scored significantly lower than the norm (mean 100) on all 3 subscales (cognitive mean 96.5, language mean 93.8, motor mean 94.0, all p<0.03). Children who lived with foster/adoptive families at follow up had higher cognitive scores (median 100 vs 95, p=0.03) than those who lived with biological relatives, and were less likely to have motor scores <85 (p = 0.02). Eight percent of children required treatment for strabismus.
Conclusions
Children treated for NAS are at risk for lower developmental scores and higher rates of strabismus at age 2 than the general population.
Introduction
Due to the opioid misuse epidemic across the nation, more infants are being exposed to narcotics during fetal life and developing neonatal abstinence syndrome (NAS) in the neonatal period. The rate of NICU admissions for NAS increased from 7 cases per 1000 admissions in 2004 to 27 cases per 1000 admissions in 20131, and average hospital charges for infants with NAS amount to more than five times those for healthy infants2. Later effects of NAS on neurodevelopment are largely unknown, although studies have suggested that independent of socioeconomic status, children exposed to opioids in utero and in the neonatal period are at risk for later cognitive, language, attention, and visual problems3, 4, 5, 6, 7, 8 and poorer academic achievement9. NAS is therefore a pressing public health issue with significant social and economic implications.
No large-scale prospective studies have been published on treatment and long term outcomes of infants with NAS. A recent meta-analysis evaluating long term outcomes (>2 years) in opioid-exposed children found only 5 articles in the literature10. The few studies that evaluate subsequent outcomes of infants exposed to opioids in utero have not distinguished between infants who did or did not require pharmacologic treatment. It is not known which factors impact neurodevelopment in infants with pharmacologically treated NAS. In this retrospective study, our objective was to evaluate outcomes at age 2 of infants treated for NAS in the neonatal period. We hypothesized that infants with NAS would have lower scores than the normative sample on the Bayley Scales of Infant Development, 3rd edition (BSID-III), that infants with NAS would have adverse visual outcomes, and that type of NAS treatment in the neonatal period would not affect outcomes.
Subjects and Methods
This retrospective chart review was approved by the Cincinnati Children’s Hospital IRB with no parental consent required. We performed an electronic medical record search for all patients initially seen in the Cincinnati Children’s NICU Follow-Up Clinic between the years 2011–2015 with a diagnosis of NAS who had a Bayley performed after 18 months of age. Children who were not treated with opioids for NAS in the neonatal period and children with iatrogenic NAS were excluded. Choice of first line pharmacologic treatment for NAS in our region is based on the policy of each NICU, and all units used the same standardized protocols for initiating and weaning methadone, morphine, or buprenorphine as well as the same non-pharmacologic bundle11. In the last 4 years, two NICUs in Cincinnati have moved toward using sublingual buprenorphine as the drug of choice to treat NAS for all babies, including those whose mothers were taking heroin or methadone. Infants are not discharged home on opioid medications, but infants can be discharged home on phenobarbital. Since 2013, every child treated for NAS in our region, which includes 27,000 annual births, has been referred to our Follow-Up clinic for medical and developmental care and offered a BSID-III exam at 2 years of age. In this cohort, a BSID-III was performed by a single examiner who was not blinded to the child’s main diagnosis. BSID-III was offered to all families of infants with NAS. Data collected from the neonatal period included gender, race/ethnicity, maternal age, maternal substance use, gestational age, birth weight, breastfeeding, type of treatment for NAS, length of hospital stay, and with whom the infant was discharged home. Infants were considered exposed to a substance in utero if maternal urine at delivery or infant urine, meconium, or umbilical cord toxicology screens were positive for that substance. Due to universal maternal toxicology screening in our region, all mothers and infants had toxicology screens. Infants were considered exposed to polysubstances if they were exposed to drugs from more than one class. Follow up data included living situation at follow up, age at Bayley, Bayley scores, enrollment in Early Intervention services or center-based therapies, self-reported behavioral issues at follow up, and a diagnosis of strabismus made by a pediatric ophthalmologist.
Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina). The Bayley subscale means of our study cohort were compared to the population mean (mean=100, SD=15) using a one-sample two-sided t-test. Because the data had a non- normal distribution we performed non-parametric statistical testing for assessing associations between Bayley subscales and clinical factors. Differences in the median Bayley subscale values by factors such as gender, who child lived with (biological versus foster/adoptive family), and treatment protocol were tested using the Mann-Whitney U test or Kruskal Wallis test. Spearman correlations between the Bayley subscales and continuous variables, such as birthweight and gestational age, were also conducted.
Results
Initial search revealed 455 patients born between 2011 and 2015 with a diagnosis of NAS seen in our NICU follow up clinic for at least one visit. BSID-III was performed on 102 of those infants and 99 had scorable exams (3 infants did not comply with testing). Infants not actually treated for NAS, those <34 weeks, those with iatrogenic NAS, and those with significant other comorbid conditions (e.g. meconium aspiration syndrome requiring prolonged ventilation, Pierre-Robin syndrome requiring jaw distraction) were excluded, leaving a total of 87 patients for analysis (Figure 1).
Figure 1.
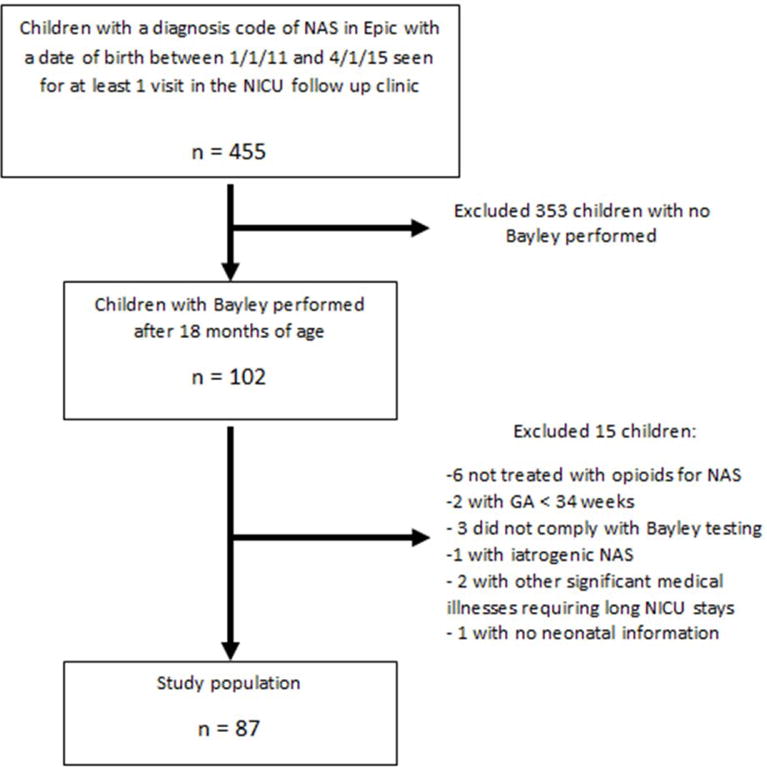
Patient flowsheet
Median birth weight was 2.87 kg (range 1.94–3.80) with 14% having birth weight <10th percentile. Median gestational age was 38 weeks (range 34–41), with 4 babies born at 34 weeks, 1 baby born at 35 weeks, and 6 babies born at 36 weeks. All mothers were Caucasian with a median maternal age of 26 years (range 18–38), which reflects the demographics of the opioid epidemic in our area. Although almost all women used illicit substances during pregnancy (Table 1), the majority were on maintenance therapy with methadone (38%) or buprenorphine (24%) at the time of delivery.
Table 1.
Maternal characteristics
| Characteristic | n | percent |
|---|---|---|
| Caucasian | 87 | 100% |
| Maternal polysubstance use | 50 | 57% |
| Maternal known Hepatitis C | 46 | 53% |
| In utero heroin | 58 | 67% |
| In utero cocaine | 19 | 22% |
| In utero benzodiazepines | 20 | 23% |
| In utero marijuana | 25 | 29% |
| In utero methadone | 33 | 38% |
| In utero buprenorphine | 21 | 24% |
| In utero amphetamines | 9 | 10% |
| In utero other opioid (oxycodone etc) |
30 | 36% |
| Any breastfeeding | 8 | 9% |
In this cohort, 72% of infants were treated with methadone, 16% were treated with morphine, and 13% were treated with buprenorphine. One infant received buprenorphine then was switched to methadone. 53% of infants received phenobarbital as adjuvant treatment, and 3% of infants received clonidine. Median NICU stay was 18 days (range 5–104 days). Child Protective Services were involved in all cases. Only 26% of infants went home in the primary care of their mother. 30% went home in the primary care of the father or another relative, and 44% went home in foster care or with an adoptive family.
The median age at the time of the Bayley was 23 months (range 18–28 months). At follow up, 22% of children were in the primary care of their mother, 34% lived with another relative, and 44% were with foster/adoptive families. However, 16 of the 87 infants had changed custody from the time of discharge (Figure 2). Of note, no babies started out in foster care and ended up in the care of their biological mother at 2 years of age.
Figure 2.
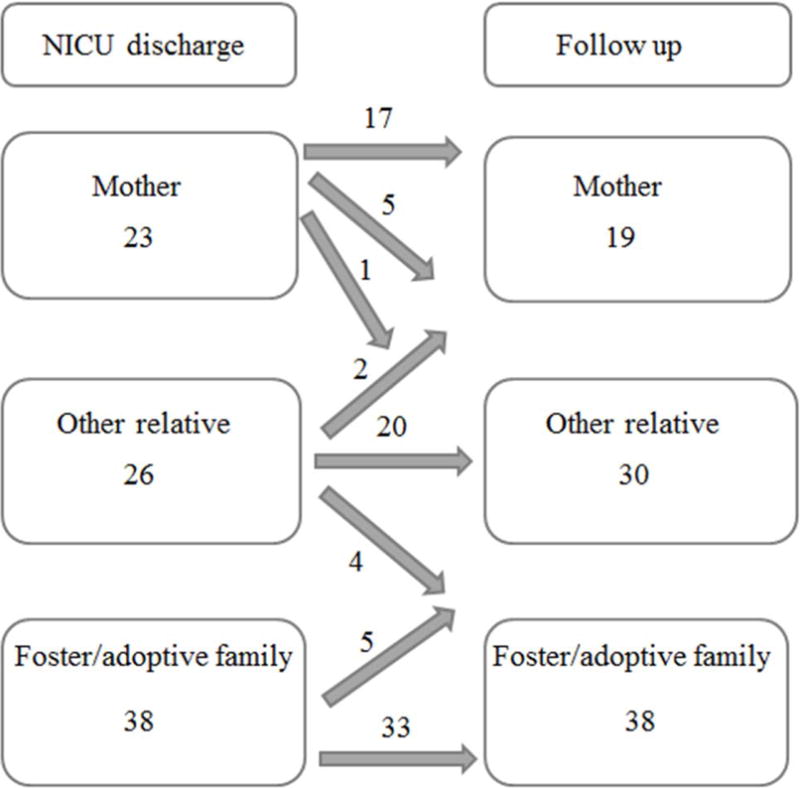
Disposition of children at discharge versus 2 year follow up
Most children had Bayley scores within the normal range for all 3 subscales, although a large proportion did have scores at least 1 standard deviation below the mean in at least 1 subscale (Table 2). Compared to the normative Bayley data (mean of 100, SD of 15), children with NAS scored significantly lower on the cognitive, language, and motor subscales (means 96.5, 93.8, 94.0 respectively, p<0.03 for all). Children who lived with foster/adoptive families at follow up scored significantly higher on the cognitive subscale than those who lived with their mother or a biological relative at follow up (median 100 vs median 95, p = 0.03, Figure 3). Language scores were not different based on living situation, but children who lived with biological relatives were also significantly more likely to have motor scores <85 (p = 0.02).
Table 2.
Bayley scores
| Median (range) | Mean (SD) | Score < 85 | Score < 70 | |
|---|---|---|---|---|
| Cognitive | 95 (65–115) | 96.5 (10.6) | 9 | 3 |
| Language | 94 (62–132) | 93.8 (13.3) | 17 | 5 |
| Motor | 94 (70–112) | 94.0 (9.4) | 11 | 0 |
Figure 3.
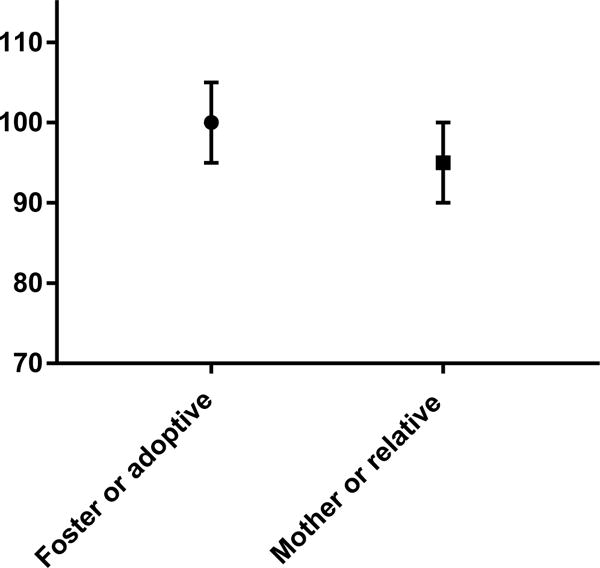
Median Bayley cognitive score in infants with NAS living with foster or adoptive families versus biological relatives
Girls with NAS scored higher than boys with NAS on the cognitive (median cognitive score 100 vs 95, p = 0.002) and language (median language score 97 vs 94, p=0.04) subscales, but not motor (median motor score 97 vs 94, p=0.16), (Figure 4). There was no difference in Bayley scores between children who required adjuvant therapy with phenobarbital or clonidine and those who did not. Type of primary treatment for NAS (methadone vs morphine vs buprenorphine) also did not affect Bayley scores, although numbers for morphine (n=14) and buprenorphine (n=11) were small. Children who were exposed to maternal polysubstance use in utero did not score differently than those who were exposed to a single drug class. Length of hospital stay, gestational age, and birth weight were not significantly correlated with later Bayley scores.
Figure 4.
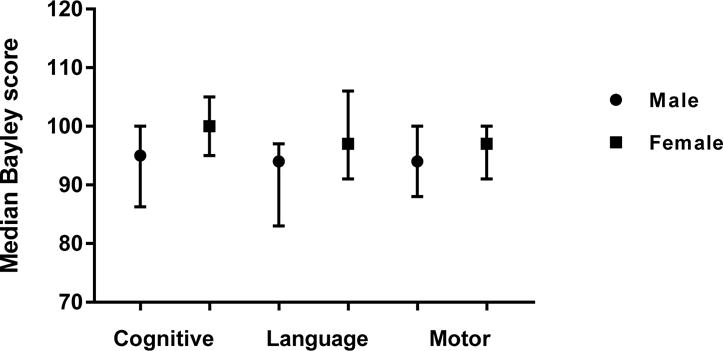
Median Bayley scores of children with NAS based on gender
All families were asked about therapies at each visit to our follow up clinic, and charts were also reviewed for therapy notes and referrals to county Early Intervention programs. Forty percent of children received county Early Intervention services with a developmental specialist and/or therapist prior to their follow up visit at 2 years of age. A significant proportion of children received one or more hospital-based therapies as well (speech/language therapy 22%, occupational therapy 7%, physical therapy 16%). Most children were enrolled in therapies before their 2 year visit, although some children were referred for the first time based on their Bayley results.
At the time of the 2 year visit, families were queried about sleep and behavioral issues. 26% of families reported significant behavioral or sleep issues, most commonly tantrums, hyperactivity, sensory issues, and difficulty falling/staying asleep. Due to the reported association of in utero opioid exposure with later strabismus, we reviewed all charts for ophthalmology notes and found that 8% of children in this cohort received treatment (patching, atropine, and/or surgery) for strabismus in the first 3 years of life, which is significantly higher than the expected population incidence of about 3%12. Nystagmus is another reported association with in utero opioid exposure, but nystagmus was not diagnosed in this cohort.
Discussion
In this retrospective study, we evaluated outcomes at 2 years of age of a regional cohort of infants treated for NAS due to maternal opioid use. We found that children with NAS performed lower than the normative Bayley sample, although still within the normal range in most cases. We do believe that the 4–6 point difference in Bayley scores is clinically significant, although it is difficult to say whether this will translate into later problems with school performance or IQ. Our findings are consistent with previous literature on opioid-exposed infants, although most previous studies have not distinguished between those exposed in utero and those exposed and also treated for NAS postnatally. A study which evaluated infants at six months of age showed that infants exposed to methadone in utero (n=81) had lower scores in all domains of the Griffith scales (similar to the Bayley) than controls (p<0.001), and infants requiring pharmacologic treatment performed more poorly than infants exposed but not treated5. A longitudinal study assessed 72 children with prenatal opioid exposure and 58 controls from age 1 to age 8, and found that exposed boys had stable, lower levels of cognitive functioning than controls at all time points, but the exposed girls had increasing differences over time from the controls6. Even children adopted at birth had lower cognitive scores; however, the controls were not matched for socioeconomic status(SES)6. In a 2003 study with multiple control groups, young children born to heroin-dependent mothers as well as unexposed children of low SES had lower intellectual skills and higher rate of inattention than controls of higher SES7. Children exposed in utero but adopted at birth had normal intellectual function but a higher rate of inattention and behavioral problems at school age7.
School age outcomes have been evaluated in several recent studies. One study specifically followed infants of mothers who took buprenorphine during pregnancy to school age (5–6 years)13. This study did not have a control group, but found that buprenorphine-exposed children had visual-motor, attention, motor, and memory problems as well as increased hyperactivity, impulsivity, and attention problems. No significant differences were found between infants exposed to buprenorphine who did and did not require pharmacologic treatment for NAS. Another recent study linked a neonatal discharge diagnosis of NAS with standardized test scores in 2236 children with NAS born in Australia9. They found that a diagnosis of NAS was associated with lower mean standardized test scores in all 5 test domains in grade 3, 5, and 7 and that the achievement gap increased as the children progressed in school.
The mechanism behind neurodevelopmental delays in children exposed to opioids in utero and postnatally is not entirely clear. Animal studies have shown that both buprenorphine14 and methadone15 alter myelination of the developing brain and affect various neurotransmitter systems16, 17, so there may be a biological cause. Increased social stressors, such as poverty, poor nutrition, and lower levels of education, are likely present in the families of children affected by NAS. Our finding of higher cognitive scores in children raised by foster/adoptive families suggests that socioeconomic factors do significantly affect outcomes in this population. Girls with NAS having higher scores than boys is consistent with previous studies6, 9 and may reflect increased resiliency to family stressors in girls18, 19, 20. These findings underscore the need for early intervention programs and family education and support for children affected by the opioid epidemic.
We also found on chart review that 8% of children in our cohort had been treated for strabismus by age 3. Previous studies have shown that infants exposed to opioids in utero are at risk for adverse visual outcomes. In one study, infants with in utero methadone exposure were more likely to have immature visual evoked potentials within the first several days of birth than unexposed infants, suggesting that in utero opiate exposure may affect the development of the fetal visual cortex4. Other reports of visual problems in this population come from retrospective case series, which show reduced visual acuity, nystagmus, delayed visual maturation, strabismus, refractive errors, and cortical visual impairment3, 21, 22. It is unknown whether postnatal opioid treatment increases the risk for visual problems, although one study found that infants who required pharmacologic treatment for NAS had more nystagmus than those who were exposed but did not require treatment3. In animal models, opioids are known to affect the development of the visual cortex23, 24, which may be the underlying mechanism for the visual issues seen in infants exposed to opioids in utero.
We acknowledge that our study has significant limitations, notably the retrospective observational design. We did not have a control group, and we did not have accurate information on socioeconomic status of the families. Of all the children with NAS in our region, only about 25% actually had a Bayley performed due to caregiver preference/no-shows, which could have led to selection bias. Our findings could well be due to the postnatal environment experienced by these children, and warrant a prospective study using standardized measures of behavior and visual function in addition to neurodevelopment, as well as standardized data collection of socioeconomic status.
We conclude that children with NAS are at risk for lower developmental scores than the test normative population at 2 years of age. As infants with NAS are identified at hospital discharge, there is a strong potential for early intervention in these families, as well as families whose infants are exposed to opioids in utero but do not withdraw significantly enough to require treatment for NAS. Social support for mothers and relatives caring for infants with NAS is vital. In Ohio, NAS is currently not an automatic inclusion criteria for county Early Intervention programs, but based on the results of our study we encourage close medical follow up and screening early on for delays so that intervention can be initiated in a timely fashion. We also recommend close attention to the visual exam, possibly with the newer visual screeners which can detect strabismus and refractive error in the general pediatrician’s office.
Acknowledgments
Funding source: NIH UG1HD027853-27S1 (Poindexter/Merhar)
Abbreviations
- NAS
neonatal abstinence syndrome
- BSID-III
Bayley Scales of Infant Development, 3rd edition
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest Statement: None of the authors has competing financial interests in relation to the work described.
References
- 1.Tolia VN, Patrick SW, Bennett MM, Murthy K, Sousa J, Smith PB, et al. Increasing incidence of the neonatal abstinence syndrome in U.S. neonatal ICUs. N Engl J Med. 2015;372(22):2118–2126. doi: 10.1056/NEJMsa1500439. [DOI] [PubMed] [Google Scholar]
- 2.Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. JAMA. 2012;307(18):1934–1940. doi: 10.1001/jama.2012.3951. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton R, McGlone L, MacKinnon JR, Russell HC, Bradnam MS, Mactier H. Ophthalmic, clinical and visual electrophysiological findings in children born to mothers prescribed substitute methadone in pregnancy. Br J Ophthalmol. 2010;94(6):696–700. doi: 10.1136/bjo.2009.169284. [DOI] [PubMed] [Google Scholar]
- 4.McGlone L, Hamilton R, McCulloch DL, MacKinnon JR, Bradnam M, Mactier H. Visual outcome in infants born to drug-misusing mothers prescribed methadone in pregnancy. Br J Ophthalmol. 2014;98(2):238–245. doi: 10.1136/bjophthalmol-2013-303967. [DOI] [PubMed] [Google Scholar]
- 5.McGlone L, Mactier H. Infants of opioid-dependent mothers: neurodevelopment at six months. Early Hum Dev. 2015;91(1):19–21. doi: 10.1016/j.earlhumdev.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Nygaard E, Moe V, Slinning K, Walhovd KB. Longitudinal cognitive development of children born to mothers with opioid and polysubstance use. Pediatr Res. 2015;78(3):330–335. doi: 10.1038/pr.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ornoy A. The impact of intrauterine exposure versus postnatal environment in neurodevelopmental toxicity: long-term neurobehavioral studies in children at risk for developmental disorders. Toxicol Lett. 2003;140–141:171–181. doi: 10.1016/s0378-4274(02)00505-2. [DOI] [PubMed] [Google Scholar]
- 8.Hunt RW, Tzioumi D, Collins E, Jeffery HE. Adverse neurodevelopmental outcome of infants exposed to opiate in-utero. Early Hum Dev. 2008;84(1):29–35. doi: 10.1016/j.earlhumdev.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Oei JL, Melhuish E, Uebel H, Azzam N, Breen C, Burns L, et al. Neonatal Abstinence Syndrome and High School Performance. Pediatrics. 2017;139(2) doi: 10.1542/peds.2016-2651. [DOI] [PubMed] [Google Scholar]
- 10.Baldacchino A, Arbuckle K, Petrie DJ, McCowan C. Neurobehavioral consequences of chronic intrauterine opioid exposure in infants and preschool children: a systematic review and meta-analysis. BMC Psychiatry. 2014;14:104. doi: 10.1186/1471-244X-14-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall ES, Isemann BT, Wexelblatt SL, Meinzen-Derr J, Wiles JR, Harvey S, et al. A Cohort Comparison of Buprenorphine versus Methadone Treatment for Neonatal Abstinence Syndrome. J Pediatr. 2016;170:39–44.e31. doi: 10.1016/j.jpeds.2015.11.039. [DOI] [PubMed] [Google Scholar]
- 12.Friedman DS, Repka MX, Katz J, Giordano L, Ibironke J, Hawse P, et al. Prevalence of amblyopia and strabismus in white and African American children aged 6 through 71 months the Baltimore Pediatric Eye Disease Study. Ophthalmology. 2009;116(11):2128–2134. e2121–2122. doi: 10.1016/j.ophtha.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sundelin Wahlsten V, Sarman I. Neurobehavioural development of preschool-age children born to addicted mothers given opiate maintenance treatment with buprenorphine during pregnancy. Acta Paediatr. 2013;102(5):544–549. doi: 10.1111/apa.12210. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez ES, Bigbee JW, Fobbs W, Robinson SE, Sato-Bigbee C. Opioid addiction and pregnancy: perinatal exposure to buprenorphine affects myelination in the developing brain. Glia. 2008;56(9):1017–1027. doi: 10.1002/glia.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vestal-Laborde AA, Eschenroeder AC, Bigbee JW, Robinson SE, Sato-Bigbee C. The opioid system and brain development: effects of methadone on the oligodendrocyte lineage and the early stages of myelination. Dev Neurosci. 2014;36(5):409–421. doi: 10.1159/000365074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson SE, Maher JR, Wallace MJ, Kunko PM. Perinatal methadone exposure affects dopamine, norepinephrine, and serotonin in the weanling rat. Neurotoxicol Teratol. 1997;19(4):295–303. doi: 10.1016/s0892-0362(97)00018-4. [DOI] [PubMed] [Google Scholar]
- 17.Robinson SE. Effect of prenatal opioid exposure on cholinergic development. J Biomed Sci. 2000;7(3):253–257. doi: 10.1007/BF02255474. [DOI] [PubMed] [Google Scholar]
- 18.Luine V, Gomez J, Beck K, Bowman R. Sex differences in chronic stress effects on cognition in rodents. Pharmacol Biochem Behav. 2017;152:13–19. doi: 10.1016/j.pbb.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samplin E, Ikuta T, Malhotra AK, Szeszko PR, Derosse P. Sex differences in resilience to childhood maltreatment: effects of trauma history on hippocampal volume, general cognition and subclinical psychosis in healthy adults. J Psychiatr Res. 2013;47(9):1174–1179. doi: 10.1016/j.jpsychires.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bale TL, Epperson CN. Sex differences and stress across the lifespan. Nat Neurosci. 2015;18(10):1413–1420. doi: 10.1038/nn.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta M, Mulvihill AO, Lascaratos G, Fleck BW, George ND. Nystagmus and reduced visual acuity secondary to drug exposure in utero: long-term follow-up. J Pediatr Ophthalmol Strabismus. 2012;49(1):58–63. doi: 10.3928/01913913-20110308-01. [DOI] [PubMed] [Google Scholar]
- 22.Gill AC, Oei J, Lewis NL, Younan N, Kennedy I, Lui K. Strabismus in infants of opiate-dependent mothers. Acta Paediatr. 2003;92(3):379–385. [PubMed] [Google Scholar]
- 23.Song T, Li G, Liang Z, Tang Y, Yang Y, Xia J, et al. Chronic morphine exposure affects contrast response functions of V1 neurons in cats. Neuroscience. 2012;226:451–458. doi: 10.1016/j.neuroscience.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 24.Mei B, Niu L, Cao B, Huang D, Zhou Y. Prenatal morphine exposure alters the layer II/III pyramidal neurons morphology in lateral secondary visual cortex of juvenile rats. Synapse. 2009;63(12):1154–1161. doi: 10.1002/syn.20694. [DOI] [PubMed] [Google Scholar]


