Abstract
Rhythm is an important aspect of both human speech and birdsong. Adult zebra finches show increased neural activity following exposure to arrhythmic compared to rhythmic song in regions similar to the mammalian auditory association cortex and amygdala. This pattern may indicate that birds are detecting errors in the arrhythmic song relative to their learned song template or to more general expectations of song structure. Here we exposed juvenile zebra finches to natural conspecific song (rhythmic) or song with altered inter-syllable intervals (arrhythmic) prior to or during template formation, or afterward as males are matching vocal production to a memorized song template (sensorimotor integration). Before template formation, expression of the immediate early gene ZENK was increased in the caudomedial nidopallium (NCM) of birds exposed to rhythmic relative to arrhythmic song. During template formation, ZENK expression was increased in the caudomedial mesopallium (CMM) of birds exposed to arrhythmic relative to rhythmic song. These results suggest that the youngest birds may be predisposed to respond to a more natural stimulus, and a template may be required for arrhythmic song to elicit increased neural activity. Compared to data from adults, it also appears that functional development across the brain regions investigated continues to maturity.
1. Introduction
Evidence is increasing for an association between rhythm skills and language development. For example, in typical speakers, there is a positive relation between language and literacy skills and the ability to analyze beat-based rhythmic sequences (Grube, Cooper, and Griffiths 2013). Conversely, children with specific language impairment have difficulties processing and synchronizing tapping with a rhythmic stimulus (Corriveau, Pasquini, and Goswami 2007; Corriveau and Goswami 2009). People who stutter appear to have deficits in the internal generation of rhythm, and both adults and children who stutter can dramatically reduce the rate of disfluencies when they synchronize their speech with an external rhythmic stimulus such as a metronome (Hanna and Morris 1977; Greenberg 1970) or music (Johnson and Rosen 1937). Children who stutter have a reduced ability to discriminate complex rhythms as compared to their age-matched peers (Wieland et al. 2015). Thus, the development of rhythmic processing abilities is important for typical speech and language development. A deeper understanding of how rhythm is processed in the brains of individuals at all stages of development should aid in understanding a range of developmental speech and language disorders, as well as other conditions that involve deficits in rhythm processing such as Parkinson’s disease (Grahn and Brett 2009).
An appropriate animal model allows for levels of investigation not possible in human subjects, and songbirds such as zebra finches can be particularly useful for the study of vocal development, reviewed in (Doupe and Kuhl 1999). For example, in both species, vocalizations of adult tutors are memorized to form a template and then juveniles practice vocalizations to make modifications that increasingly match the template. This process includes babbling in humans as well as subsong and plastic song in birds such as zebra finches. Ultimately this process results in adult speech in humans and crystalized song in zebra finches. Vocal learning is limited to a critical period in both species.
It has been theorized that vocal learning is a necessary factor for the capacity to perceive and entrain to rhythmic auditory stimuli (Patel 2006). A broad range of species have been identified as having this capacity, including elephants (Poole et al. 2005; Stoeger and Manger 2014), cetaceans (Janik 2014), bats (Esser 1994; Prat, Taub, and Yovel 2015), parrots (Pepperberg 2010), hummingbirds, and songbirds (Marler and Tamura 1964; Bolhuis, Okanoya, and Scharff 2010; Doupe and Kuhl 1999). Recent studies have indicated that at least one species that does not learn vocalizations has the capacity to entrain to a beat (Cook et al. 2013; Wilson and Cook 2016). While the extent to which vocal learning is required for rhythmic entrainment is still unclear (Ten Cate et al. 2016), a far greater number of vocal-learning species than non-vocal learners have demonstrated a capacity to entrain to a beat (Schachner et al. 2009). As one of the few of these species amenable to breeding in laboratory conditions, the zebra finch is a good model to study the juvenile development of rhythm perception. Their songs, which are used for nest site defense and courtship, are highly rhythmic (Norton and Scharff 2016; Zann 1996). A zebra finch song bout consists of a sequence of repetitive introductory notes, followed by repetitions of an ordered set of syllables called a motif. The intervals between notes in these songs are very regular (Figure 1). The natural rhythmic structure of zebra finch song adds to their value as a model species to improve our understanding of how rhythm perception develops in a vocal-learning species.
Figure 1.
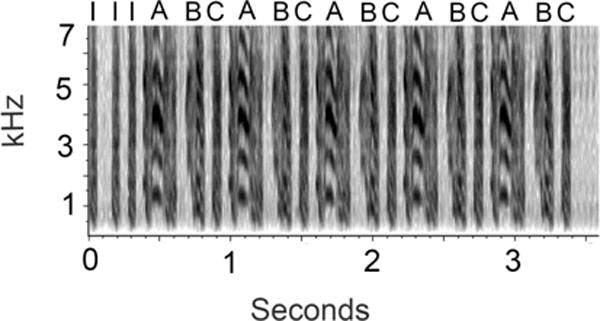
A natural zebra finch song used to generate one of the stimuli from the present study is depicted. Introductory notes are labeled with I. A, B, and C labels indicate separate syllables in the song, with each repetition of the three syllables constituting a motif. Modified from Figure 1 in (Lampen et al. 2014).
Whether zebra finches and other birds are capable of perceiving higher order rhythms or are simply focused on local timing elements is an issue still under debate (Benichov, Globerson, and Tchernichovski 2016; Ten Cate et al. 2016). In a go/no go paradigm where birds were asked to discriminate between regular and irregular beat patterns, zebra finches seemed to default to making decisions based on local temporal structure, such as duration of notes or inter-onset intervals, but also appeared capable of detecting broader temporal structure, encompassing multiple shorter intervals (Ten Cate et al. 2016). A similar study judged birds’ capacity to discriminate between isochronous and irregular tone sequences (van der Aa, Honing, and ten Cate 2015). While zebra finches could discriminate between the training stimuli, they were not able to generalize to isochronous rhythms at different tempi, which was interpreted to mean that they were only able to attend to local timing features (van der Aa, Honing, and ten Cate 2015). In addition, both of these studies utilize stimuli composed of pure tones to assess rhythmic discrimination capabilities in zebra finches. NCM and CMM do not respond as strongly to tones as conspecific vocalizations (Stripling, Kruse, and Clayton 2001; Mello, Vicario, and Clayton 1992; Bailey, Rosebush, and Wade 2002; Bailey and Wade 2003, 2006), thus the capacity to process global rhythms may be different if the sound pattern is composed of natural zebra finch sounds which induce more activity in auditory processing areas of the brain. The overall capacity of zebra finches to attend to different levels of timing and rhythm requires further investigation. We used the immediate early gene ZENK with relatively natural song stimuli to assess differences in neural responses to ecologically relevant rhythmic and arrhythmic stimuli.
Expression of this gene and/or its protein product is frequently used to study neural activity in zebra finches. ZENK is an acronym for the multiple names that have been assigned to this evolutionarily conserved protein, zif-268 (Christy, Lau, and Nathans 1988), egr-1 (Sukhatme et al. 1988), NGFI-A (Milbrandt 1987), and Krox-24 (Lemaire et al. 1988). ZENK is involved in learning and synaptic plasticity (Mello, Velho, and Pinaud 2004), and can act through regulation of other genes through a DNA binding site (Christy and Nathans 1989). Inhibition of ZENK expression in juvenile zebra finches during tutor song exposure prevents normal song learning (London and Clayton 2008).
A previous study in our lab focused on rhythm effects on ZENK expression in the adult zebra finch brain (Lampen et al. 2014). ZENK expression was assessed in the caudomedial nidopallium (NCM), the caudomedial mesopallium (CMM), which while anatomically distinct in the zebra finch brain, are both considered analogous to the auditory association cortex in humans (Bolhuis and Gahr 2006), and nucleus taeniae (Tn) which is analogous to the mammalian amygdala (Riters et al. 2004). NCM is likely the location where the learned song template is stored in the brain (London and Clayton 2008; Gobes, Zandbergen, and Bolhuis 2010; Yanagihara and Yazaki-Sugiyama 2016). We hypothesize that this template may contain information about the proper timing of songs, allowing for discrimination of timing and rhythmicity. In our study on adults (Lampen et al. 2014), birds exposed to song that was modified to disrupt its natural rhythmic structure had significantly more ZENK expression in all three brain regions compared to birds exposed to song with the same syllables presented with the original (unmodified) rhythm. These different levels of activity in secondary auditory areas may suggest that birds perceive errors in the arrhythmic song relative to the learned template. The increased activity in Tn, which is involved in pair bonding and mate selection (Riters et al. 2004; Dios et al. 2013; Svec, Licht, and Wade 2009), may indicate an aversion to the disrupted rhythm and an assessment of poor quality as a potential mate.
The general timeline of vocal development in zebra finches is agreed upon, but the exact ages at which specific milestones occur is still debated to some degree (Doupe 1993; Doupe and Solis 1997; Mooney 2009; Tomaszycki et al. 2009). Template formation occurs between approximately post-hatching days 20 and 60 (Tomaszycki et al. 2009; Mooney 2009; Doupe and Solis 1997). In males, vocalizations begin as food begging, then develop into subsong, an immature form of vocalization that is quiet and contains poorly formed notes with greatly variable structure and sequence (Zann 1996). As the males mature, these songs are practiced and modified to relatively closely match the learned template. This phase of sensorimotor integration occurs between about post hatching day 25 and sexual maturity which occurs around 90 days of age (Doupe and Solis 1997; Mooney 2009; Tomaszycki et al. 2009). After sensorimotor integration, males have a crystalized song that will not undergo significant changes throughout the rest of their lives under normal circumstances (Doupe 1993). In addition to learning to produce specific song syllables, juvenile males also learn a specific grammar to structure their song (Menyhart et al. 2015).
Understanding the developmental trajectory of neural responses to rhythm is useful in elucidating their relationship to function. We exposed birds to rhythmic or arrhythmic song prior to and during the template formation period, as well as during early sensorimotor integration. Differences in neural activity following stimulation with the two types of songs prior to acquisition of a template could indicate an innate capacity to perceive song-related rhythms. Discrimination during and after template formation, but not before, would suggest that characteristics of rhythm are learned. If rhythm discrimination emerges during sensorimotor integration in males, it would suggest that a motor component is required for distinct neural responses to rhythmicity. Differences between the sexes may be informative here as well, as both males and females appear to form templates (Lauay et al. 2004), even though only males engage in the production of song (Zann 1996).
2. Material and methods
2.1 Subjects
Zebra finches hatched and were reared in large, walk-in aviaries containing 5–7 adult male and female pairs and their offspring. On day of hatching, toes were clipped as a means of unique identification. Tissue from the toes was used to identify the sex of the birds through polymerase chain reaction (Agate, Perlman, and Arnold 2002). A total of 108 birds were used (18 males and 18 females at 15–17 days post hatching (d15), 25–27 days post hatching (d25), and 45–47 days post hatching (d45)). All animals were maintained on at 12:12 hour light/dark cycle and had ad libitum access to seed (Kaytee Finch Feed; Chilton, WI, USA), water, cuttle bone and gravel. Their diet was supplemented weekly with hard boiled chicken eggs, bread, spinach, and oranges. All procedures were approved by the Institutional Animal Care and Use Committee of Michigan State University.
2.2 Stimulus Exposure
Rhythmic and arrhythmic zebra finch song stimuli were used from our study on adults (Lampen et al. 2014). Details regarding their creation are reported in that paper. Briefly, rhythmic song stimuli were generated from recordings of natural zebra finch song, and arrhythmic stimuli used the same recordings with the silent intervals between notes altered to disrupt the temporal pattern of the song. Each interval was randomly changed to one of three durations: 1) 10 ms, 2) the average duration (based on all intervals in a song except those between introductory notes), or 3) double the average duration, minus 10 ms. Because the intervals added between notes were silence rather than background sound as present in the rhythmic song recordings, it is possible that the difference in contrast contributed to the perceptual distinction between stimulus types. Three different stimuli of each type were created, each of which contained three of nine different songs that were generated (rhythmic stimuli depicted in Supplemental Figure 1, arrhythmic stimuli depicted in Supplemental Figure 2). Each song was presented for 30 seconds followed by 30 seconds of silence, with the three songs repeating in random order for 30 minutes. Audio files of example rhythmic and arrhythmic songs are available as supplementary materials. All songs of each type were novel to all subjects.
Nine males and 9 females of each age group were exposed to each stimulus type (rhythmic or arrhythmic). One bird was exposed at a time between 9am and 3pm. Birds were placed into a sound isolation chamber (252-Mini Sound Shelter, IAC Acoustics, Bronx, New York, USA) to habituate for one hour. Following the habituation period, the stimulus was presented at approximately 70dB. Birds remained in the chamber for one hour following song exposure to allow for peak ZENK protein expression. Brains were then immediately collected and flash frozen in methylbutane.
All song exposures were recorded using a Canon Vixia HF R300 camcorder. Recordings were reviewed to ensure birds were not producing subsong and no extraneous background noise existed that might influence the results.
2.3 Tissue Processing
Brains were sectioned coronally at 20μm and collected in 6 series. Sections were thaw mounted onto SuperFrost Plus slides (Fisher Scientific, Hampton, NH). One set of tissue from each bird was thionin stained to allow for clear identification of anatomy.
An alternate set of tissue from each bird was processed for immunohistochemistry for ZENK. Tissue was processed in five groups due to the large number of slides. All experimental groups were evenly represented in all immunohistochemistry runs. The protocol was conducted as described in (Lampen et al. 2014). Briefly, slides were warmed to room temperature, fixed 4% paraformaldehyde, and rinsed in 0.9% hydrogen peroxide in methanol. Slides were then incubated in 5% normal goat serum in 0.1M phosphate buffered saline (PBS) with 0.3% Triton X-100. Slides were incubated overnight at 4°C in ZENK (Egr-1) rabbit polyclonal antibody (0.5 μg/ml, sc-189, Santa Cruz Biotechnology, Inc., Dallas, TX) in 5% normal goat serum in PBS with 0.3% Triton X-100. The next day slides were incubated in biotin conjugated goat anti-rabbit polyclonal antibody (0.5 mg/ml; Vector Labs, Burlingame, CA) in PBS with 0.3% Triton X-100, followed by Elite ABC reagents (Vector Labs, Burlingame, CA), and then treated with diaminobenzidine with 0.003% H2O2 to produce a color reaction.
An investigator blind to the stimulus exposure condition, sex, and age of the birds conducted analysis of all slides. Images of all areas of interest were captured using a 10X objective on an Olympus BX60 microscope (Olympus, Center Valley, PA), a MicroPublisher 5.0 RTV camera (QImaging, Surry, BC, Canada) and ImageJ software (National Institutes of Health, Bethesda MD). All regions of interest were analyzed bilaterally in two adjacent sections. Boxes were placed within NCM, CMM and Tn as described and depicted in (Lampen et al. 2014), and all cells containing a dark brown stained nucleus that was clearly distinguishable from the surrounding tissue were counted. For NCM, a 0.525 mm*0.393 mm box was placed with the medial corner under the hippocampus at the point where the ventricle begins to curve ventrally to run parallel with the midline. For CMM, a 0.496 mm*0.205 mm box was placed the ventricle just lateral to where it curves ventrally toward the midline between A 1.6 and A 1.2 from a songbird brain atlas (Stokes, Leonard, and Nottebohm 1974). For Tn, a 0.238 mm*0.244 mm box was placed near the ventral edge of the telencephalic lobe where a corner is formed by the ventral and medal edges of the lobe. Images depicting the location within each brain region where ZENK was analyzed were previously published (Lampen et al. 2014). The density of ZENK expressing cells (labeled nuclei per unit area) was calculated for each brain region. Due to high levels of baseline labeling apparent in these juvenile brains, values were also assessed in the control region nucleus rotundus (Rt), a thalamic visual area that should show no ZENK induction specifically from the auditory stimuli. A 0.268mm*0.268mm box was placed in the center of the region bilaterally in two adjacent sections and density of ZENK expressing cells was calculated. For each animal, average density values were calculated for each region of interest, collapsing across the hemispheres.
In order to confirm consistency of analysis by the investigator, the density of ZENK expression was assessed in replicates of a subset of the images in order to enable calculation of a coefficient of variance. For each combination of age, sex and rhythm condition, one image of NCM was randomly selected and copied in triplicate. The images were coded so the investigator was blind to the identity of the images (both the group each represented and which were replicates). The average coefficient of variance across replicates was 8.05%.
2.4 Data analysis
The brains in the final run of immunohistochemistry were excluded from analysis due to unusually high levels of background staining unique to this set of tissue, reducing the total number of animals in each group by one. Occasionally individual brain regions were not able to be analyzed in particular birds due to damage to the tissue sections. Final sample sizes are indicated in Table 1. Compared to our study in adults, constitutive levels of ZENK expression were increased across brain region and ages. Therefore, labeling in Rt was used as a covariate (Supplemental Figure 3). The density of ZENK+ cells in Rt correlated significantly with the values obtained from NCM, CMM, and Tn (all r >0.642, p<0.001). Separate three-way ANCOVAs were run for NCM, CMM, and Tn, with age, sex, and stimulus type (rhythmicity) as factors. To provide pairwise comparisons probing main effects of age detected in NCM and CMM, separate one-way ANCOVAs, with rhythmicity as the factor, were conducted within each region. To investigate interactions of age and stimulus type detected in NCM and CMM, individual one-way ANCOVAs were conducted within each age group, as well as across all ages within each rhythm condition, with further one-way ANCOVAs used to provide pairwise comparisons between each combination of two ages within a rhythm condition. For all sets of pairwise comparisons, a Holm’s Bonferroni correction was used (Holm 1979), with adjusted α-levels indicated with each result. The Holm’s correction provides α-values at which to assess the significance of multiple comparisons, with the most significant result analyzed at the most strict α-value of 0.05/total number of comparisons, as in a traditional Bonferroni correction, and further comparisons assessed in rank order of their significance, reducing the value of the denominator in the α equation by one with each comparison. All statistics were calculated using SPSS (IBM, Armonk, NY).
Table 1.
Unadjusted ZENK+ cells/mm2 means and SEM for the caudomedial nidopallium (NCM), the caudomedial mesopallium (CMM), and nucleus taeniae (Tn). Values within each cell indicate the mean with the standard error in parentheses and n on the row below.
| d15 | d25 | d45 | |||||
|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | ||
| NCM | Rhythmic | 671.8 (92.3) 9 |
791.7 (55.2) 9 |
387.6 (104.3) 9 |
334.6 (128.7) 9 |
430.6 (153.3) 8 |
273.2 (83.1) 9 |
| Arrhythmic | 630.0(131.7) 9 |
629.5 (109.6) 9 |
481.7 (117.0) 8 |
394.9 (86.5) 8 |
351.6 (107.3) 9 |
352.8 (117.4) 9 |
|
| CMM | Rhythmic | 1063.6 (132.6) 9 |
944.4 (128.8) 9 |
560.1 (143.0) 9 |
485.0 (150.5) 9 |
430.0 (151.7) 9 |
428.9 (147.4) 9 |
| Arrhythmic | 973.9 (158.0) 9 |
1020.3 (180.9) 9 |
561.3 (77.8) 9 |
765.8 (147.2) 9 |
538.4 (144.4) 9 |
513.9 (158.8) 9 |
|
| Tn | Rhythmic | 840.4 (158.1) 9 |
864.8 (84.8) 9 |
592.0(126.1) 9 |
532.9 (123.0) 9 |
558.7 (163.4) 8 |
535.6 (170.3) 9 |
| Arrhythmic | 749.4 (169.6) 9 |
952.1 (168.0) 9 |
681.6 (112.2) 8 |
641.2 (99.0) 8 |
421.1 (97.9) 9 |
586.2 (126.0) 9 |
|
3. Results
No main effects of sex were detected in NCM (F1,79=1.079, p=0.302), CMM (F1,82=0.288, p=0.593), or Tn (F1,79=0.341, p=0.561). However, in NCM, there was a main effect of age (F2,85=3.388, p=0.039; Figure 2). D15 birds had a higher density of ZENK expressing cells than those at d25 (F1,58=8.415, p=0.005, α=0.0167), and a trend existed for greater ZENK expression at d15 compared to d45 (F1,60=4.413, p=0.040, α=0.025). ZENK expression did not differ significantly between d25 and d45 (F1,57=0.960, p=0.331, α=0.050). While a main effect of rhythm condition did not exist (F1,85=0.458, p=0.501), age and stimulus type interacted (F2,85=3.189 p=0.047; Figure 2). At d15, a trend toward a greater density of ZENK+ cells was detected following exposure to rhythmic compared to arrhythmic song (F1,29=6.038, p=0.020, α=0.0167). However, no effect of the stimulus type was detected at d25 (F1,29=1.383, p=0.250, α=0.025) or d45 (F1,29=0.300, p=0.865, α=0.050). In addition, there was an effect of age within birds exposed to rhythmic (F2,42=6.983, p=0.002, α=0.025), but not arrhythmic (F2,42=0.057, p=0.945, α=.050) song. In birds that heard the rhythmic song, a greater density of ZENK expressing cells was observed at d15 compared to both d25 (F1,28=14.023, p=0.001, α=0.0167) and d45 (F1,28=5.784, p=0.023, α=0.025), and in animals at d45 compared to d25 (F1,27=4.817, p=0.037, α=.050).
Figure 2.
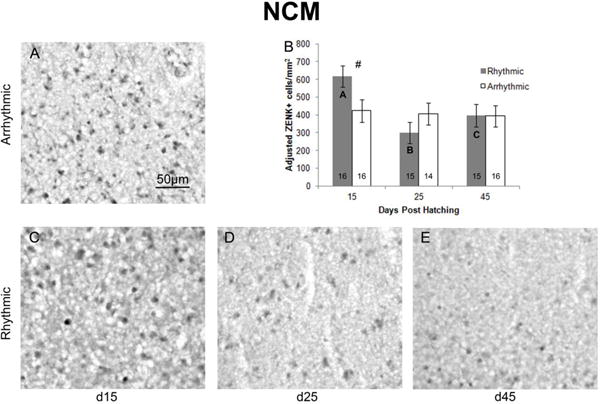
Density of ZENK expressing cells in NCM. The photographs depict representative samples of ZENK expression in birds at A) d15 exposed to arrhythmic song, and birds exposed to rhythmic song at C) d15, D) d25, and E) d45. All photographs are of male brains, but are representative of both sexes, as no sex differences were detected. Panel B depicts the density of ZENK expressing cells across ages and rhythmic conditions. Marginal means from the overall ANCOVA were used to graph adjusted means ± SEM. Group sizes are listed within the bars. Data are collapsed across sexes, as no significant effects were detected. A main effect of age was detected, with greater density of ZENK expressing cells at d15 compared to d25 and a trend for greater expression at d15 compared to d45. Age and stimulus type (rhythmicity) also interacted. Bold letters within the bars indicate significant differences between all age groups of rhythmic song exposed birds, with the greatest density detected in d15 birds, and the least in d25. # = Trend toward an increase in ZENK expression following exposure to rhythmic song at d15.
Similarly, a main effect of age was detected in CMM (F2,82=8.446, p<0.001; Figure 3). D15 birds had greater ZENK expression than those at d25 (F1,60=11.900, p=0.001, α=0.025) and d45 (F1,61=13.805, p<0.001, α=0.0167), whereas no difference was detected between birds at d25 and d45 (F1,60=0.544, p=0.464, α=0.050). A main effect of rhythm condition was not seen (F1,82=0.470, p=0.495), but age and stimulus type interacted (F2,82= 3.485, p=0.035; Figure 3). At d25, a greater density of ZENK expressing cells was detected in birds that heard arrhythmic compared to rhythmic song (F1,28=7.061, p=0.013, α=0.0167). However, there was no effect of rhythm condition at d15 (F1,29=1.671, p=0.206, α=0.025) or d45 (F1,29=0.001, p=0.973, α=0.050). In addition, there was an effect of age in birds exposed to both rhythmic (F2,43=7.788, p=0.001, α=0.025) and arrhythmic (F2,44=3.397, p=0.042, α=0.050) song. In those hearing rhythmic song, an increased density of ZENK+ cells was observed in d15 compared to d25 (F1,28=17.591, p<0.001, α=0.0167), but no differences were seen between d15 and d45 (F1,29=2.928, p=0.098, α=0.050), or in animals at d25 compared to d45 (F1,28=3.678, p=0.065, α=0.025). Within the birds exposed to arrhythmic song, a trend was detected toward greater ZENK expression in birds at d15 compared to d45 (F1,29=6.016, p=0.020, α=0.0167), but no differences between d15 and d25 (F1,29=0.787, p=0.382, α=0.050) or d25 and d45 (F1,29=3.673, p=0.065, α=0.025) were seen.
Figure 3.
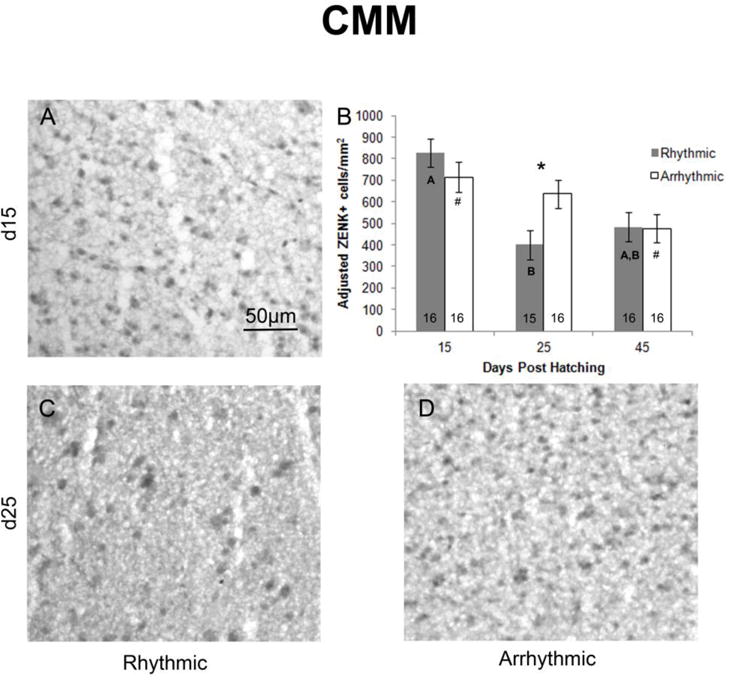
Density of ZENK expressing cells in CMM. Photographs depict representative samples of ZENK expression in birds exposed to rhythmic song at A) d15, and C) d25, and D) a bird exposed to arrhythmic song at d25. All photographs are of male brains, but are representative of both sexes, as no sex differences were detected. Panel B indicates the density of ZENK expressing cells across ages and rhythmic conditions. Marginal means from the overall ANCOVA were used to graph adjusted means ± SEM. Group sizes are listed within the bars. Data are collapsed across sexes, as no significant effects were seen. A main effect of age was detected, such that a greater density of ZENK expressing cells was present a d15 compared to both d25 and d45. Effects of age and stimulus type (rhythmicity) also interacted. Bold letters within the bars indicate significant differences between ages of rhythmic song exposed birds, with greater ZENK expression in d15 compared to d25, but no differences between d45 and either of the other ages. # = a trend for an increase at d15 compared to d45 within birds exposed to arrhythmic stimuli. * = Significant increase in ZENK expression following exposure to arrhythmic compared to rhythmic song at d25.
Main effects of rhythm and age were not detected in Tn (F1,79=0.021, p=0.884; F2,79=0.992, p=0.376 respectively), and no age by rhythm interaction existed (F2,79=1.617, p=0.205) (Table 1).
4. Discussion
4.1 Summary of Specific Effects
Expression of the immediate early gene ZENK in across the two sexes was affected by developmental stage and rhythmicity of song stimuli in two auditory regions of the zebra finch brain, NCM and CMM. In contrast, neither variable influenced the density of ZENK expressing cells in Tn, a homologue of the mammalian amygdala. Several results were parallel across the two auditory regions. For example, expression was greater at d15 compared to d25 in both NCM and CMM and at d15 compared to d45 in CMM; a trend for this age difference also existed in NCM. Similarly, within birds exposed to rhythmic stimuli, increased ZENK in both regions was detected at d15 compared to d25, effects that were not detected in either area in birds exposed to the arrhythmic songs. The magnitude of other effects differed between the NCM and CMM, although the patterns were quite similar across the two regions (compare figures 2B and 3B). Specifically, in NCM only, the density of ZENK+ cells in birds exposed to rhythmic song was greater at d15 than d45 and at d45 than d25, whereas in CMM ZENK was only increased in d15 compared to d25 in birds that heard the rhythmic stimuli. While significant differences between pairs of ages were not detected in either cortical region, a trend was detected for an increase at d15 compared to d45 in birds exposed to the arrhythmic stimulus. Finally, effects of stimulus type within specific ages differed across the two regions, such that at d25 arrhythmic song resulted in greater ZENK expression in CMM but not NCM, and a trend for an increase following rhythmic song existed at d15 in NCM but not CMM.
The increased ZENK expression generally detected in the youngest birds in this study parallels results from work documenting relatively high levels of constitutive expression of this protein at post-hatching day 20 in both NCM (Jin and Clayton 1997; Stripling, Kruse, and Clayton 2001) and the broader auditory lobule (London et al. 2009). These earlier studies suggested that ZENK was not inducible with conspecific song in these regions. The present work cannot address this issue specifically, but using expression in Rt as a covariate suggests that ZENK is induced in NCM in response to typical song at d15 and to a greater degree than at later developmental stages. The lack of age and rhythm effects in Tn indicate that the effects detected in NCM and CMM are specific to these auditory areas, and not indications of global increases in activity or constitutive high ZENK expression in the early juvenile period.
While it remains possible that differences in baseline ZENK expression among the regions of interest contributed to the effects of age we detected, and this issue could be investigated directly in future studies, it seems unlikely because in both NCM and CMM, the overall effect of age appears to be driven by data from birds that heard rhythmic song. This suggests that developmental changes in auditory processing occur primarily in natural stimuli. The substantial reduction between d15 and d25 in ZENK expression following rhythmic song in both regions could be related to the birds’ focus on memorizing song from tutors at the later age rather than reacting to the novel vocalizations presented in the current study. ZENK expression is associated with synaptic plasticity and learning (Mello, Velho, and Pinaud 2004), and during template formation substantial synaptic changes would be required for acquisition of the tutor song memory.
4.2 NCM
The trend we detected toward greater ZENK expression following rhythmic compared to arrhythmic song in NCM is consistent with other data collected during song development suggesting an innate preference toward more natural, species typical sounds. For example, during the critical period for template memorization, birds reared in isolation show a behavioral preference for conspecific over heterospecific song by landing more frequently on a perch that elicits playback of conspecific song (Braaten and Reynolds 1999). Additionally, juvenile zebra finches that have not been exposed to adult song prefer songs with more common elements, as shown by time spent near a speaker (ter Haar et al. 2014). Similar to the results of the present study, in the primary auditory area, field L, a group of neurons that code for the silent intervals between syllables seems to respond selectively to zebra finch songs, even in birds that have been cross fostered by Bengalese finches or raised in isolation from their fathers (Araki, Bandi, and Yazaki-Sugiyama 2016). Behavioral drift toward natural wild-type zebra finch song can be seen in colonies tutored by birds reared in isolation, again indicating a natural tendency toward the most species typical sounds (Feher et al. 2009).
The pattern of ZENK expression following exposure to the same auditory stimuli used in the present study differed in the NCM of adult zebra finches. In the older birds, greater ZENK expression was detected in individuals hearing arrhythmic compared to rhythmic song (Lampen et al. 2014). Such a difference across ages is not surprising, as substantial development occurs within NCM in the late juvenile period. For example, estrogens synthesized within NCM become sexually dimorphic between 46 and 80 days post hatching, with greater estrogen levels present in males compared to females (Chao, Paon, and Remage-Healey 2015). Estradiol facilitates improved discrimination of auditory stimuli within NCM (Remage-Healey et al. 2010; Remage-Healey et al. 2012; Maney, Cho, and Goode 2006; Maney et al. 2008). While effects of this hormone on rhythm perception in songbirds are unknown, it is possible that adult responses may require levels of estradiol availability not present in the juvenile birds in the present study.
It is also possible that relative levels of habituation influenced the ZENK responses in juveniles and adults. Faster habituation of electrophysiological responses to songs is seen in NCM in 35 day-old compared to adult zebra finches (Miller-Sims and Bottjer 2014). Reduction of the ZENK response following repeated presentations of auditory stimuli also occurs in adult zebra finches (Mello, Nottebohm, and Clayton 1995). While the ZENK expression in juvenile birds exposed to repeated presentations of the same stimulus has not yet been assessed, it is possible that the pattern of increased habituation of neural responses during the juvenile period extends to ZENK expression. More rapid habituation of neural responses to auditory stimuli, including those in the present study, may result in reduced ZENK expression within the juvenile NCM relative to its potential peak level, which could result in diminished ability to detect differences in ZENK expression to the different stimuli.
A third possible explanation for the different pattern seen between d15 and adult birds (Lampen et al. 2014) relates to the phenotype of the cells. Many GABAergic neurons are present in NCM, and some of them express ZENK in response to song in adult birds (Pinaud et al. 2004; Pinaud et al. 2008). The GABAergic neurons in NCM are involved in maintaining the selectivity tutor song responsive neurons, and in the late juvenile period the inhibition of GABAergic cells can allow tutor song selective neurons to fire in response to a broader range of song stimuli (Yanagihara and Yazaki-Sugiyama 2016). In zebra finches 51–83 days post-hatching, injection of GABA agonists into the auditory nucleus NIf is sufficient to disrupt the rhythm and stereotypy of plastic song (Naie and Hahnloser 2011). Together these results indicate that by late in juvenile development GABAergic neurons are active within the auditory system and are important for song control and specificity of auditory responses. It is possible that in the present study the birds were too young for the inhibitory network of GABAergic neurons to be sufficiently developed to facilitate auditory discrimination.
4.3 CMM
The pattern of ZENK expression at d25 in CMM was the same as we detected in adults, with arrhythmic song producing an increase compared to rhythmic song (Lampen et al. 2014). That this pattern was detected at d25 during template formation as well as in adulthood, but not at d45 during sensorimotor integration in males, leads to a challenge for interpretation. One possibility is that birds are focused around d25 on accurately perceiving external stimuli for creation of the highest fidelity match and this focus on auditory perception is important again in adulthood when the quality of a song is important for mate attraction and nest site defense. In contrast, during sensorimotor integration around d45, male zebra finches are more focused on the motor task of matching their own vocalizations to a preexisting template. The zebra finch brain may have a greater capacity to discriminate auditory stimuli during times when primary attention toward auditory signals is ecologically relevant, and both sexes may show a reduced capacity to discriminate rhythms at ages important for sensorimotor integration in males. If this explanation is valid, it is unclear why the CMM of females would not exhibit differential responses to the two song types at d45, as they do not practice vocalizations. One possibility is that while males at this age are focused on sensorimotor integration, females may undergo a different developmental process that may reduce their attention toward auditory discrimination. It is also possible that the developmental trajectories are parallel without obvious functional consequences for females.
Similar to the pattern we saw in CMM across d25 birds in the present experiment and our study on adults (Lampen et al. 2014), electrophysiological responses within NCM differed across a range of song stimuli at d20 and in adulthood, but not at d35 (Miller-Sims and Bottjer 2014). While this effect was found in NCM rather than CMM, it is evidence that response patterns within the brain emerge during early stages of development, regress in later stages and are present again in adulthood.
In the present study, no effect of sex was seen in CMM in juvenile zebra finches which contrasts with adult zebra finches in which ZENK expression was enhanced in females compared to males across the same auditory stimuli (Lampen et al. 2014). The higher ZENK expression in CMM in adult females may be due to involvement of CMM in evaluating the quality of a male’s song for the purpose of mate selection which would not occur prior to sexual maturity.
4.4 Tn
The differences in results in Tn compared to the adult study (Lampen et al. 2014) may also relate to sexual maturity. While no effects of age or rhythm were seen in this region in the present study on juveniles, a greater density of ZENK expressing cells was observed in the Tn of adult birds that heard arrhythmic compared to rhythmic song (Lampen et al. 2014). Populations of cells in this brain region in adult Bengalese finches are also responsive to particular types of vocalizations such as song or calls generated by only males or females (Fujii, Ikebuchi, and Okanoya 2016), suggesting its importance in processing socially relevant stimuli. Tn appears to be involved in mate selection and pair bonding; it responds in multiple avian species to acts of mating or the presence of a partner, and can indicate the strength of a pair bond (Svec, Licht, and Wade 2009; Dios et al. 2013; Riters et al. 2004). As for the other regions in this experiment, it will eventually be important to investigate responses to differences in the rhythmicity of song during the transition into adulthood.
4.5 Conclusions
Overall, the results of the present study, based on relative densities of cells expressing ZENK protein, suggest that the ability to discriminate temporal regularity in auditory stimuli fully develops between the stages of sensorimotor integration and adulthood. Some degree of innate rhythmic discrimination may exist in NCM, but the pattern of activity following rhythmic vs. arrhythmic stimulus exposure is reversed in adults compared to 15 day-old birds (Lampen et al. 2014), leaving the possibility that there is also a learned component, as error detection in arrhythmic song hearing adults could be facilitated by memorization of rhythmic regularity as a component of the song template. In CMM, a learned component to rhythm discrimination is likely since differences in ZENK expression first appear during template formation. The development of rhythm discrimination is a complex process that develops at different rates across these brain regions. As in the present study, most research has focused on zebra finch neural development during the time periods surrounding major milestones in the development of vocal learning. Further work is necessary to understand how the auditory responsive brain nuclei mature during the late juvenile period in order to provide adult responses to varied stimuli.
Supplementary Material
Highlights.
Prior to template formation, NCM ZENK is greater after normal vs arrhythmic song exposure.
ZENK is increased in CMM following arrhythmic song during template memorization.
Greater ZENK expression occurs at d15 than d25 in NCM and CMM.
ZENK expression associated with song rhythmicity continues to mature after d45.
Acknowledgments
The authors thank Levi Storks for analyzing the videos, and Katherine Jones for creating the stimuli used in this experiment. This work was partially funded by National Institutes of Health R01-MH096705 and Michigan State University’s program for Research in Autism, Intellectual and Neurodevelopmental Disabilities (RAIND).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agate RJ, Perlman WR, Arnold AP. Cloning and expression of zebra finch (Taeniopygia guttata) steroidogenic factor 1: overlap with hypothalamic but not with telencephalic aromatase. Biol Reprod. 2002;66:1127–33. doi: 10.1095/biolreprod66.4.1127. [DOI] [PubMed] [Google Scholar]
- Araki M, Bandi M, Yazaki-Sugiyama Y. Mind the gap: Neural coding of species identity in birdsong prosody. Science. 2016;354:1282–1287. doi: 10.1126/science.aah6799. [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Rosebush JC, Wade J. The hippocampus and caudomedial neostriatum show selective responsiveness to conspecific song in the female zebra finch. J Neurobiol. 2002;52:43–51. doi: 10.1002/neu.10070. [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Wade J. Differential expression of the immediate early genes FOS and ZENK following auditory stimulation in the juvenile male and female zebra finch. Brain Res Mol Brain Res. 2003;116:147–54. doi: 10.1016/s0169-328x(03)00288-2. [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Wade J. Sexual dimorphism in song-induced ZENK expression in the medial striatum of juvenile zebra finches. Neurosci Lett. 2006;401:86–91. doi: 10.1016/j.neulet.2006.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benichov JI, Globerson E, Tchernichovski O. Finding the Beat: From Socially Coordinated Vocalizations in Songbirds to Rhythmic Entrainment in Humans. Front Hum Neurosci. 2016;10:255. doi: 10.3389/fnhum.2016.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhuis JJ, Gahr M. Neural mechanisms of birdsong memory. Nat Rev Neurosci. 2006;7:347–57. doi: 10.1038/nrn1904. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Okanoya K, Scharff C. Twitter evolution: converging mechanisms in birdsong and human speech. Nat Rev Neurosci. 2010;11:747–59. doi: 10.1038/nrn2931. [DOI] [PubMed] [Google Scholar]
- Braaten RF, Reynolds K. Auditory preference for conspecific song in isolation-reared zebra finches. Anim Behav. 1999;58:105–111. doi: 10.1006/anbe.1999.1134. [DOI] [PubMed] [Google Scholar]
- Chao A, Paon A, Remage-Healey L. Dynamic variation in forebrain estradiol levels during song learning. Dev Neurobiol. 2015;75:271–86. doi: 10.1002/dneu.22228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy B, Nathans D. DNA binding site of the growth factor-inducible protein Zif268. Proc Natl Acad Sci U S A. 1989;86:8737–41. doi: 10.1073/pnas.86.22.8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy BA, Lau LF, Nathans D. A gene activated in mouse 3T3 cells by serum growth factors encodes a protein with “zinc finger” sequences. Proc Natl Acad Sci U S A. 1988;85:7857–61. doi: 10.1073/pnas.85.21.7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook P, Rouse A, Wilson M, Reichmuth C. A California sea lion (Zalophus californianus) can keep the beat: motor entrainment to rhythmic auditory stimuli in a non vocal mimic. J Comp Psychol. 2013;127:412–27. doi: 10.1037/a0032345. [DOI] [PubMed] [Google Scholar]
- Corriveau K, Pasquini E, Goswami U. Basic auditory processing skills and specific language impairment: a new look at an old hypothesis. J Speech Lang Hear Res. 2007;50:647–66. doi: 10.1044/1092-4388(2007/046). [DOI] [PubMed] [Google Scholar]
- Corriveau KH, Goswami U. Rhythmic motor entrainment in children with speech and language impairments: tapping to the beat. Cortex. 2009;45:119–30. doi: 10.1016/j.cortex.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Dios AM, Alexander K, Hanson SJ, Cheng MF. Specific neural representation for conceptual set of behavior: pair bonding. Research and Reports in Biology. 2013;4:33–38. [Google Scholar]
- Doupe AJ. A neural circuit specialized for vocal learning. Curr Opin Neurobiol. 1993;3:104–11. doi: 10.1016/0959-4388(93)90043-x. [DOI] [PubMed] [Google Scholar]
- Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annu Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- Doupe AJ, Solis MM. Song- and order-selective neurons develop in the songbird anterior forebrain during vocal learning. J Neurobiol. 1997;33:694–709. [PubMed] [Google Scholar]
- Esser KH. Audio-vocal learning in a non-human mammal: the lesser spear-nosed bat Phyllostomus discolor. Neuroreport. 1994;5:1718–20. doi: 10.1097/00001756-199409080-00007. [DOI] [PubMed] [Google Scholar]
- Feher O, Wang H, Saar S, Mitra PP, Tchernichovski O. De novo establishment of wild-type song culture in the zebra finch. Nature. 2009;459:564–8. doi: 10.1038/nature07994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii TG, Ikebuchi M, Okanoya K. Auditory Responses to Vocal Sounds in the Songbird Nucleus Taeniae of the Amygdala and the Adjacent Arcopallium. Brain Behav Evol. 2016;87:275–89. doi: 10.1159/000447233. [DOI] [PubMed] [Google Scholar]
- Gobes SM, Zandbergen MA, Bolhuis JJ. Memory in the making: localized brain activation related to song learning in young songbirds. Proc Biol Sci. 2010;277:3343–51. doi: 10.1098/rspb.2010.0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn JA, Brett M. Impairment of beat-based rhythm discrimination in Parkinson’s disease. Cortex. 2009;45:54–61. doi: 10.1016/j.cortex.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Greenberg JB. The Effect of a Metronome on the Speech of Young Stutterers. Behavior Therapy. 1970;1:240–244. [Google Scholar]
- Grube M, Cooper FE, Griffiths TD. Auditory temporal-regularity processing correlates with language and literacy skill in early adulthood. Cogn Neurosci. 2013;4:225–30. doi: 10.1080/17588928.2013.825236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna R, Morris S. Stuttering, speech rate, and the metronome effect. Perceptual and Motor Skills. 1977;44:452–454. doi: 10.2466/pms.1977.44.2.452. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian journal of statistics. 1979:65–70. [Google Scholar]
- Janik VM. Cetacean vocal learning and communication. Curr Opin Neurobiol. 2014;28:60–5. doi: 10.1016/j.conb.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Jin H, Clayton DF. Localized changes in immediate-early gene regulation during sensory and motor learning in zebra finches. Neuron. 1997;19:1049–59. doi: 10.1016/s0896-6273(00)80396-7. [DOI] [PubMed] [Google Scholar]
- Johnson W, Rosen L. Studies in the Psychology of Stuttering: VII. Effects of Certain Changes in Speech Pattern upon Frequency of Stuttering. Journal of Speech and Hearing Disorders. 1937;2:105–110. [Google Scholar]
- Lampen J, Jones K, McAuley JD, Chang SE, Wade J. Arrhythmic song exposure increases ZENK expression in auditory cortical areas and nucleus taeniae of the adult zebra Finch. PLoS One. 2014;9:e108841. doi: 10.1371/journal.pone.0108841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauay C, Gerlach NM, Adkins-Regan E, Devoogd TJ. Female zebra finches require early song exposure to prefer high-quality song as adults. Animal Behaviour. 2004;68:1249–1255. [Google Scholar]
- Lemaire P, Revelant O, Bravo R, Charnay P. Two mouse genes encoding potential transcription factors with identical DNA-binding domains are activated by growth factors in cultured cells. Proc Natl Acad Sci U S A. 1988;85:4691–5. doi: 10.1073/pnas.85.13.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London SE, Clayton DF. Functional identification of sensory mechanisms required for developmental song learning. Nat Neurosci. 2008;11:579–86. doi: 10.1038/nn.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London SE, Dong S, Replogle K, Clayton DF. Developmental shifts in gene expression in the auditory forebrain during the sensitive period for song learning. Dev Neurobiol. 2009;69:437–50. doi: 10.1002/dneu.20719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney DL, Cho E, Goode CT. Estrogen-dependent selectivity of genomic responses to birdsong. Eur J Neurosci. 2006;23:1523–9. doi: 10.1111/j.1460-9568.2006.04673.x. [DOI] [PubMed] [Google Scholar]
- Maney DL, Goode CT, Lange HS, Sanford SE, Solomon BL. Estradiol modulates neural responses to song in a seasonal songbird. J Comp Neurol. 2008;511:173–86. doi: 10.1002/cne.21830. [DOI] [PubMed] [Google Scholar]
- Marler P, Tamura M. CULTURALLY TRANSMITTED PATTERNS OF VOCAL BEHAVIOR IN SPARROWS. Science. 1964;146:1483–6. doi: 10.1126/science.146.3650.1483. [DOI] [PubMed] [Google Scholar]
- Mello C, Nottebohm F, Clayton D. Repeated exposure to one song leads to a rapid and persistent decline in an immediate early gene’s response to that song in zebra finch telencephalon. J Neurosci. 1995;15:6919–25. doi: 10.1523/JNEUROSCI.15-10-06919.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Velho TA, Pinaud R. Song-induced gene expression: a window on song auditory processing and perception. Ann N Y Acad Sci. 2004;1016:263–81. doi: 10.1196/annals.1298.021. [DOI] [PubMed] [Google Scholar]
- Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc Natl Acad Sci U S A. 1992;89:6818–22. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menyhart O, Kolodny O, Goldstein MH, DeVoogd TJ, Edelman S. Juvenile zebra finches learn the underlying structural regularities of their fathers’ song. Front Psychol. 2015;6:571. doi: 10.3389/fpsyg.2015.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrandt J. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science. 1987;238:797–9. doi: 10.1126/science.3672127. [DOI] [PubMed] [Google Scholar]
- Miller-Sims VC, Bottjer SW. Development of neural responsivity to vocal sounds in higher level auditory cortex of songbirds. J Neurophysiol. 2014;112:81–94. doi: 10.1152/jn.00484.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R. Neural mechanisms for learned birdsong. Learn Mem. 2009;16:655–69. doi: 10.1101/lm.1065209. [DOI] [PubMed] [Google Scholar]
- Naie K, Hahnloser RH. Regulation of learned vocal behavior by an auditory motor cortical nucleus in juvenile zebra finches. J Neurophysiol. 2011;106:291–300. doi: 10.1152/jn.01035.2010. [DOI] [PubMed] [Google Scholar]
- Norton P, Scharff C. “Bird Song Metronomics”: Isochronous Organization of Zebra Finch Song Rhythm. Frontiers in Neuroscience. 2016;10 doi: 10.3389/fnins.2016.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AD. Musical rhythm, linguistic rhythm, and human evolution. Music Perception. 2006;24:99–103. [Google Scholar]
- Pepperberg IM. Vocal learning in Grey parrots: A brief review of perception, production, and cross-species comparisons. Brain Lang. 2010;115:81–91. doi: 10.1016/j.bandl.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Pinaud R, Terleph TA, Tremere LA, Phan ML, Dagostin AA, Leao RM, Mello CV, Vicario DS. Inhibitory network interactions shape the auditory processing of natural communication signals in the songbird auditory forebrain. J Neurophysiol. 2008;100:441–55. doi: 10.1152/jn.01239.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinaud R, Velho TA, Jeong JK, Tremere LA, Leao RM, von Gersdorff H, Mello CV. GABAergic neurons participate in the brain’s response to birdsong auditory stimulation. Eur J Neurosci. 2004;20:1318–30. doi: 10.1111/j.1460-9568.2004.03585.x. [DOI] [PubMed] [Google Scholar]
- Poole JH, Tyack PL, Stoeger-Horwath AS, Watwood S. Animal behaviour: elephants are capable of vocal learning. Nature. 2005;434:455–6. doi: 10.1038/434455a. [DOI] [PubMed] [Google Scholar]
- Prat Y, Taub M, Yovel Y. Vocal learning in a social mammal: Demonstrated by isolation and playback experiments in bats. Sci Adv. 2015;1:e1500019. doi: 10.1126/sciadv.1500019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci U S A. 2010;107:3852–7. doi: 10.1073/pnas.0906572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong SM, Chao A, Schlinger BA. Sex-specific, rapid neuroestrogen fluctuations and neurophysiological actions in the songbird auditory forebrain. J Neurophysiol. 2012;107:1621–31. doi: 10.1152/jn.00749.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV, Teague DP, Schroeder MB, Cummings SE. Vocal production in different social contexts relates to variation in immediate early gene immunoreactivity within and outside of the song control system. Behav Brain Res. 2004;155:307–18. doi: 10.1016/j.bbr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Schachner A, Brady TF, Pepperberg IM, Hauser MD. Spontaneous motor entrainment to music in multiple vocal mimicking species. Curr Biol. 2009;19:831–6. doi: 10.1016/j.cub.2009.03.061. [DOI] [PubMed] [Google Scholar]
- Stoeger AS, Manger P. Vocal learning in elephants: neural bases and adaptive context. Curr Opin Neurobiol. 2014;28:101–7. doi: 10.1016/j.conb.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes TM, Leonard CM, Nottebohm F. The telencephalon, diencephalon, and mesencephalon of the canary, Serinus canaria, in stereotaxic coordinates. J Comp Neurol. 1974;156:337–74. doi: 10.1002/cne.901560305. [DOI] [PubMed] [Google Scholar]
- Stripling R, Kruse AA, Clayton DF. Development of song responses in the zebra finch caudomedial neostriatum: role of genomic and electrophysiological activities. J Neurobiol. 2001;48:163–80. doi: 10.1002/neu.1049. [DOI] [PubMed] [Google Scholar]
- Sukhatme VP, Cao XM, Chang LC, Tsai-Morris CH, Stamenkovich D, Ferreira PC, Cohen DR, Edwards SA, Shows TB, Curran T, et al. A zinc finger-encoding gene coregulated with c-fos during growth and differentiation, and after cellular depolarization. Cell. 1988;53:37–43. doi: 10.1016/0092-8674(88)90485-0. [DOI] [PubMed] [Google Scholar]
- Svec LA, Licht KM, Wade J. Pair bonding in the female zebra finch: a potential role for the nucleus taeniae. Neuroscience. 2009;160:275–83. doi: 10.1016/j.neuroscience.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Cate C, Spierings M, Hubert J, Honing H. Can Birds Perceive Rhythmic Patterns? A Review and Experiments on a Songbird and a Parrot Species. Front Psychol. 2016;7:730. doi: 10.3389/fpsyg.2016.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Haar SM, Kaemper W, Stam K, Levelt CC, ten Cate C. The interplay of within-species perceptual predispositions and experience during song ontogeny in zebra finches (Taeniopygia guttata) Proc Biol Sci. 2014;281:20141860. doi: 10.1098/rspb.2014.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaszycki ML, Peabody C, Replogle K, Clayton DF, Tempelman RJ, Wade J. Sexual differentiation of the zebra finch song system: potential roles for sex chromosome genes. BMC Neurosci. 2009;10:24. doi: 10.1186/1471-2202-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Aa J, Honing H, ten Cate C. The perception of regularity in an isochronous stimulus in zebra finches (Taeniopygia guttata) and humans. Behav Processes. 2015;115:37–45. doi: 10.1016/j.beproc.2015.02.018. [DOI] [PubMed] [Google Scholar]
- Wieland EA, McAuley JD, Dilley LC, Chang SE. Evidence for a rhythm perception deficit in children who stutter. Brain Lang. 2015;144:26–34. doi: 10.1016/j.bandl.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M, Cook PF. Rhythmic entrainment: Why humans want to, fireflies can’t help it, pet birds try, and sea lions have to be bribed. Psychon Bull Rev. 2016;23:1647–1659. doi: 10.3758/s13423-016-1013-x. [DOI] [PubMed] [Google Scholar]
- Yanagihara S, Yazaki-Sugiyama Y. Auditory experience-dependent cortical circuit shaping for memory formation in bird song learning. Nat Commun. 2016;7:11946. doi: 10.1038/ncomms11946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zann RA. The Zebra Finch. Oxford University Press; New York: 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


