Abstract
Objectives
We aimed to test if EEG responses to novel events reliably dissociated individuals with Parkinson’s disease and controls, and if this dissociation was sensitive and specific enough to be a candidate biomarker of cognitive dysfunction in Parkinson’s disease.
Methods
Participants included N=25 individuals with Parkinson’s disease and an equal number of well-matched controls. EEG was recorded during a three-stimulus auditory oddball paradigm both ON and OFF medication.
Results
While control participants showed reliable EEG habituation to novel events over time, individuals with Parkinson’s did not. In the OFF condition, individual differences in habituation correlated with years since diagnosis. Pattern classifiers achieved high sensitivity and specificity in discriminating patients from controls, with a maximum accuracy of 82%. Most importantly, the confidence of the classifier was related to years since diagnosis, and this correlation increased as the time course of differential habituation increasingly distinguished the groups.
Conclusions
These findings identify systemic alteration in an obligatory neural mechanism that may contribute to higher-level cognitive dysfunction in Parkinson’s disease.
Significance
These findings suggest that EEG responses to novel events in this rapid, simple, and inexpensive test have tremendous promise for tracking individual trajectories of cognitive dysfunction in Parkinson’s disease.
Keywords: Parkinson’s, EEG, Novelty, Classification, Habituation
Introduction
Some of the most debilitating aspects of Parkinson’s disease include cognitive and mood disturbances. While it is widely appreciated that cell death in Parkinson’s disease somehow contributes to deficits in higher cognitive functioning, the mechanisms underlying these deficits remain unclear. Executive dysfunction is common in Parkinson’s disease (Dirnberger and Jahanshahi, 2013; Eberling et al., 2014; Robbins and Cools, 2014), yet one sub-process stands out as being specifically compromised: diminished orienting responses to novel stimuli (Kingstone et al., 2002; Poliakoff et al., 2003; Yamaguchi and Kobayashi, 1998; Zhou et al., 2012).
Frontal orienting responses are well-represented in the EEG. The event-related potential (ERP) component known as the P3a has often been defined as a central nervous system reflection of the orienting response (Barceló et al., 2002; Friedman et al., 2001; Nieuwenhuis et al., 2011). The P3a occurs to novel stimuli, it has a mid-frontal distribution, and it habituates rapidly (Polich, 2007). Individuals with Parkinson’s disease have a smaller P3a than controls (Polich, 2007; Tsuchiya et al., 2000; Zeng et al., 2002), and this component has been advanced as a candidate biomarker of Parkinson’s disease progression, as it is smaller in more severe patients (Solís-Vivanco et al., 2015). It should be noted that the P3a is a part of a family of evoked brain responses that can be difficult to disentangle; recent findings suggest that a later frontal novelty-specific component may be a better representation of the orienting response (Barry et al., 2016). To date, this novelty-P3 has not yet been investigated in Parkinson’s disease.
Importantly, the orienting response is not a unitary phenomenon, as it is influenced by competing processes of sensitization and habituation (Barry, 2009; Groves and Thompson, 1970). Individuals with Parkinson’s disease show reduced habituation in the blink reflex following glabellar tap (Penders and Delwaide, 1971) or startling sounds (Chen et al., 2016; Nieuwenhuijzen et al., 2006). Chen et al. (2016) recently described how mid-frontal EEG orienting responses predicted both the degree of startle blink habituation as well as separate measures of executive functioning in individuals with Parkinson’s disease. Indeed, midfrontal dopaminergic dysfunction may be a common denominator underlying deficits amongst these processes (Parker et al., 2015; Popescu et al., 2016). It remains unknown if differential habituation trajectories alter the neural marker of the orienting response in Parkinson’s disease.
A recent review suggests that EEG may be able to dissociate different disease states within Parkinsonism: while frontal novelty-related components like the P3a are sensitive to executive impairments, the posterior target-related P3b is more sensitive to dementia (Seer et al., 2016). Given the dearth of viable tools for assessing mild cognitive impairment and progression to Parkinson’s disease dementia (Eberling et al., 2014; Halliday et al., 2014), these EEG features appear highly promising for further study. P3a amplitude is highly reliable across test-retest sessions (Debener et al., 2002) and P3b has been shown to have moderate heritability (Smit et al., 2007) and has comparable variance with biomedical serum measures in a variety of diagnostic assays (Polich and Herbst, 2000).
While the relationship between orienting, P3a amplitude, frontal dopamine, and Parkinson’s disease is complex and non-specific (see Discussion), these collective findings converge to offer a candidate biomarker for cognitive dysfunction in Parkinson’s disease that is statistically reliable and linked to well-known neurobiological and psychological mechanisms. In this report we used machine learning pattern classifiers to define the sensitivity and specificity of EEG responses to novelty as a biomarker in Parkinson’s disease.
Methods
Experimental Design
The University of New Mexico Office of the Institutional Review Board approved the study and all participants provided written informed consent. Participants were paid $20/hr for participation. Participants included N=25 individuals with Parkinson’s disease (PD group) recruited from the Albuquerque, New Mexico community and an equal number of sex and age matched controls (CTL group). This set of participants was taken from a slightly larger sample with behavioral data reported elsewhere (Cavanagh et al., 2017). The PD group visited the lab twice, seven days apart: once on medication and once after a 15-hour overnight withdrawal from their individual prescriptions of dopaminergic medication used to treat PD. Hereafter these conditions are referred to as ON or OFF, respectively.
All patient and control sessions were run at 9 AM; 13 patients were ON in their first session, 12 were OFF. The PD group completed neuropsychological and questionnaire assessments in their ON state (see Table 1). United Parkinson’s Disease Rating Scale (UPDRS) motor scores were videotaped in each patient session and were scored by a neurologist. All participants had Mini Mental State Exam (MMSE) scores above 26. Parkinson’s and control participants did not differ on any measurements of education or premorbid intelligence (see Table 1).
Table 1.
Patient and control participant demographics (mean +/− SD). All controls were age and sex matched to a patient. BDI = Beck Depression Inventory, MMSE = Mini Mental State Exam, NAART = North American Adult Reading Test, UPDRS = United Parkinson’s Disease Rating Scale (motor), LED = L-Dopa equivalence dose in mg.
| PD | CTL | Statistic | |
|---|---|---|---|
|
| |||
| Sex | 16 M, 9 F | 16 M, 9 F | |
| Age | 69.68 (8.73) | 69.32 (9.58) | t(48)=.14, p=.89 |
| Years of Education | 17.24 (2.95) | 16.42 (3.20) | t(48)=.98, p=.33 |
| Parent’s Years Ed | 12.98 (3.21) | 12.11 (3.33) | t(48)=1.07, p=.29 |
| MMSE | 28.68 (1.03) | 28.76 (1.05) | t(48)=−.27, p=.79 |
| NAART | 45.92 (9.29) | 46.80 (7.64) | t(48)=−.37, p=.72 |
| BDI | 7.00 (4.77) | 5.24 (4.74) | t(48)=1.31, p=.20 |
| UPDRS ON | 23.36 (9.87) | ||
| UPDRS OFF | 24.80 (8.66) | ||
| LED | 685 (452) | ||
| Years since Diagnosis | 5.40 (4.09) | ||
Auditory Oddball Task
The 3-auditory oddball task was programmed in Matlab using Psychtoolbox. Standards were 440Hz sinusoidal tones (70% of trials), targets were 660 Hz sinusoidal tones (15% of trials) and novel distractors (15% of trials) were unique sections from a naturalistic sounds dataset (Bradley and Lang, 1999). All sounds were presented for 200 ms, tones were presented at 80 dB and naturalistic novel sounds had a mean of 65 dB with an inter-quartile range of +/− 6.5 dB. A random inter-trial-interval (ITI) was selected from a uniform distribution of 500 to 1000 ms. Due to a delay in loading standard and target sine-wave based sounds, these conditions were delayed by an extra 450 ms following the inter-trial-interval. Thus, the novel conditions had an ITI of 500 to 1000 ms but the standard and target conditions had an ITI of 950 to 1450 ms (this between-condition difference was not noticeable and does not affect our findings, which are largely based on group differences to the novelty condition). Sounds were played on stereo speakers. Participants were instructed to count the targets and ignore standards and novels. This passive paradigm removes potential confounding influences of motor system activity and decision making on P3 amplitudes (Johnson, 1986; O’Connell et al., 2012). There were two blocks of 100 trials each; participants reported their target counts after each block. The task took an average of 12 minutes to complete.
EEG Recording and Preprocessing
EEG was recorded continuously from sintered Ag/AgCl electrodes across .1 to 100 Hz with a sampling rate 500 Hz, an online CPz reference, and a ground at AFz on a 64 channel Brain Vision system. The vertical electrooculogram (VEOG) was recorded from bipolar auxiliary inputs. All analyses were performed using Matlab R2016b and EEGlab version 14_0_0b (Delorme and Makeig, 2004). First, very ventral temporal sites were removed, as they tend to be unreliable, leaving 60 electrodes. Data were epoched around the stimulus onset (−2000 to 2000 ms), from which the associated stimulus responses were isolated. Activity at the reference electrode CPz was re-created and bad channels and bad epochs were identified using a conjunction of the FASTER algorithm (Nolan et al., 2010) and pop_rejchan from EEGlab and were subsequently interpolated and rejected respectively. Eye blinks were removed following ICA. Data were then re-referenced to an average reference.
Single trial EEG epochs were baseline corrected from −200 to 0 ms pre-stimulus, ERPs were filtered from .1 to 20 Hz. The P3a was defined as the peak from 325 to 375 ms at FCz in the novelty condition. For some analyses, a surface Laplacian ( laplacian_perrinX.m) was computed on the ERPs, which acts as a reference-free spatial filter (Cohen, 2014). Following a recent analysis of the orienting response (Barry et al., 2016), temporal principal components analysis was performed on the novelty condition ERPs for all participants. Observations were concatenated across groups (CTL, ON, OFF), participants (25 per group), and electrodes (60 per participant), yielding 4500 observations across 1001 samples (−500:2:1500). ERPs were first detrended then mean centered prior to PCA ( pca.m). Seven factors were selected for promax rotation ( rotatefactors.m) based on comparison to shuffled data (Dien et al., 2007). Factors were re-scaled to microvolts by multiplying by the standard deviation of the raw data and temporal factors were reconstructed by multiplying those weights with the ERPs (Dien, 2012).
Time-Frequency measures were computed by multiplying the fast Fourier transformed (FFT) power spectrum of single trial EEG data with the FFT power spectrum of a set of complex Morlet wavelets (defined as a Gaussian-windowed complex sine wave: ei2πtfe-tˆ2/(2×σ^2), where t is time, f is frequency (which increase from 1-50Hz in 50 logarithmically spaced steps), and defines the width (or ‘cycles’) of each frequency band, set according to 4/(2πf)), and taking the inverse FFT. Inter trial phase consistency (ITPC) was quantified as the length of the average of unit-length vectors that were distributed according to their phase angles (Lachaux et al., 1999). ITPC quantifies the consistency of phase values for a given frequency band at each point in time, with values varying from 0 to 1 where 0 indicates random phases at that time-frequency point across trials, and 1 indicates identical phase values at that time-frequency point across trials.
Statistical Analyses
To specifically examine change over time, the last 1/3 of trials were subtracted from the first 1/3 of trials in the novelty condition for each participant (95% of all participants had 9 or 10 trials per tertile condition for this contrast). Differences in time-dependent ERPs and time-frequency plots were tested between conditions or groups using t-tests that were corrected for multiple comparisons by thresholding the mass of the statistical cluster (sum of absolute t values) against 5000 permutations of group labels and taking the one-dimensional cluster mass at the 95th percentile as the threshold for chance occurrence. Only activities after the time locking event were statistically contrasted. This pair-wise statistical testing procedure was chosen instead of an Analysis of Variance since it was expected that ON and OFF differences from CTL should be very similar and this yielded a more useful comparison of medication differences compared to an inclusive model (there were no ON vs. OFF differences in any statistical contrasts of EEG activities). All topographical plots were displayed as the average activity from 400 to 700 ms post-stimulus, significant differences are indicated by black diamonds. There were no multiple comparisons for the topographical plots since the spatial hypothesis was specific to mid-frontal sites. The number of years since the diagnosis of Parkinson’s disease was used as a proxy for disease severity following a recent report from this same cohort where years diagnosed was the major explanatory variable of inter-subject variability (Cavanagh et al., 2017).
Pattern Classification
For classification, EEG epochs were down-sampled to 100 Hz and high pass filtered at .1 Hz. Trials were then matched between conditions and participant pairs so that every pair of patient and matched control had the same number of trials in standard, target, and novel conditions. First, thirty random standard condition trials were selected for each participant. Second, trial counts were matched between each participant and their age and sex matched pair. The trial count minimum across all conditions (i.e. due to rejection of bad epochs) in ON, OFF, and matched CTL sets was determined and this number of epochs was randomly selected from any sets that were larger. This minimum ranged from 23 to 29 across participants (mode = 28). Segregation of the data in this manner not only controlled for potential biases between groups, but it allows direct comparison between conditions without spurious influences of training set size.
Data from each condition were taken from the first 50 coefficients (1 to 50 Hz power) of the fast Fourier Transform (FFT) of single trial data from 250 to 1000 ms at each of 60 electrodes, which were then averaged across trials for each participant (yielding a 60 channels *50 Fourier coefficients = 3000 point linearized vector for each participant). Cross validation was performed as a controlled leave-one-out procedure, where a single individual from the PD group was held out of the training set along with their age and sex matched individual from the CTL group. The other 24 participants in each group constituted the training set, which were z-score normalized across participants. The two test set holdouts were normalized to training set mean and standard deviation. Classification of the training set was performed with a linear support vector machine (SVM) ( fitcsvm.m); out of sample prediction of the training weights on the test sample holdouts was then performed ( predict.m).
ON and OFF medication groups were separately tested against the CTL group. Both medication groups were expected to reveal similar patterns. While we expected the novelty condition to maximally dissociate groups, all three conditions were tested separately to confirm this assumption. Motivated by the ERP analyses of novelty condition change over time, the data from the novelty condition were split into first 3rd, middle 3rd, and last 3rd sets, as well as the difference between the last 3rd and first 3rd sets. The confidence in SVM classification was derived from the absolute distance from the decision boundary; this participant-specific measure was then correlated with years diagnosed.
To determine the most important features that contributed to classification, select contrasts were subjected to an iterative procedure for feature removal. In this process, each individual channel*coefficient feature was removed and the classification accuracy was computed. If the accuracy changed by less than 5% compared to the full feature set, the feature was removed. This iterative procedure then tested the next feature. At the end of all 3000 iterations, only the most predictive features were left. This process was repeated 50 times for each contrast; features were selected randomly in each permutation. Summary statistics of the retained features were averaged across five major EEG frequency bands and displayed topographically (delta: DC to 3 Hz, theta: 4 to 7 Hz, alpha: 8 to 11 Hz, beta: 12 to 29 Hz, gamma: 30 to 49 Hz). It was hypothesized that low-frequency frontal midline activities should maximally discriminate PD from CTL groups.
Results
All participants reported reasonably accurate target counts (ON and CTL were 100% accurate within a range of +/− 1; OFF was 92% accurate in this range), indicating that they understood the task and remained alert during each block. UPDRS motor scores did not significantly change between ON and OFF sessions (t(24)=−1.02, p=.32). Years since diagnosis correlated with daily Levodopa-equivalent dose (r(25)=.60, p=.002).
ERPs
Permutation-corrected statistical contrasts of ERP time courses revealed significant differences between PD and CTL groups, with larger amplitudes in each PD group (Fig 1). Specifically, the brain response to standard tones was enhanced in the PD group (ON or OFF) vs. the CTL group over much of the late duration of the event, and novel trials were specifically enhanced in the ON vs. CTL contrast (OFF vs. CTL had a similar trend, but with lower statistical power). Topographical plots revealed a common pattern of comparatively enhanced frontal midline activity in the PD group regardless of condition or medication status.
Figure 1.
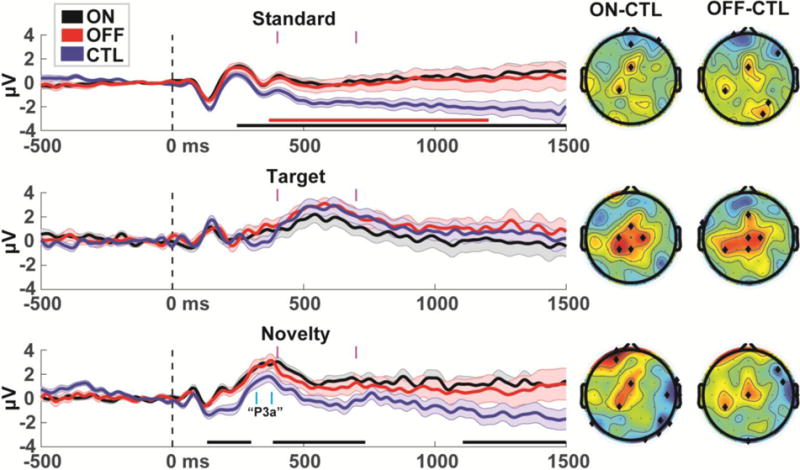
ERPs in individuals with Parkinson’s disease ON and OFF medication vs. a well-matched CTL group. Standard and Novel ERPs are from the FCz electrode, Target ERPs are from POz. Horizontal bars under each ERP indicate permutation-corrected statistically significant differences between PD and CTL (black=ON>CTL, red=OFF>CTL). Major differences between groups occurred over much of the duration of standard and novel trials. PD and CTL groups reliably differed in frontal midline electrodes regardless of medication status. The time range of the P3a is marked in cyan. Topoplots are shown in time ranges indicated by vertical magenta bars (400-700 ms), significant differences are indicated by black diamonds. Error bars are _+/− SEM
Surprisingly, the P3a component trended towards being larger in the PD group than the CTL group at the FCz electrode (ON vs. CTL: t(48)=1.90, p=.06; OFF vs. CTL: t(48)=1.56, p=.13), in contrast to some previous findings of a diminished P3a (Polich, 2007; Solís-Vivanco et al., 2015; Tsuchiya et al., 2000; Zeng et al., 2002). However, this small component is riding on top of a larger underlying trend; high-pass filtering the data at 1 Hz (as in Solís-Vivanco et al., 2015) removes this shift and reverses these trends (ON vs. CTL: t(48)=−.25, p=.81, OFF vs. CTL t(48)=−.75, p=.46). Collectively, these findings bolster the hypothesis of altered mediofrontal activities in Parkinsonism, and indicate that this tendency transcends traditional ERP component activities like the P3a.
Habituation to Novel Events
In order to specifically examine habituation to novel events, the last 1/3 of novel trials were subtracted from the first 1/3 of novel trials; these difference ERPs were then tested in a similar manner as Fig 1. Figure 2a shows how control participants had a diminishment in ERP amplitudes from ~400 to 700 ms, demonstrating a change to novel sounds over time. The PD group actually had enhanced activity over time (ON: ~700 to 900 ms), and both ON and OFF conditions were significantly larger than CTL, particularly over mid-frontal electrodes. A Laplacian spatial filter verified that these responses were specific to frontal midline areas (Fig 2b). This specific measure of habituation (400 to 700 ms) correlated with years since diagnosis, but only in the OFF group (rho=.50, p=.01; without outlier rho=.43, p=.04) and not in the ON group (rho=.10, p=.63). This positive correlation in the OFF group was specific to frontal midline sites (Fig 2c). No statistical contrasts for habituation to standard or target conditions were significantly different than chance or different between groups, as expected.
Figure 2.
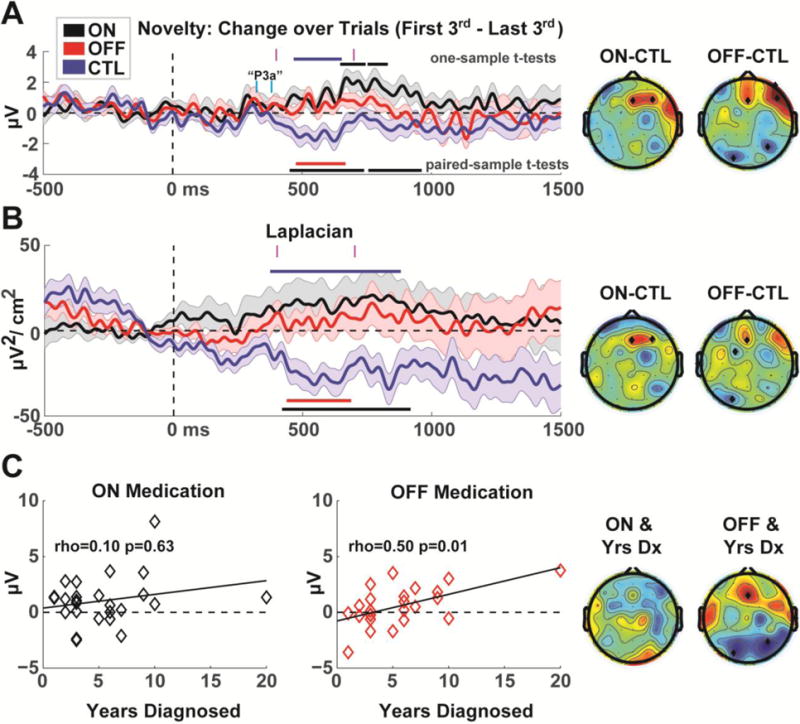
Habituation during novel trials (last 1/3 of trials minus first 1/3 of trials). A) ERP timecourses from the Fz electrode. Horizontal bars indicate permutation-corrected statistically significant differences: under the ERPs these reflect tests between PD and CTL groups (black=ON>CTL, red=OFF>CTL), bars above each ERP indicate similarly corrected one-sample t-tests for each group (black=ON, blue=CTL). These contrasts indicate that individuals with Parkinson’s disease have diminished habituation compared to controls, particularly over frontal midline sites. Indeed, patients ON medication show sensitization over time (higher amplitudes) instead of habituation. The time range of the P3a is marked in cyan; major effects clearly followed the P3a. Topoplots are shown in time ranges indicated by vertical magenta bars (400 to 700 ms), significant differences are indicated by black diamonds. Error bars are _+/− SEM B) A surface Laplacian (spatial filter) verified that the voltage effects shown in Figure 2A were local to frontal midline areas. C) Habituation correlated with a proxy of disease progression (years since diagnosis) OFF but not ON medication.
While this change over time is robust, the alternating polarities of ERP features can obscure whether a lower voltage reflects a diminished response (e.g. less positivity) or an enhanced response (e.g. greater negativity). For an objective interpretation of neural activities, we investigated the inter-trial phase consistency (ITPC) of these responses (Fig 3). At the FCz electrode, novelty conditions were represented by ~2-4 Hz phase consistency. This response was larger in the first 1/3 than the last 1/3 of trials in the CTL group, demonstrating that these ERP effects shown in Figure 2 represent a decline in neural responsivity over time in the CTL group. There was no significant change over time in the ON or OFF groups and the change over time was significantly different between CTL and OFF groups. While this contrast was also different between CTL and ON groups, this latter contrast did not survive multiple comparisons correction. However, this failure of multiple comparisons correction should be interpreted in the context of the extremely strong a priori time and frequency regions of interest. These findings strongly support the specific hypothesis that diminished habituation to novelty is a sensitive indicator of altered mediofrontal habituation in Parkinsonism.
Figure 3.
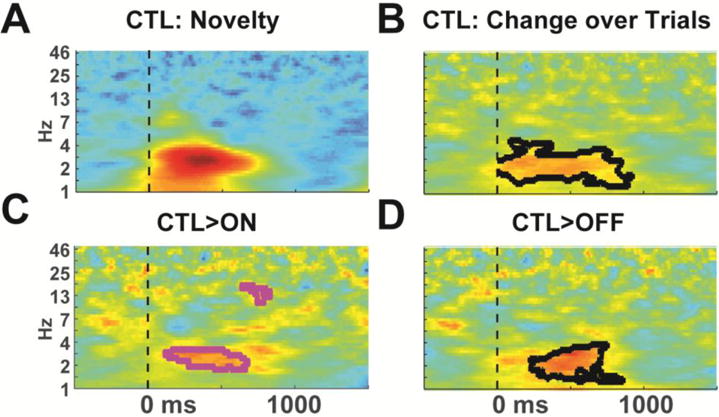
Time-frequency plots of inter-trial phase consistency. A) Novel trials are characterized by ~2-4 Hz phase consistency. Plot is scaled from 0 to .5. B) In the CTL group, the first 1/3 of trials had significantly greater phase consistency than the last 1/3 of trials, demonstrating that the ERP change over time reflects a decrease in neural activities. There was no significant change over time in ON or OFF groups. C-D) The CTL group had significantly greater change in phase consistency than the PD groups, although the contrast with the ON group did not survive multiple comparisons correction (thus is shown with magenta outline). All difference plots are scaled from −.4 to .4.
Principal Components Analysis
Findings suggest that mediofrontal alteration and diminished habituation in Parkinson’s disease may be reflected by broader responses to novelty than the P3a. To investigate how these phenomena relate to existing ERP components, we computed a temporal PCA of the novelty condition, as in Barry et al. (2016), see Figure 4a. Three temporal factors (TFs) stood out in the time ranges of interest: TF1 appears to reflect the P3a component; TF2 appears to reflect the P3b component, and TF6 may reflect the novelty P3 (nP3) component that Barry et al. (2016) revealed to be specific to a smaller-variance late TF that only occurs due to distracting novel stimuli (Fig, 4b). While the timing of this nP3 leads the P3b here instead of following it as in Barry et al. (2016), we urge caution in comparing the relative temporal order of events between healthy young students and an aged patient population. Following our findings of mediofrontal alteration that transcends the P3a, these components demonstrate that the change over time is common to late (post P3a) mid-frontal activities that are not specific to P3b or nP3 features (Fig 4c). In sum, the midfrontal EEG responses to habituation that differentiate CTL and PD groups do not appear to be specific to any single ERP component.
Figure 4.
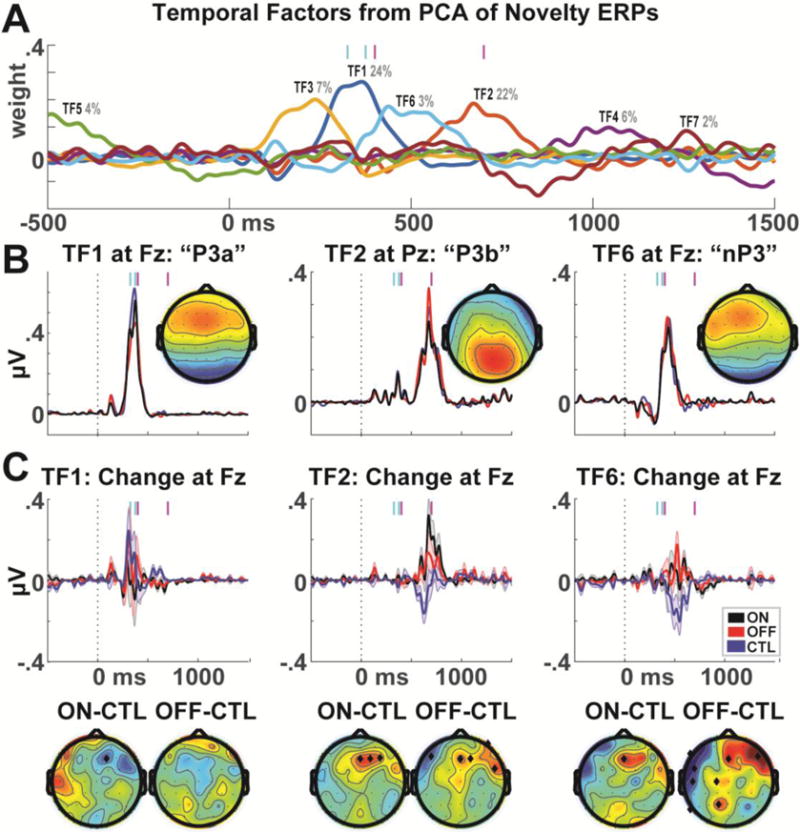
Principal components analysis of novelty condition ERPs. A) A seven-factor solution with promax rotation revealed three major temporal factors in the relevant time ranges. Cyan bars represent the P3a time range; magenta bars represent the time range of major habituation effects. B) TF1 corresponded to the P3a time range and had a frontal midline maximum. TF2 corresponded to a P3b time range and had a parietal midline maximum. TF6 had a late time range and corresponded to a frontal midline maximum; this may reflect a novelty P3 component. C) When the TF weights for each different component were applied to the difference between the first 1/3 minus last 1/3 trials, the previously observed pattern of results is observed for both TF2 and TF6, suggesting that this altered brain dynamic is not specific to a specific ERP component. Error bars are _+/− SEM. Topoplots are shown in time ranges indicated by vertical magenta bars, significant differences are indicated by black diamonds.
Pattern Classification
As described in the methods, efforts were made to ensure that any variance available to the classification process was not due to demographic variables or spurious influences of signal to noise based on available epochs. The novelty condition maximally dissociated the PD group from the CTL group; ON and OFF groups were similarly dissociated (Fig 5a). Maximal total accuracy was 82% (OFF vs. CTL in the novelty condition), see Fig 5b. Figure 5c shows how SVM confidence significantly correlated with years since diagnosis for patients ON but not OFF medication.
Figure 5.
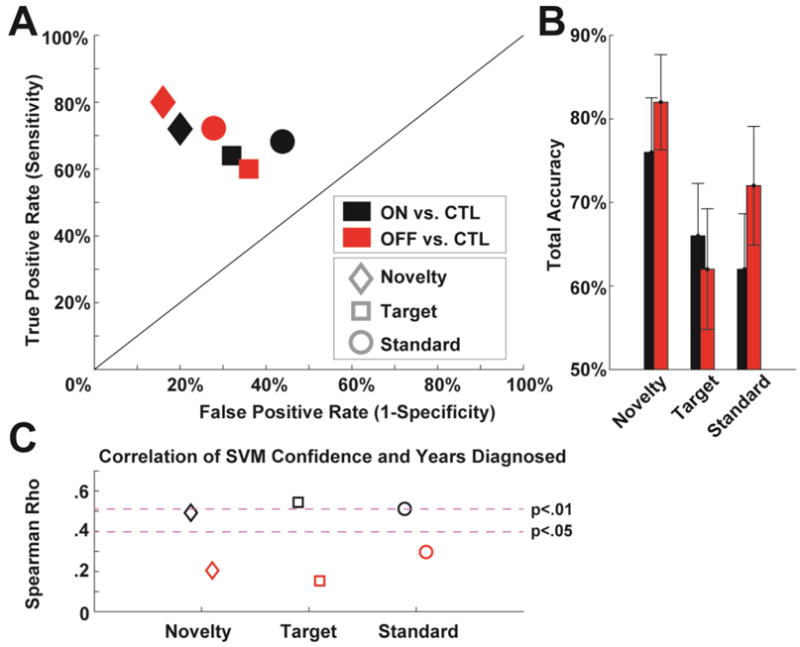
Classification of individuals with Parkinson’s disease based on different condition ERPs. A) A receiver operating characteristic plot shows the true vs. false positive rates of PD vs. CTL discrimination for each medication and task condition. B) Total accuracy (average of sensitivity and specificity) for each condition. C) Correlation of SVM confidence and years diagnosed for each condition; only patients ON medication had significant relationships between ease of classification and years diagnosed.
Bolstered by the ERP findings from the test of habituation to novelty, Figure 6a shows classification outcomes of the novelty condition split by tertiles as well as the difference between the last and first tertiles (i.e. the test of habituation shown in Fig 2). Compared to the novelty condition classification in Fig 5, the tertiles had slightly lower success in dissociating groups and the difference measure had the most modest effects (likely due to decreasing signal to noise across all these contrasts), see Fig 6b. However, these conditions were differently correlated with years diagnosed when patients were ON vs. OFF meds (Fig 6c). The OFF group showed an expected pattern of increasing correlation as time elapses: as differences in habituation increasingly dissociate groups, the most symptomatic patients are best classified. Unexpectedly, the ON group showed the opposite pattern over trials.
Figure 6.
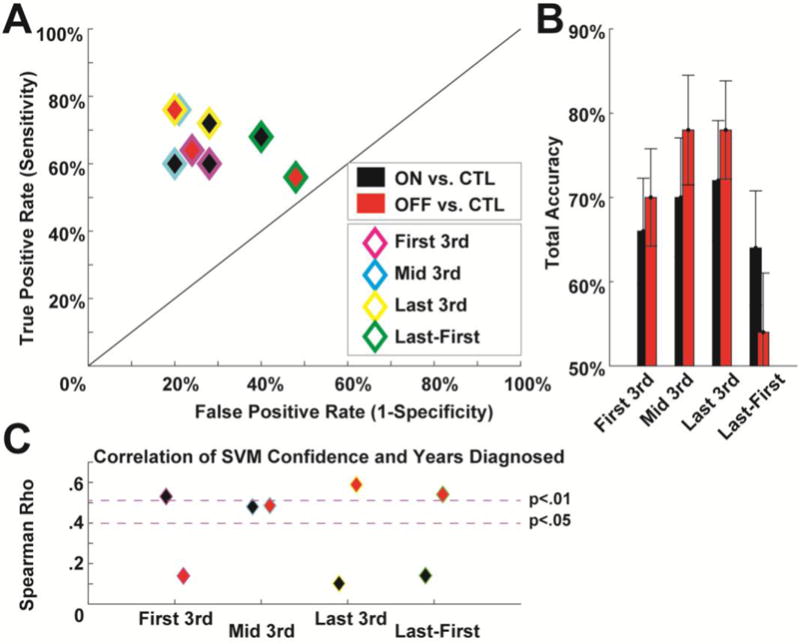
Classification based on habituation to novelty. A) Tertiles of the novelty condition over the course of the experiment, as well as the difference between the last and first tertiles. B) Total accuracy in classification increased over tertiles, commensurate with the hypothesis that lack of habituation maximally discriminates groups. Interestingly, the difference measure designed to highlight habituation was not particularly effective in discrimination, suggesting that overall responses to novelty are also important in discriminating groups. C) The success of these discriminations was dependent on years diagnosed. Whereas patients OFF medication had the expected increase in classification accuracy over time and in the habituation contrast, patients ON medication had the opposite pattern.
Figure 7 shows the most discriminating features for major classification contrasts. Each iterative procedure reduced the feature set to around 1% of the size (the median number of retained features was just above 30). As predicted, the delta band was the most discriminating frequency band. Topographical features were somewhat widespread, but the frontal, central, and parietal midline areas were heavily represented.
Figure 7.
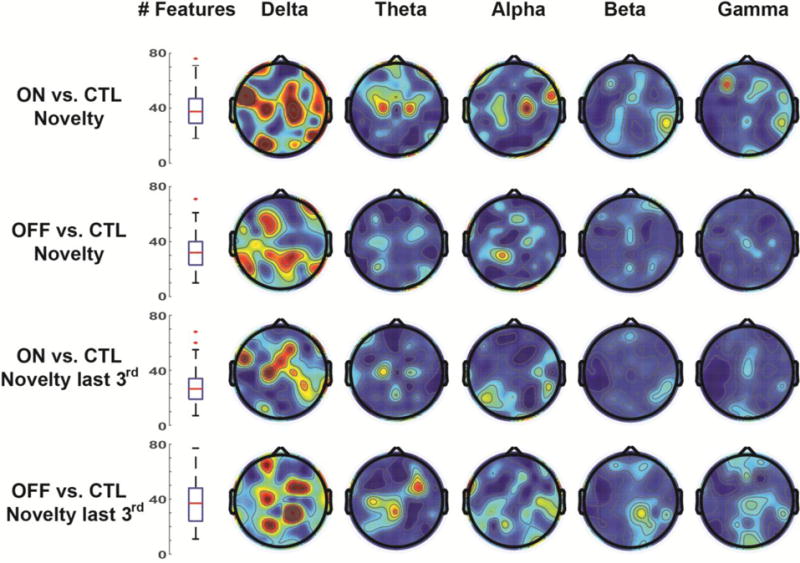
Maximally discriminating features after iterative reduction. For each of four major contrasts (rows), features were removed from the classification if they did not contribute to overall accuracy. Over 50 permutations, each contrast relied on a median of ~30 features for accurate classification. Topoplots show the average number of discriminating features, which were primarily in the delta band over midline areas. Topoplots are all scaled from 0 (blue) to .05 (red).
Discussion
These findings reveal that brain responses to novel sounds, particularly over time, effectively differentiate individuals with Parkinson’s disease from well-matched controls. In this very short task with minimal performance requirements we were able to discriminate the PD group from the CTL group at 82% accuracy while holding all other potential spurious variables (age, sex, SES, time of day, signal to noise) constant. These findings are important for two reasons. First, they identify systemic alteration in an obligatory neural mechanism that may contribute to higher-level cognitive dysfunction in Parkinson’s disease. Second, they demonstrate that this neural mechanism has considerable promise as a biomarker of Parkinson’s disease symptom progression.
Patients ON medications were a less variable population than when OFF medication, effectively differentiating from controls in condition-specific ERPs (Fig 1) and diminished habituation to novelty (Fig 2a). However, the increased variability OFF medication was clinically relevant: years since diagnosis was related to individual variability in habituation (Fig 2c) and classification due to habituation (Fig 6c). This feature stands in contrast to individual variability ON medication, which was not related to habituation (Fig 2c; Fig 6c), although it was related to overall condition-specific discriminability (Fig 5c, Fig 6c). In summary, medication status appears to have different effects on the brain response to novelty, where patients ON medication had a reliably altered orienting response but patients OFF medication were more idiosyncratically sensitive to the habituation to novelty. Importantly, years since diagnosis moderated both of these effects.
The orienting response is a primitive component of executive control, and it contributes to obligatory motor slowing via a route including the subthalamic nucleus (Wessel and Aron, 2017). An altered orienting response is suggested to contribute to quality of life issues in patients, including distractibility (Sharpe, 1990) and risk for falls (Nieuwenhuijzen et al., 2006). In this study we defined habituation as the response decrement to novel stimuli over time. However, other studies have advanced more sophisticated measures for assessing habituation which allow dissociation from fatigue or other spurious influences (Barry, 2009; Rankin et al., 2009; Steiner and Barry, 2011). It would be very beneficial to replicate the findings reported here with habituation-specific stimulus-response patterns, and it could be very important to consider how this basic deficit contributes to alteration in higher-level cognition.
While previous studies have found reduced P3a amplitudes in individuals with Parkinson’s disease (Polich, 2007; Tsuchiya et al., 2000; Zeng et al., 2002), the evidence is somewhat mixed across studies (Seer et al., 2016). We demonstrate here that signal processing choices (e.g. high pass filtering) can have profound effects on this difference. Indeed, the findings reported here suggest that differences in medication status, task durations, and symptom severity all contribute important variance to this phenomenon. While years since diagnosis is a crude measure of symptom severity, the use of this measure was motivated by previous findings in this same cohort that it moderated behaviorally-expressed learning alterations due to medication status (Cavanagh et al., 2017). None of the aforementioned studies of the orienting response have completed a within-patient longitudinal study to examine within-patient symptom progression in tandem with frontal EEG activities; however an emerging consensus suggests this would be very beneficial (Cavanagh et al., 2017; Seer et al., 2016; Solís-Vivanco et al., 2015).
It is important to note that EEG reflects cortical operations and is unlikely to be sensitive to the pathology of midbrain cell death in PD. While monoaminergic projections strongly modify cortical operations, this relationship is poorly understood and emergent EEG operations are likely to be non-specifically related to single neurotransmitters. The P3a is sensitive to a variety of monoaminergic activities, including noradrenaline, serotonin, and acetylcholine (Brown et al., 2015; Heitland et al., 2013), although Parkinson’s disease is associated with alterations in all of these systems as well as alpha synuclein load across varied levels of the neural hierarchy (Halliday et al., 2014; Weingarten et al., 2015). In sum, it is unlikely that EEG will be a viable stand-alone biomarker for diagnosing Parkinson’s disease. However, as a sensitive index of high-level canonical circuit operations, EEG is an excellent candidate tool for sensitively and specifically discriminating of the presence and trajectory of cognitive dysfunction in Parkinson’s disease. This sets it apart from molecular biomarkers (like serum or cerebrospinal fluid markers) or neuroimaging biomarkers (like the DaT scan) which can be successful in diagnosis yet limited in ability to follow disease progression (Vogt et al., 2011). Since most large hospitals already have ample capabilities for assessing EEG, this method has tremendous promise for biomedical utility in a variety of neurological disorders.
Conclusion
In conclusion, the 3-auditory oddball task has considerable promise as a simple, rapid, and inexpensive biomarker for assessing individual disease course trajectories in individuals with Parkinson’s disease. Two steps need to be taken to realize this goal. First, a within-patient longitudinal study would verify this hypothesis. Second, maximal accuracy needs to be strengthened in the classification process. Other studies have classified Parkinson’s patients from controls with similar accuracy using resting EEG activities (Chaturvedi et al., 2017; Lainscsek et al., 2013), which could be integrated with the approach used here with very minor demands on patient time. However, sophisticated data processing techniques and large scale datasets are required to achieve maximal classification. To facilitate this goal, we have made all data and code for this experiment open source and available online (see Acknowledgements).
Highlights.
Parkinson’s patients do not show EEG habituation to novel events over time.
This neural response classifies patients at 82% accuracy.
These findings identify a systemic alteration that contributes to cognitive dysfunction.
Acknowledgments
The authors thank Pat Thalhammer and the New Mexico Parkinson’s Coalition for help recruiting participants. JFC and AM are supported by NIGMS 1P20GM109089-01A1. JFC is also supported by NIMH 1UH2MH109168-01 and NIAAA R21AA0023947-01A1. This work was partially supported by a grant from the University of New Mexico Office of the Vice President of Research. Sponsors had no role in the collection, analysis, interpretation of data, or writing of the manuscript. All data and code for this experiment are available on the PRED+CT website: www.predictsite.com
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure
None of the authors have potential conflicts of interest to be disclosed.
References
- Barceló F, Periáñez Ja, Knight RT. Think differently: a brain orienting response to task novelty. Neuroreport. 2002;13:1887–92. doi: 10.1097/00001756-200210280-00011. [DOI] [PubMed] [Google Scholar]
- Barry RJ. Habituation of the orienting reflex and the development of Preliminary Process Theory. Neurobiol Learn Mem. 2009;92:235–42. doi: 10.1016/j.nlm.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Steiner GZ, De Blasio FM. Reinstating the Novelty P3. Sci Rep. 2016;6:31200. doi: 10.1038/srep31200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. International affective digitized sounds (IADS): Stimuli, instruction manual and affective ratings. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 1999. (Tech. Rep. No. B-2). [Google Scholar]
- Brown SBRE, van der Wee NJA, van Noorden MS, Giltay EJ, Nieuwenhuis S. Noradrenergic and cholinergic modulation of late ERP responses to deviant stimuli. Psychophysiology. 2015;52:1620–31. doi: 10.1111/psyp.12544. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Mueller AA, Brown DR. Cognitive states influence dopamine-driven aberrant learning in Parkinson’s Disease. Cortex. 2017 doi: 10.1016/j.cortex.2017.02.021. doi: http://dx.doi.org/10.1016. [DOI] [PMC free article] [PubMed]
- Chaturvedi M, Hatz F, Gschwandtner U, Bogaarts JG. Quantitative EEG (QEEG) Measures Differentiate Parkinson’s Disease (PD) Patients from Healthy Controls (HC) Front Aging Neurosci. 2017;9:1–7. doi: 10.3389/fnagi.2017.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K-H, Okerstrom KL, Kingyon JR, Anderson SW, Cavanagh JF, Narayanan NS. Startle Habituation and Midfrontal Theta Activity in Parkinson Disease. J Cogn Neurosci. 2016;28:1923–32. doi: 10.1162/jocn_a_01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX. Analyzing Neural Time Series Data: Theory and Practice. Cambridge, MA: MIT Press; 2014. [Google Scholar]
- Debener S, Kranczioch C, Herrmann CS, Engel AK. Auditory novelty oddball allows reliable distinction of top-down and bottom-up processes of attention. Int J Psychophysiol. 2002;46:77–84. doi: 10.1016/S0167-8760(02)00072-7. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. doi:10.1016/j.jneumeth.2003.10.009S0165027003003479 [pii] [DOI] [PubMed] [Google Scholar]
- Dien J, Khoe W, Mangun GR. Evaluation of PCA and ICA of simulated ERPs: Promax vs infomax rotations. Hum Brain Mapp. 2007;28:742–63. doi: 10.1002/hbm.20304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dien J. Applying Principal Components Analysis to Event-Related Potentials: A Tutorial. Dev Neuropsychol. 2012;37:497–517. doi: 10.1080/87565641.2012.697503. [DOI] [PubMed] [Google Scholar]
- Dirnberger G, Jahanshahi M. Executive dysfunction in Parkinson’s disease: A review. J Neuropsychol. 2013;7:193–224. doi: 10.1111/jnp.12028. [DOI] [PubMed] [Google Scholar]
- Eberling J, Vincent L, Goldman JG, Weintraub D, Kulisevsky J, Marras C, et al. Therapeutic development paths for cognitive impairment in Parkinson’s disease: Report of a regulatory roundtable. J Parkinsons Dis. 2014;4:585–9. doi: 10.3233/JPD-140385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D, Cycowicz YM, Gaeta H. The novelty P3: an event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neurosci Biobehav Rev. 2001;25:355–73. doi: 10.1016/S0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Groves PM, Thompson RF. Habituation: A dual-process theory. Psychol Rev. 1970;77:419–50. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Halliday GM, Leverenz JB, Schneider JS, Adler CH. The neurobiological basis of cognitive impairment in Parkinson’s disease. Mov Disord. 2014;29:634–50. doi: 10.1002/mds.25857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitland I, Kenemans JL, Oosting RS, Baas JMP, Böcker KBE. Auditory event-related potentials (P3a, P3b) and genetic variants within the dopamine and serotonin system in healthy females. Behav Brain Res. 2013;249:55–64. doi: 10.1016/j.bbr.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Johnson R. A triarchic model of P300 amplitude. Psychophysiology. 1986;23:367–84. doi: 10.1111/j.1469-8986.1986.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Kingstone A, Klein R, Morein-Zamir S, Hunt A, Fisk J, Maxner C. Orienting Attention in Aging and Parkinson’s Disease: Distinguishing Modes of Control. J Clin Exp Neuropsychol. 2002;24:951–67. doi: 10.1076/jcen.24.7.951.8387. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Hum Brain Mapp. 1999;8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainscsek C, Hernandez ME, Weyhenmeyer J, Sejnowski TJ, Poizner H. Non-linear dynamical analysis of EEG time series distinguishes patients with Parkinson’s disease from healthy individuals. Front Neurol. 2013;4:200. doi: 10.3389/fneur.2013.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, De Geus EJ, Aston-Jones G. The anatomical and functional relationship between the P3 and autonomic components of the orienting response. Psychophysiology. 2011;48:162–75. doi: 10.1111/j.1469-8986.2010.01057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuijzen PHJA, Horstink MW, Bloem BR, Duysens J. Startle responses in Parkinson patients during human gait. Exp Brain Res. 2006;171:215–24. doi: 10.1007/s00221-005-0270-0. [DOI] [PubMed] [Google Scholar]
- Nolan H, Whelan R, Reilly RB. FASTER: Fully Automated Statistical Thresholding for EEG artifact Rejection. J Neurosci Methods. 2010;192:152–62. doi: 10.1016/j.jneumeth.2010.07.015. [DOI] [PubMed] [Google Scholar]
- O’Connell RG, Dockree PM, Kelly SP. A supramodal accumulation-to-bound signal that determines perceptual decisions in humans. Nat Neurosci. 2012;15:1729–35. doi: 10.1038/nn.3248. [DOI] [PubMed] [Google Scholar]
- Penders CA, Delwaide PJ. Blink reflex studies in patients with Parkinsonism before and during therapy. J Neurol Neurosurg Psychiatry. 1971;34:674–8. doi: 10.1136/jnnp.34.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Chen K-H, Kingyon JR, Cavanagh JF, Narayanan NS. Medial frontal ∼4-Hz activity in humans and rodents is attenuated in PD patients and in rodents with cortical dopamine depletion. J Neurophysiol. 2015;114:1310–20. doi: 10.1152/jn.00412.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliakoff E, O’Boyle DJ, Moore AP, McGlone FP, Cody FWJ, Spence C. Orienting of attention and Parkinson’s disease: Tactile inhibition of return and response inhibition. Brain. 2003;126:2081–92. doi: 10.1093/brain/awg210. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–48. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Herbst KL. P300 as a clinical assay: Rationale, evaluation, and findings. Int J Psychophysiol. 2000;38:3–19. doi: 10.1016/S0167-8760(00)00127-6. [DOI] [PubMed] [Google Scholar]
- Popescu AT, Zhou MR, Poo M-M. Phasic dopamine release in the medial prefrontal cortex enhances stimulus discrimination. Proc Natl Acad Sci USA. 2016;113:E3169–3176. doi: 10.1073/pnas.1606098113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin CH, Abrams T, Barry RJ, Bhatnagar S, Clayton DF, Colombo J, et al. Habituation revisited: An updated and revised description of the behavioral characteristics of habituation. Neurobiol Learn Mem. 2009;92:135–8. doi: 10.1016/j.nlm.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Cools R. Cognitive deficits in Parkinson’s disease: A cognitive neuroscience perspective. Mov Disord. 2014;29:597–607. doi: 10.1002/mds.25853. [DOI] [PubMed] [Google Scholar]
- Seer C, Lange F, Georgiev D, Jahanshahi M, Kopp B. Event-Related Potentials and Cognition in Parkinson’s Disease: An Integrative Review. Neurosci Biobehav Rev. 2016;71:691–714. doi: 10.1016/j.neubiorev.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Sharpe MH. Distractibility in Early Parkinson’s Disease. Cortex. 1990;26:239–46. doi: 10.1016/S0010-9452(13)80353-X. [DOI] [PubMed] [Google Scholar]
- Smit DJa, Posthuma D, Boomsma DI, deGeus EJC. Genetic contribution to the P3 in young and middle-aged adults. Twin Res Hum Genet. 2007;10:335–47. doi: 10.1375/twin.10.2.335. [DOI] [PubMed] [Google Scholar]
- Solís-Vivanco R, Rodríguez-Violante M, Rodríguez-Agudelo Y, Schilmann A, Rodríguez-Ortiz U, Ricardo-Garcell J. The P3a wave: A reliable neurophysiological measure of Parkinson’s disease duration and severity. Clin Neurophysiol. 2015;126:2142–9. doi: 10.1016/j.clinph.2014.12.024. [DOI] [PubMed] [Google Scholar]
- Steiner GZ, Barry RJ. Pupillary responses and event-related potentials as indices of the orienting reflex. Psychophysiology. 2011;48:1648–55. doi: 10.1111/j.1469-8986.2011.01271.x. [DOI] [PubMed] [Google Scholar]
- Tsuchiya H, Yamaguchi S, Kobayashi S. Impaired novelty detection and frontal lobe dysfunction in Parkinson’s disease. Neuropsychologia. 2000;38:645–54. doi: 10.1016/S0028-3932(99)00108-6. [DOI] [PubMed] [Google Scholar]
- Vogt T, Kramer K, Gartenschlaeger M, Schreckenberger M. Estimation of further disease progression of Parkinson’s disease by dopamin transporter scan vs clinical rating. Park Relat Disord. 2011;17:459–63. doi: 10.1016/j.parkreldis.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Weingarten CP, Sundman MH, Hickey P, Chen Nkuei. Neuroimaging of Parkinson’s disease: Expanding views. Neurosci Biobehav Rev. 2015;59:16–52. doi: 10.1016/j.neubiorev.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR, Aron AR. On the globality of motor suppression : unexpected events and their influence on behavior and cognition. Neuron. 2017;93:259–80. doi: 10.1016/j.neuron.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Kobayashi S. Contributions of the dopaminergic system to voluntary and automatic orienting of visuospatial attention. J Neurosci. 1998;18:1869–78. doi: 10.1523/JNEUROSCI.18-05-01869.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Hirata K, Tanaka H, Hozumi A, Yamazaki K. Insufficient Processing Resources in Parkinson’s Disease: Evaluation Using Multimodal Event-Related Potentials Paradigm. Brain Topogr. 2002;14:299–311. doi: 10.1023/a:1015704827984. [DOI] [PubMed] [Google Scholar]
- Zhou S, Chen X, Wang C, Yin C, Hu P, Wang K. Selective attention deficits in early and moderate stage Parkinson’s disease. Neurosci Lett. 2012;509:50–5. doi: 10.1016/j.neulet.2011.12.049. [DOI] [PubMed] [Google Scholar]


