Abstract
Objective
Multiple studies have shown that gut microbes contribute to atherosclerosis, and there is mounting evidence that microbial metabolism of dietary nutrients influences pathophysiology. We hypothesized that indole- and phenyl-derived metabolites that originate solely or in part from bacterial sources would differ between patients with advanced atherosclerosis and age- and sex-matched controls without clinically apparent atherosclerosis.
Methods
Plasma from the advanced atherosclerosis cohort (n=100) were from patients who underwent carotid endarterectomy, leg bypass surgery, or major leg amputation for critical limb ischemia. The controls (n=22) were age- and sex-matched participants who had no peripheral arterial disease or history of stroke or myocardial infarction. Patients with chronic kidney disease were excluded. Metabolites and internal standards were measured using tandem high performance liquid chromatography and mass spectrometry.
Results
Plasma metabolite concentrations differed significantly between the advanced atherosclerosis and control cohorts. After adjustment for traditional atherosclerosis risk factors, indole (odds ratio [OR], .84; 95% confidence interval [CI], .75–.95; P=.004), tryptophan (OR, <.001; 95% CI, <.001–.003; P<.001), indole-3-propionic acid (OR, .27; 95% CI, .019–.91; P=.02), and indole-3-aldehyde (OR, .12; 95% CI, .014–.92; P=.04) negatively associated with advanced atherosclerosis, while the kynurenine/tryptophan ratio (OR, 61.7; 95% CI, 1.9–>999; P=.02) was positively associated. Furthermore, tryptophan and indole-3-propionic acid (Spearman coefficients 0.63 and 0.56, respectively, P<.001) correlated with the ankle-brachial index, a surrogate for overall atherosclerotic disease burden. Fourteen patients experienced a major post-operative cardiac complication within 30 days in the advanced atherosclerosis cohort, which was associated with baseline kynurenine/tryptophan ratio (P=.001) and hippuric acid (P=.03). In a multivariate analysis, only the kynurenine/tryptophan ratio remained significantly associated with a post-operative cardiac complication (OR, 44.1; 95% CI, 3.3–587.1; P=.004). Twenty patients in the advanced atherosclerosis cohort experienced a major adverse cardiac event during the follow-up period, which was associated with hippuric acid (P=.002) and the kynurenine/tryptophan ratio (P<.001). Both hippuric acid and the kynurenine/tryptophan ratio were independently associated with a major adverse cardiac event in multivariate analyses that included diabetes mellitus.
Conclusions
Specific microbe-derived metabolite signatures associate with advanced human atherosclerosis and post-operative cardiac complications. We suggest that these metabolites are potential novel biomarkers for atherosclerotic disease burden and that further investigation into mechanistic links between defined microbial metabolic pathways and cardiovascular disease is warranted.
Introduction
Multiple animal and human studies have demonstrated that gut microbes contribute to human atherosclerosis and associated risk factors such as obesity and diabetes mellitus.1–9 Biologic pathways by which microbes are known to regulate atherosclerotic risk are mediated by metabolism of dietary nutrients to short chain fatty acids, secondary bile acids, and trimethylamine (reviewed in ref10). However, whether other gut microbe-derived metabolites and their biotransformants are associated with atherosclerosis is largely unknown.
An untargeted metabolomics study comparing plasma present in conventional and germ-free mice revealed distinct plasma metabolite profiles between the two sample set.11 Since germ-free mice are born and raised without microbes, any differences in the plasma metabolite profiles between conventional and germ-free mice implies a role for bacterial-dependent metabolism or for host-microbial interactions. Among the differences in plasma metabolite profiles, specific indole- and phenyl-derived metabolites that originate solely or in part from microbial sources are potentially biomarkers of advanced atherosclerosis. Some of these metabolites have known links to atherosclerosis in animal models or human studies (see Supplemental Table I), but the interrelationships among them, associations with clinical phenotypes in patients undergoing surgery for advanced atherosclerosis, and predictive correlations with post-operative outcomes have not been previously examined.
This represents a pilot targeted metabolomics study comparing plasma concentrations of these metabolites between a cohort of patients with advanced atherosclerosis and an age- and sex-matched control cohort of patients without clinically-apparent atherosclerosis. We also correlated plasma metabolite concentrations with ankle-brachial index (ABI), a surrogate for overall atherosclerosis disease burden,12 and with post-operative events in the advanced atherosclerosis cohort. We anticipate that the results of this study could be used for hypothesis generation into mechanisms by which microbes influence atherosclerosis development and to identify novel microbe-related biomarkers for atherosclerosis severity and patient outcomes.
Methods
Study population
Advanced atherosclerosis cohort
Plasma samples for the advanced atherosclerosis cohort were collected from a single institution as part of a separate study. Demographic and clinical data and blood were collected from patients undergoing carotid endarterectomy, open infrainguinal leg revascularization for peripheral arterial disease (PAD), or major leg amputation for critical limb ischemia between 2012 and 2015. The study was approved by the Partners Human Research Committee Institutional Review Board. For the purposes of the current study, all patients were classified as having “advanced atherosclerosis” by virtue of the indications for surgery, i.e., high grade carotid stenosis for the patients undergoing carotid endarterectomy, critical limb ischemia or disabling claudication for patients undergoing lower extremity revascularization, or critical limb ischemia with non-reconstructable PAD for the patients undergoing lower extremity amputation. We excluded any participant with a history of stage IV and V chronic kidney disease (CKD) or dialysis-dependence since impaired renal clearance may confound metabolite plasma concentrations. All blood samples were drawn preoperatively on the day of surgery. Blood was collected into EDTA and sodium citrate vacutainer tubes. Tubes were spun at 3,000 revolutions per minute for 20 minutes at 4° C. Plasma was immediately aliquoted into sterile cryogenic tubes and stored at −70° C. Samples had not been thawed prior to use for this study.
Control cohort
The control group without atherosclerosis was selected in a 4:1 fashion from participants in the Walking and Leg Circulation Studies (WALCS) II and III between 2009 and 201113,14 and was approved by the Northwestern University Institutional Review Board. Matching was performed on three factors, age (age ≥ 70 vs. age < 70), sex, and history of diabetes mellitus, i.e., 8 strata. Controls were selected to create equivalent proportions of patients and controls in each strata. In addition, controls had no PAD (as determined by normal ABI) or clinically-apparent atherosclerosis (history of stroke or myocardial infarction [MI]) or renal impairment. Plasma samples were prepared and stored in the same fashion as described above and had not been thawed prior to use for this study.
The Northwestern University Institutional Review Board approved the overall current study protocol and no additional informed consent was required.
Variables
Sociodemographic variables included sex and age. Plasma high-sensitivity C-reactive protein (CRP) concentrations in both cohorts were determined using an immunotechnique on a Behring BN II analyzer (Dade Behring, Wilmington, DE). Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease (MDRD) Study equation.15 Past medical history and medication use in both cohorts was collected at the time of original study enrollment based on a combination of patient report, medical record review, and medication lists. In both cohorts, smoking was defined as current or prior tobacco use. In the control cohort, bilateral resting ABI was measured at the baseline visit. In the advanced atherosclerosis cohort, bilateral resting ABI, when obtained, was also measured at the baseline visit. The method of ABI measurement for both cohorts was similar. In brief, systolic blood pressure measurements in the bilateral brachial, dorsalis pedis, and posterior tibial arteries were obtained in the supine position using a hand-held Doppler instrument. To avoid potential bias from axillary or subclavian artery stenosis, the higher of the brachial artery pressures was used as the denominator when brachial pressures differed by ≥ 10 mm Hg. For each lower extremity, the ABI numerator was the highest pressure (dorsalis pedis or posterior tibial) from that leg. The leg cuff was inflated to a maximum of 250 mm Hg, and the ABI was classified as “non-compressible” if a pulse was still detected at this level. In all patients, the lower ABI for each patient was selected for analysis. All invalid ABI (non-compressible vessels or ABI ≥ 1.3) were excluded from the analysis.
Follow-up and outcomes
All advanced atherosclerosis patients were followed for at least one year. Post-operative events, including date of death, were recorded real-time from the medical record. The main clinical outcomes were a major post-operative cardiac complication, defined as a composite of MI, arrhythmia, and/or heart failure exacerbation16 within 30 days of vascular surgical procedure, and major adverse cardiac event (MACE), defined as a composite of all-cause death, stroke, MI, or coronary revascularization during the follow-up period. Post-operative stroke was defined as any new embolic, thrombotic, or hemorrhagic cerebrovascular event with neurological deficits that persisted for at least 24 hours, as defined by an attending neurologist. Post-operative MI was defined according to the American Heart Association universal definition of acute MI.17
Detection and quantification of metabolites by high performance liquid chromatography (HPLC)-tandem mass spectrometry
See Supplemental Methods.
Statistical Analysis
Summary statistics for continuous variables are reported as median values with interquartile ranges. Categorical variables are reported as frequencies and percentages. Categorical variables were compared using Fisher’s exact testing. Continuous variables were analyzed using the Wilcoxon signed-rank test or the Student’s t test based on the normality of distribution. Associations between continuous variables were evaluated by Spearman rank correlation. Metabolite and CRP concentrations were natural log (ln)-transformed to reduce skewness before regression analyses. ROC analyses were also carried out via logistic regression to examine the sensitivity and specificity of the metabolites and other clinical factors for predicting selected outcomes. A P value < .05 was considered statistically significant. All statistical analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, NC) and GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA).
Results
Cohort characteristics at baseline
After exclusion of participants with a history of stage IV or V CKD or dialysis-dependence, 122 participants were eligible for analysis. Baseline participant characteristics are presented in Table IA. As expected, the advanced atherosclerosis cohort (n=100) had a greater prevalence of diabetes, hypertension, smoking, and history of MI and stroke; lower high density lipoprotein (HDL) cholesterol; more frequent use of statins; and worse median lowest ABI than the control cohort (n=22). Interestingly, median low density lipoprotein (LDL) cholesterol was lower in the advanced atherosclerosis group, presumably due to statin use.
Table I.
| A. Baseline characteristics of study population. | |||
|---|---|---|---|
|
| |||
| Covariates | Control cohort (n = 22) |
Advanced atherosclerosis cohort (n = 100) |
P value |
|
| |||
| Median age (years) | 70 (68, 76) | 69 (63, 74) | .14 |
|
| |||
| Female sex | 9 (40.9%) | 37 (37%) | .81 |
|
| |||
| Non-Caucasian race | 1 (4.6%) | 12 (12%) | .46 |
|
| |||
| Body mass index (kg/m2) | 26.8 (24.8, 28.4) | 27.3 (24.4, 31.7) | .88 |
|
| |||
| Past medical history | |||
|
| |||
| Diabetes mellitus | 2 (9.1%) | 35 (35%) | .006 |
|
| |||
| Hypertension | 15 (78.2%) | 91 (91%) | .01 |
|
| |||
| Prior MI | 0 | 25 (25%) | .007 |
|
| |||
| CHF | 0 | 13 (13%) | .12 |
|
| |||
| Stroke | 0 | 19 (19%) | .07 |
|
| |||
| Current or former smoker | 10 (45.5%) | 79 (79%) | .001 |
|
| |||
| Statin use | 9 (40.9%) | 86 (86%) | <.001 |
|
| |||
| Aspirin use | No data | 90 (90%) | |
|
| |||
| Lowest ABI | 1.12 (1.1, 1.2) | .5 (.22, .65) | <.001 |
|
| |||
| LDL cholesterol (mg/dL) | 115.4 (95.2, 125.8) | 81.5 (71.0, 115.0) | .007 |
|
| |||
| HDL cholesterol (mg/dL) | 57.7 (41.9, 68.0) | 42.0 (34.0, 50.0) | .005 |
|
| |||
| eGFR (mL/min/1.73mm2) | 72.4 (59.0, 96.1) | 66.6 (50.1, 81.8) | .17 |
|
| |||
| hs-CRP (mg/mL) | 2.1 (.78–3.42) | 7.4 (2.2–60.6) | .24 |
|
| |||
| Type of surgery | |||
|
| |||
| CEA | 48 (48%) | ||
| Open infrainguinal revascularization | 40 (40%) | ||
| Major leg amputation | 12 (12%) | ||
|
| |||
| Metabolites | |||
|
| |||
| Indole and indole derivatives | |||
|
| |||
| Indole (nmol) | 72.6 (0, 170.7) | 0 (0, 0) | .002 |
|
| |||
| Tryptophan (µmol) | 101.6 (97.4, 110.4) | 80.2 (72.9, 88.2) | <.001 |
|
| |||
| Kynurenine (nmol) | 10049.0 (9275.3, 11888.2) | 9175.3 (8033.8, 11063.5) | .03 |
|
| |||
| Kynurenine/tryptophan ratio | 97.8 (86.1, 108.3) | 111.11 (98.1, 134.3) | .002 |
|
| |||
| 3-Hydroxyanthranilic acid (nmol) | 238.3 (98.0, 333.0) | 339.6 (251.4, 466.9) | .005 |
|
| |||
| Indole-3-propionic acid (µmol) | .41 (.27, .90) | .22 (.16, .34) | <.001 |
|
| |||
| Indole-3-aldehyde (nmol) | 124.0 (103.3, 158.4) | 96.4 (62.0, 117.1) | <.001 |
|
| |||
| Indoxyl sulfate (µmol) | 7.7 (6.6, 9.1) | 7.5 (6.1, 9.0) | .46 |
|
| |||
| Phenyl derivatives | |||
|
| |||
| Hippuric acid (µmol) | 9.0 (7.9, 10.4) | 7.9 (7.2, 10.9) | .16 |
|
| |||
| p-Cresyl sulfate (µmol) | 94.0 (67.7, 130.3) | 7.5 (6.1, 9.0) | .83 |
| B. Odds ratios for advanced atherosclerosis obtained from logistic models, each involving individual metabolites separately, adjusted for diabetes mellitus, hypertension, smoking (current/former), and HDL cholesterol. | |||
|---|---|---|---|
| Metabolite | Odds ratio | 95% CI | P value |
| ln indole (nmol) | .84 | .75, .95 | .004 |
| ln tryptophan (µmol) | <.001 | <.001, .003 | < .001 |
| ln kynurenine (nmol) | .11 | .005, 2.7 | .18 |
| ln kynurenine/tryptophan ratio | 61.7 | 1.9, >999 | .02 |
| ln 3-hydroxyanthranilic acid (nmol) | .95 | .73, 1.2 | .72 |
| ln indole-3-propionic acid (µmol) | .27 | .09, .91 | .02 |
| ln indole-3-aldehyde (nmol) | .12 | .014, .92 | .04 |
All values shown are median (interquartile range) or n (%). MI indicates myocardial infarction. CHF, congestive heart failure. ABI, ankle-brachial index. LDL, low density lipoprotein. HDL, high density lipoprotein. eGFR, estimated glomerular filtration rate. hs-CRP, high-sensitivity C-reactive protein. CEA, carotid endarterectomy.
The majority of patients undergoing carotid endarterectomy (n=29, 60.4%) had asymptomatic high-grade carotid artery stenosis. In the patients undergoing infrainguinal revascularization, 72% (n=28) had critical limb ischemia as the indication for surgery and the others had disabling claudication. All patients undergoing major leg amputation had critical limb ischemia but half had concomitant non-salvageable foot infections.
Patients with advanced atherosclerosis had significantly lower baseline plasma concentrations of indole and the indole derivatives tryptophan (trp), kynurenine (kyn), indole-3-propionic acid (I3P), and indole-3-aldehyde (I3A) and higher plasma concentrations of 3-hydroxyanthranilic acid than the patients in the control cohort. The indole derivative serotonin was undetected in 97 (80%) of patients and was excluded from further analysis. The kyn/trp ratio, an estimate of the activity of indole-2,3-deoxygenase (IDO1) and tryptophan-2,3-deoxygenase (TDO),18 was significantly higher in the advanced atherosclerosis cohort. Of note, we did not have adequate power to reject the null hypothesis for indoxyl sulfate, hippuric acid, and p-cresyl sulfate (PCS). After adjusting all the other metabolites for traditional risk factors for advanced atherosclerosis19 (history of diabetes mellitus, hypertension, smoking, and HDL cholesterol) (Table IB), indole (odds ratio [OR] .84; 95% confidence interval [CI] .75–.95; P=.004), trp (OR < .001; 95% CI < .001–.003; P<.001), I3P (OR .27; 95% CI .09–.91; P=.02), and I3A (OR .12; 95% CI .014–.92; P=.04) remained significantly negatively associated with advanced atherosclerosis, while the kyn/trp ratio (OR 61.7; 95% CI 1.9–>999; P=.02) was significantly positively associated with advanced atherosclerosis. Given the small odds ratio for tryptophan, we also dichotomized tryptophan concentration by its 75th percentile (94.87 mol) and found an odds ratio for advanced atherosclerosis of .06 (95% confidence interval .013–.254; P=.0002), again suggesting that tryptophan greatly reduces the risk of advanced atherosclerosis.
In order to address potential heterogeneity of patient characteristics in the advanced atherosclerosis cohort, we next compared the baseline characteristics of the subset of patients undergoing carotid endarterectomy (n=48) with the control cohort. Baseline characteristics of these two groups differed in prevalence of hypertension, smoking, statin use, median LDL and HDL cholesterol, and in median lowest ABI (Supplemental Table IIA). As noted in the advanced atherosclerosis cohort as a whole, the carotid stenosis subgroup also had significantly lower indole, trp, kyn, I3P, and I3A, and higher plasma levels of 3-hydroxyanthranilic acid. The kyn/trp ratio was also significantly higher in the carotid stenosis subgroup compared to the control cohort. Again, we were underpowered to detect a difference between the two groups in the concentrations of indoxyl sulfate, hippuric acid, and PCS. After adjustment of the all the other metabolites for risk factors for carotid stenosis (diabetes mellitus, hypertension, smoking, and HDL cholesterol) (Supplemental Table IIB), indole (OR .87; 95% CI .77–.98; P=.03) and trp (OR <.001; 95% CI <.001; P=.001) remained significantly negatively associated with carotid stenosis and the kyn/trp ratio (OR 99; 95% CI 1.5 – >999; P=.03) remained significantly positively associated with carotid stenosis.
Within the advanced atherosclerosis cohort, we also found that the plasma concentrations of certain metabolites discriminated between procedure type, as shown in Supplemental Table III. Specifically, the kyn/trp ratio was highest in the major amputation cohort (n=12) and lowest in the carotid endarterectomy cohort (n=48) (P=.02), while the opposite pattern was observed for trp (P=.008), I3P (P=.005), and I3A (P=.02).
Relationship between metabolites
These data revealed that trp was approximately 8- to 10-fold more abundant than kyn and 1000-fold more abundant than indole, which was anticipated since the kyn pathway accounts for the catabolism on 99% of ingested trp not used for protein synthesis.20 There were modest correlations between the various indole derivatives and between the two phenyl derivatives, hippuric acid and PCS, as shown in Supplemental Table IV. We observed mild but significant correlations between trp, I3P, and CRP, a marker of systemic inflammation, suggesting potential overlapping signaling pathways.
Relationship between metabolites and ABI
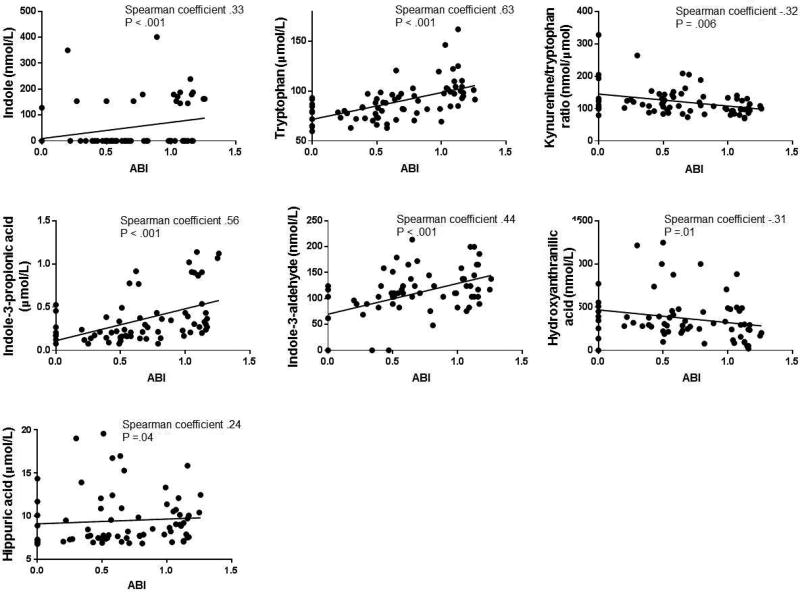
The ABI is a general measure of overall atherosclerosis disease burden and is associated with atherosclerotic risk factors.12 Thus, we examined the association between the metabolites and ABI in the entire cohort. In the advanced atherosclerosis cohort, an ABI was obtained in 50 patients (50%). Although an ABI was obtained in all but three patients undergoing lower extremity revascularization or amputation, only six patients undergoing carotid endarterectomy had an ABI measurement. In a univariate analysis of each metabolite and ABI in the entire cohort, higher indole, trp, I3P, I3A, and hippuric acid were significantly associated with higher ABI, or less severe PAD, while the kyn/trp ratio and 3-hydroxyanthranilic acid were negatively associated with ABI, as shown in Figure 1. The metabolites with the strongest correlations to ABI were trp (Spearman coefficient=0.63, P<.001), I3P (Spearman coefficient=0.56, P<.001), and I3A (Spearman coefficient=0.44, P<.001). The direction, magnitude, and significance of these relationships were preserved even after exclusion of outliers based on visual inspection of the histogram of each metabolite (data not shown). When we performed the correlation analysis of metabolite concentrations with ABI in the advanced atherosclerosis cohort only, there was a significant correlation seen only with I3A (Spearman r .37; P=.01). However, when we repeated the correlation analysis in the control cohort only, there was a significant positive correlation seen only with the kyn/trp ratio (Spearman r −.54; P=.02), demonstrating that the two groups are different, as we anticipated from Tables IA and IB. However, when we analyzed the control and advanced atherosclerosis cohorts separately, the sample size for each cohort decreased, possibly accounting for the differences in correlations with ABI with the entire combined cohort.
Figure 1.
Correlations between metabolites and ABI in the entire cohort. Only correlations with P < .05 are shown. Spearman coefficients and P values are shown for each plot. Each line represents the linear regression of each metabolite on ABI.
Relationship between metabolites and post-operative outcomes in the advanced atherosclerosis cohort
Fourteen (14%) patients in the advanced atherosclerosis cohort experienced a major post-operative cardiac complication (MI, arrhythmia, and/or heart failure exacerbation) within the first 30 days of surgery. Patients who experienced this event were more likely to be diabetic and have a history of congestive heart failure (CHF) and stroke, as shown in Table II. In addition, baseline decreased trp (P=.02), increased kyn/trp (P=.001), and increased hippuric acid (P=.03) concentrations were all associated with increased risk of major post-operative cardiac event. In a multivariate analysis that included diabetes, kyn/trp, and hippuric acid, only the kyn/trp ratio remained significantly associated with a post-operative cardiac complication (OR 44.1; 95% CI 3.3–587.1; P=.004). A predictive model for a major post-operative cardiac complication that included diabetes, history of CAD, CHF, and stroke had good discrimination, as reflected by an area under the curve (AUC) of 0.88 (SEM 0.04). This was improved by addition of ln kyn/trp and ln hippuric acid to the model, with AUC of 0.95 (SEM 0.02). The difference in these curves was marginally significant (P=.09)
Table II.
| Baseline characteristics of patients who had a major postoperative cardiac complication within 30 days of surgery in the advanced atherosclerosis cohort. | |||
|---|---|---|---|
| Covariate | No major postoperative cardiac complication (n = 86) |
Major postoperative cardiac complication (n = 14) |
P value |
| Median age (years) | 68.5 (63, 74) | 70.5 (64, 74) | .94 |
| Female sex | 32 (37.2%) | 5 (35.71%) | 1.0 |
| Non-Caucasian race | 9 (10.5%) | 3 (21.4%) | .37 |
| Body mass index (kg/m2) | 27.3 (24.4, 31.3) | 28.3 (23.6, 34.3) | .75 |
| Past medical history | |||
| Diabetes mellitus | 26 (30.2%) | 9 (64.3%) | .03 |
| Hypertension | 77 (89.5%) | 14 (100%) | .35 |
| Prior MI | 19 (22.1%) | 6 (42.9%) | .11 |
| CHF | 7 (8.1%) | 6 (42.9%) | .003 |
| Stroke | 16 (18.6%) | 7 (50%) | .02 |
| Current or former smoker | 69 (80.2%) | 10 (71.4%) | .48 |
| Statin use | 74 (86.1%) | 12 (85.7%) | 1.0 |
| Lowest ABI | .52 (.34, .69) | .22 (0, .41) | .02 |
| Type of procedure | .51 | ||
| Carotid endarterectomy | 42 (48.8%) | 6 (42.9%) | |
| Infrainguinal revascularization | 35 (40.7%) | 5 (35.7%) | |
| Major leg amputation | 9 (10.5%) | 3 (21.4%) | |
| LDL cholesterol (mg/dL) | 79 (69.5, 112) | 107 (74, 120) | .38 |
| HDL cholesterol (mg/dL) | 42 (34, 52) | 46 (32, 49) | .99 |
| eGFR (mL/min/1.73mm2) | 67.6 (51.9, 82.4) | 54.1 (46.1, 70.4) | .08 |
| CRP (mg/mL) | 7.4 (2, 86.3) | 6.7 (2.6, 33.6) | .79 |
| Indole (nmol) | 0 (0, 0) | 0 (0, 0) | .27 |
| Tryptophan (µmol) | 81.89 (74.9, 88.6) | 73.3 (71.9, 78.8) | .02 |
| Kynurenine (nmol) | 8983.7 (8010.8, 10391.3) | 11076.5 (9074.7, 14712.7) | .03 |
| Kynurenine/tryptophan ratio | 108.7 (95.5, 130.7) | 143.1 (123.9, 168.3) | .001 |
| 3-Hydroxyanthranilic acid (nmol) | 323.2 (244.9, 466.9) | 342.8 (316.7, 515.9) | .27 |
| Indole-3-proprionic acid (µmol) | .23 (.16, .36) | .18 (.12, .25) | .17 |
| Indole-3-aldehyde (µmol) | 96.4 (55.1, 117.1) | 99.89 (82.67, 124.0) | .30 |
| Indoxyl sulfate (µmol) | 7.35 (6.08, 9.0) | 7.8 (5.4, 12.9) | .84 |
| Hippuric acid (µmol) | 7.7 (7.2, 9.9) | 9.2 (7.8, 15.3) | .03 |
| p-Cresyl sulfate (µmol) | 102.6 (53.6, 148.5) | 142.0 (47.7, 183.9) | .40 |
During a median follow-up period of 14.5 months (IQR 3, 23.8), 20 patients in the advanced atherosclerosis cohort experienced a MACE. As observed for patients who experienced a major post-operative cardiac complication, patients who experienced MACE were more likely to be diabetic (P<.001) and have a history of CHF (P=.004) and stroke (P=.02) (Table IIIA). Baseline trp (P=.007), kyn/trp ratio (P≤.001), and hippuric acid (P=.002) were all linked to MACE on univariate analysis. In a multivariate analysis that included diabetes and the kyn/trp ratio, both diabetes and the kyn/trp ratio were independently associated with MACE (Table IIIB). Similarly, in a multivariate analysis that included diabetes and hippuric acid, both diabetes and hippuric acid were significantly associated with MACE. However, in a model that included diabetes and both the kyn/trp ratio and hippuric acid, only diabetes remained an independent predictor of MACE, suggesting that the effect of kyn and trp potentially negated the effect of hippuric acid.
Table III.
| A. Baseline characteristics of patients who had a major adverse cardiac event (MACE) during the follow-up period in the advanced atherosclerosis cohort | |||
|---|---|---|---|
| Covariate | No MACE (n = 80) | MACE (n = 20) | P value |
| Median age (years) | 67.5 (63, 74) | 71 (67, 74) | .31 |
| Female sex | 30 (37.0%) | 7 (36.8%) | .80 |
| Non-Caucasian race | 9 (9.9%) | 4 (21.1%) | .25 |
| Body mass index (kg/m2) | 27.5 (24.8, 31.9) | 25.5 (23.10 29.5) | .23 |
| Past medical history | |||
| Diabetes mellitus | 21 (25.9%) | 14 (73.7%) | <.001 |
| Hypertension | 73 (90.1%) | 18 (94.7%) | .68 |
| Prior MI | 19 (23.5%) | 6 (31.6%) | .26 |
| CHF | 6 (7.4%) | 7 (36.8%) | .004 |
| Stroke | 15 (18.5%) | 8 (42.1%) | .02 |
| Current or former smoker | 64 (79.0%) | 15 (79.0%) | 1.0 |
| Statin use | 72 (88.9%) | 14 (73.7%) | .15 |
| Lowest ABI | .54 (.27, .69) | .34 (.22, .49) | .09 |
| Type of procedure | .56 | ||
| Carotid endarterectomy | 41 (85.4%) | 7 (14.6%) | |
| Infrainguinal revascularization | 30 (75%) | 10 (25%) | |
| Major leg amputation | 9 (75%) | 3 (25%) | |
| LDL cholesterol (mg/dL) | 79 (71, 111) | 102.5 (68.5, 120.5) | .43 |
| HDL cholesterol (mg/dL) | 42 (34, 51) | 42 (33, 49) | .94 |
| eGFR (mL/min/1.73mm2) | 67.0 (51.6, 81.8) | 58.9 (40.1, 86.3) | .35 |
| CRP (mg/mL) | 5.4 (2, 86.3) | 8.9 (2.6, 35.9) | .97 |
| Indole (nmol) | 0 (0, 0) | 0 (0, 0) | .16 |
| Tryptophan (µmol) | 82.0 (74.9, 90.1) | 73.3 (71.8, 79.5) | .007 |
| Kynurenine (nmol) | 8980.1 (7774.5, 10414.8) | 9989.3 (8972.7, 12020.1) | .03 |
| Kynurenine/tryptophan ratio | 107.5 (94.4, 126.0) | 131.7 (119.2, 159.1) | <.001 |
| 3-Hydroxyanthranilic acid (nmol) | 320.0 (241.6, 457.1) | 391.8 (306.9, 959. 9) | .06 |
| Indole-3-proprionic acid (µmol) | .23 (.16, .36) | .20 (.14, .30) | .50 |
| Indole-3-aldehyde (µmol) | 96.4 (55.1, 117.1) | 89.6 (75.8, 124.0) | .39 |
| Indoxyl sulfate (µmol) | 7.1 (6.1, 8.7) | 8.7 (5.9, 14.6) | .09 |
| Hippuric acid (µmol) | 7.7 (7.1, 9.5) | 11.1 (7.9, 17.0) | .002 |
| p-Cresyl sulfate (µmol) | 102.6 (52.4, 139.2) | 148.4 (52.8, 219.9) | .14 |
| B. Multivariate analysis of MACE after adjustment for diabetes and metabolites. | |||
|---|---|---|---|
| Model A | Odds ratio | 95% CI | P value |
| Diabetes mellitus | 5.4 | 1.7, 16.7 | .003 |
| ln kynurenine/tryptophan ratio | 14.9 | 1.8, 120.8 | .01 |
| Model B | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Diabetes mellitus | 7.2 | 2.2, 23.1 | <.001 |
| ln hippuric acid | 7.7 | 1.7, 34.6 | .008 |
| Model C | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Diabetes mellitus | 6.1 | 1.9, 19.9 | .003 |
| ln hippuric acid | 4.4 | .86, 22.1 | .08 |
| ln kynurenine/tryptophan ratio | 5.4 | .51, 56.6 | .16 |
Discussion
In this exploratory study, we demonstrate that baseline plasma concentrations of multiple gut microbiome-modulated indole- and phenyl-derived metabolites are associated with advanced atherosclerosis and predict risk of post-operative cardiac events and mortality in patients with advanced atherosclerosis.
Specifically, we show that patients with advanced atherosclerosis (patients undergoing either carotid endarterectomy; leg revascularization; or major amputation for critical limb ischemia) have significantly lower baseline plasma concentrations of trp, indole, and indole derivatives I3A and I3P, and higher plasma concentrations of the kyn/trp ratio compared to an age- and sex-matched cohort without clinically apparent atherosclerosis after adjustment for traditional risk factors for atherosclerosis. There was also a moderately strong but significant relationship between these metabolites and ABI in the entire cohort. Similarly, the subset of patients undergoing carotid endarterectomy had lower indole and trp levels and higher kyn/trp ratios compared to the control cohort after adjustment for traditional risk factors. Furthermore, in the advanced atherosclerosis cohort as a whole, there was a significant association between the baseline kyn/trp ratio with post-operative major cardiac complications and a significant association between the baseline kyn/trp ratio and hippuric acid with MACE during the median follow-up period of 14 months that was preserved after adjustment for diabetes. This is the first report associating any of these metabolites to PAD or to major post-operative adverse cardiac events.
There are data in the literature supporting a link between some indole derivatives and atherosclerosis. Trp is an essential amino acid that is supplied entirely by the diet. The metabolism of trp proceeds via either the serotonin pathway or the kyn pathway.21 Upon entering the kyn pathway, trp is converted to N-formyl-l-kynurenine by TDO (expressed in liver) or IDO1 (ubiquitously expressed) and then to kyn. Interestingly, trp depletion activates the stress pathway22 and IDO is known to be upregulated by interferon-gamma, IL-2, and IL-10 activity. The kyn/trp ratio, which is an index of IDO1 or TDO activity, has been found to be elevated in states of immune stimulation, such as infection, malignancy, endotoxin administration, neurodegenerative disease, and autoimmunity.22–27 The kyn/trp ratio also takes into account differences in trp concentrations based on dietary intake of tryptophan.18. Furthermore, downstream kyn catabolites including 3-hydroxyanthranilic acid reduce Th1 and Th17 responses and affect T cell apoptosis.28–30 As atherosclerosis is a chronic inflammatory disease, it is not surprising that trp metabolism to kyn has been correlated with cardiovascular mortality and with coronary artery disease.14,31–33 Furthermore, IDO1 deficiency decreased atherosclerotic plaque in apolipoprotein E knockout mice, a genetic model of atherosclerosis.34 Our findings that trp was negatively and the kyn/trp ratio was positively associated with advanced atherosclerosis are in concordance with these data. In addition, we show a moderate but significant unadjusted relationship with ABI in the entire cohort that included patients without clinically significant atherosclerosis, as well as a correlation with perioperative cardiac adverse events within 30 days and with MACE in the first year after vascular surgery in the surgical cohort.
Trp is also regulated by gut microbes. Trp levels are elevated and kyn levels are decreased in germ-free mice, which is reversed after colonization by microbes.35,36 Unlike eukaryotes, bacteria can synthesize trp37 and some bacteria can also produce serotonin.38 In addition, trp is converted to indole, I3A, and I3P by bacterial enzymatic pathways.39,40 Indole compounds are ligands of the aryl hydrocarbon receptor (AHR),41 and prior work by others has demonstrated that AHR activation promotes atherosclerosis in ApoE knockout mice.42 In contrast, however, we observed an inverse relationship between indole, I3P, and I3A and advanced atherosclerosis as well as with ABI, suggesting that the effect of these metabolites on human atherosclerosis may be mediated by a different receptor, or that ky upregulation of AHR activation43 overshadows the production or the protective activity of indole, I3A, and/or I3P on atherosclerosis. Although I3P and I3A suppress central nervous system inflammation via AHR,44 the direct effect of these metabolites on vascular inflammation or atherogenesis is currently unknown and is actively under study. IS, the final other indole metabolite in this study, is produced by hepatic sulfonation of indole. It is normally excreted in the urine and is considered a uremic toxin since it accumulates in the plasma in chronic kidney disease.45 IS induces free radicals in vascular smooth muscle cells and vascular endothelial cells, inhibits viability and nitric oxide production of vascular endothelial cells, and promotes aortic calcification and aortic wall thickening in hypertensive rats.46 However, we were underpowered to detect a correlation between IS plasma concentration and atherosclerosis, possibly because we excluded all patients with stage IV and V CKD and dialysis-dependence.
We also studied the relationship of two phenyl-derived metabolites, PCS and hippuric acid, with advanced atherosclerosis. PCS is an end-product of protein breakdown.47 It is produced by hepatic sulfonation of p-cresyl, which is synthesized from tyrosine and phenylalanine by hydroxyphenylacetate decarboxylase in aerobes (mainly enterobacteria) and anaerobes.48 While there is evidence in the literature that PCS correlates with cardiovascular mortality in humans and with atherogenesis in mice (see Supplemental Table I references), we were underpowered to detect a correlation between plasma PCS concentration with atherosclerosis, possibly because we excluded patients with stage IV and V CKD and dialysis-dependence. Hippuric acid is a conjugate of glycine with benzoic acid and is excreted in the urine. Diets high in protein and polyphenols (e.g. fruits, vegetables, coffee, tea, and chocolate) are degraded by gut microbes to quinic and benzoic acid, which is then oxidized in the host liver to hippuric acid.49 Urine hippuric acid was elevated in atherosclerotic rats in a single study.50 However, while we observed a significant correlation between hippuric acid with post-operative adverse cardiac events and with MACE in the advanced atherosclerosis cohort, we were underpowered to detect a significant correlation with advanced atherosclerosis. We also found a weak correlation between hippuric acid with ABI in the entire cohort, suggesting that the relationship between hippuric acid and atherosclerosis is complex and requires further study in a larger cohort.
These findings are important as they support further exploration of these metabolites as novel and reliable biomarkers of advanced atherosclerosis and of clinically relevant post-operative outcomes in patients undergoing major vascular surgery. For instance, while a simple model of a history of diabetes, CAD, CHF, and stroke clearly stratified risk of a major post-operative cardiac complication, the addition of hippuric acid and kyn/trp ratio improved the AUC. Thus, whether a combination of metabolite biomarkers have additive value for clinical risk stratification in vascular surgery patients with advanced atherosclerosis is worthy of further testing. Our findings from real-world human specimens also point to the possibility that indole-and phenyl-derived metabolites, which are regulated by both host and microbial metabolic pathways, interact in a complex manner that animal studies focused on a single metabolic pathway or ligand-receptor relationship cannot discern. These interactions would be critical to consider for the development of any potential therapeutic interventions for atherosclerosis utilizing these metabolic pathways.
The limitations of this study include its non-concurrent cohorts and modest sample size with low event rate. Unequal follow-up times amongst all the patients in the advanced atherosclerosis cohort, which encompasses three different surgical procedures, could have contributed to bias in the event rate. In addition, we did not have dietary information or direct microbiome data from our patients to explore how differences in microbial communities correlate with the metabolite observations as we used banked plasma specimens from prior studies. We also chose these metabolites based on a metabolomics study performed in mice. There are obviously known microbial community differences and differences in innate immunity between mice and humans which preclude direct translation of experimental data across the species, but the contribution of the gut microbiome to the production of multiple metabolites in our study (trp, IS, I3P, I3A, hippuric acid, and PCS) have been observed in animal and/or human studies, and hence we felt that an exploration of their link to human atherosclerosis was warranted. In addition, metabolites of microbes that reach the circulation are likely more relevant than microbes themselves because they cross epithelial barriers8 to have systemic, rather than local intestinal effects. Furthermore, the clinical phenotype of our advanced atherosclerosis cohort, which represents an amalgam of patients undergoing vascular reconstruction or major amputation, may not be representative of all atherosclerosis populations, and thus our findings may not be generalizable and require further validation. For instance, the metabolites might have much stronger discriminatory ability for disease or clinical endpoints in a future, larger cohort of patients representing a single defined clinical entity, such as claudication or symptomatic carotid stenosis, with age- and sex-matched controls. We also emphasize that this is not a definitive study and multivariable models, which included traditional risk factors for each outcome, suggest an independent effect on the outcomes that needs to be validated in a larger prospective study. Finally, as an observational study, we cannot infer causality, and confounding by one or many unrecognized factors, including the interactions between diet, medications (including prior antibiotic exposure, which have long-lasting effects of gut microbial communities51), ethnicity, geographic location, and the microbiome require further investigation. However, the absence of an interventional study to confirm causality does not limit the potential value of these metabolites as biomarkers of atherosclerotic disease burden and cardiovascular event risk.
In conclusion, we demonstrate that indole and indole-derived metabolites trp, kyn/trp ratio, I3P, and I3A associate with advanced atherosclerosis, while the kyn/trp ratio and the phenyl derivative hippuric acid associate with post-operative major cardiac events and with MACE. These findings affirm the importance of investigating the mechanisms by which gut microbial metabolic pathways and host-microbe interactions contribute to atherosclerosis and its end-stage complications.
Supplementary Material
Take Home Message.
Concentration of gut-derived metabolites was significantly different in the plasma of patients with advanced atherosclerosis vs controls and the kynurenine/tryptophan ratio was positively asssociated with advanced atherosclerosis and with post operative cardiac complications
Recommendation.
The authors suggest to consider gut microbiome metabolites as biomarkers of advanced atherosclerotis and cardiovascular disease.
Clinical relevance.
Multiple studies have shown that the gut microbiome contributes to atherosclerosis, potentially through bioactive metabolites derived from commensal organisms. We performed a targeted metabolomic study of specific indole- and phenyl-derived metabolites that originate solely or in part from gut microbes. We found that the plasma concentrations of many of these metabolites differed in patients with advanced atherosclerosis and age- and gender-matched controls, and that the concentrations of some of these metabolites correlated with postoperative outcomes in the advanced atherosclerosis cohort. These metabolites and their biotransformants are worthy of further investigation as potentially important biomarkers or modulators of atherosclerosis.
Acknowledgments
We gratefully acknowledge the assistance of Dr. Timothy C. Morton at University of Chicago with LC-MS/MS, Liqun Xiong and Dr. Qun Jiang with sample preparation, Dr. Christopher Owens with statistical analysis, and the Northwestern University Simpson Querrey Institute for BioNanotechnology equipment core facility.
Grant support
This work was funded in part by T32HL094293 (to C.C.); R01HL58099, R01HL64739, R01HL109244 (to M.M.), R01 HL133500 (to C.O.); and K08HL130601 (to K.H.) from the National Heart, Lung, and Blood Institute; Medical Student Scholarship in Cardiovascular Surgery (to R.K.) and 16GRNT27090006 (to C.O.) from the American Heart Association; UL1TR001422 (to C.C., K.H. and M.A.) from the National Center for Advancing Translational Sciences; T32DK007074, R01DK097268, P30DK42086, and R56DK102872 (to E.C.) from the National Institute of Diabetes and Digestive and Kidney Diseases; American College of Surgeons/Society of Vascular Surgery (to K.H.); and Vascular Cures (to K.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lin HV, Frassetto A, Kowalik EJ, Jr, Nawrocki AR, Lu MM, Kosinski JR, et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PloS one. 2012;7(4):e35240. doi: 10.1371/journal.pone.0035240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy EF, Cotter PD, Hogan A, O'Sullivan O, Joyce A, Fouhy F, et al. Divergent metabolic outcomes arising from targeted manipulation of the gut microbiota in diet-induced obesity. Gut. 2013;62(2):220–226. doi: 10.1136/gutjnl-2011-300705. [DOI] [PubMed] [Google Scholar]
- 3.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 4.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 5.Cani PD, Delzenne NM, Amar J, Burcelin R. Role of gut microflora in the development of obesity and insulin resistance following high-fat diet feeding. Pathologie-biologie. 2008;56(5):305–309. doi: 10.1016/j.patbio.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 7.Karlsson FH, Fak F, Nookaew I, Tremaroli V, Fagerberg B, Petranovic D, et al. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nature communications. 2012;3:1245. doi: 10.1038/ncomms2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang WH, Kitai T, Hazen SL. Gut Microbiota in Cardiovascular Health and Disease. Circulation research. 2017;120(7):1183–1196. doi: 10.1161/CIRCRESAHA.117.309715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh SS, Bie J, Wang J, Ghosh S. Oral supplementation with non-absorbable antibiotics or curcumin attenuates western diet-induced atherosclerosis and glucose intolerance in LDLR−/− mice--role of intestinal permeability and macrophage activation. PloS one. 2014;9(9):e108577. doi: 10.1371/journal.pone.0108577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown JM, Hazen SL. The gut microbial endocrine organ: bacterially derived signals driving cardiometabolic diseases. Annual review of medicine. 2015;66:343–359. doi: 10.1146/annurev-med-060513-093205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(10):3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126(24):2890–2909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 13.McDermott MM, Hoff F, Ferrucci L, Pearce WH, Guralnik JM, Tian L, et al. Lower extremity ischemia, calf skeletal muscle characteristics, and functional impairment in peripheral arterial disease. Journal of the American Geriatrics Society. 2007;55(3):400–406. doi: 10.1111/j.1532-5415.2007.01092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDermott MM, Liu K, Carr J, Criqui MH, Tian L, Li D, et al. Superficial femoral artery plaque, the ankle-brachial index, and leg symptoms in peripheral arterial disease: the walking and leg circulation study (WALCS) III. Circulation. Cardiovascular imaging. 2011;4(3):246–252. doi: 10.1161/CIRCIMAGING.110.962183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Annals of internal medicine. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 16.Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043–1049. doi: 10.1161/01.cir.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 17.Thygesen K, Alpert JS, White HD, Joint ESCAAHAWHFTFftRoMI. Jaffe AS, Apple FS, et al. Universal definition of myocardial infarction. Circulation. 2007;116(22):2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 18.Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nature reviews. Drug discovery. 2002;1(8):609–620. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- 19.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 20.Peters JC. Tryptophan nutrition and metabolism: an overview. Advances in experimental medicine and biology. 1991;294:345–358. doi: 10.1007/978-1-4684-5952-4_32. [DOI] [PubMed] [Google Scholar]
- 21.Le Floc'h N, Otten W, Merlot E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino acids. 2011;41(5):1195–1205. doi: 10.1007/s00726-010-0752-7. [DOI] [PubMed] [Google Scholar]
- 22.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22(5):633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1991;5(11):2516–2522. [PubMed] [Google Scholar]
- 24.Takikawa O, Habara-Ohkubo A, Yoshida R. IFN-gamma is the inducer of indoleamine 2,3-dioxygenase in allografted tumor cells undergoing rejection. Journal of immunology. 1990;145(4):1246–1250. [PubMed] [Google Scholar]
- 25.Ozaki Y, Edelstein MP, Duch DS. The actions of interferon and antiinflammatory agents of induction of indoleamine 2,3-dioxygenase in human peripheral blood monocytes. Biochemical and biophysical research communications. 1987;144(3):1147–1153. doi: 10.1016/0006-291x(87)91431-8. [DOI] [PubMed] [Google Scholar]
- 26.Vecsei L, Szalardy L, Fulop F, Toldi J. Kynurenines in the CNS: recent advances and new questions. Nature reviews. Drug discovery. 2013;12(1):64–82. doi: 10.1038/nrd3793. [DOI] [PubMed] [Google Scholar]
- 27.Zelante T, Fallarino F, Bistoni F, Puccetti P, Romani L. Indoleamine 2,3-dioxygenase in infection: the paradox of an evasive strategy that benefits the host. Microbes and infection. 2009;11(1):133–141. doi: 10.1016/j.micinf.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Platten M, Ho PP, Youssef S, Fontoura P, Garren H, Hur EM, et al. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science. 2005;310(5749):850–855. doi: 10.1126/science.1117634. [DOI] [PubMed] [Google Scholar]
- 29.Romani L, Fallarino F, De Luca A, Montagnoli C, D'Angelo C, Zelante T, et al. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451(7175):211–215. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- 30.Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. The Journal of experimental medicine. 2002;196(4):447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wirleitner B, Rudzite V, Neurauter G, Murr C, Kalnins U, Erglis A, et al. Immune activation and degradation of tryptophan in coronary heart disease. European journal of clinical investigation. 2003;33(7):550–554. doi: 10.1046/j.1365-2362.2003.01186.x. [DOI] [PubMed] [Google Scholar]
- 32.Zuo H, Ueland PM, Ulvik A, Eussen SJ, Vollset SE, Nygard O, et al. Plasma Biomarkers of Inflammation, the Kynurenine Pathway, and Risks of All-Cause, Cancer, and Cardiovascular Disease Mortality: The Hordaland Health Study. American journal of epidemiology. 2016 doi: 10.1093/aje/kwv242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Metghalchi S, Ponnuswamy P, Simon T, Haddad Y, Laurans L, Clement M, et al. Indoleamine 2,3-Dioxygenase Fine-Tunes Immune Homeostasis in Atherosclerosis and Colitis through Repression of Interleukin-10 Production. Cell metabolism. 2015;22(3):460–471. doi: 10.1016/j.cmet.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Cole JE, Astola N, Cribbs AP, Goddard ME, Park I, Green P, et al. Indoleamine 2,3-dioxygenase-1 is protective in atherosclerosis and its metabolites provide new opportunities for drug development. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(42):13033–13038. doi: 10.1073/pnas.1517820112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Molecular psychiatry. 2013;18(6):666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 36.El Aidy S, Kunze W, Bienenstock J, Kleerebezem M. The microbiota and the gut-brain axis: insights from the temporal and spatial mucosal alterations during colonisation of the germfree mouse intestine. Beneficial microbes. 2012;3(4):251–259. doi: 10.3920/BM2012.0042. [DOI] [PubMed] [Google Scholar]
- 37.Raboni S, Bettati S, Mozzarelli A. Tryptophan synthase: a mine for enzymologists. Cellular and molecular life sciences : CMLS. 2009;66(14):2391–2403. doi: 10.1007/s00018-009-0028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JH, Lee J. Indole as an intercellular signal in microbial communities. FEMS microbiology reviews. 2010;34(4):426–444. doi: 10.1111/j.1574-6976.2009.00204.x. [DOI] [PubMed] [Google Scholar]
- 40.Zhang LS, Davies SS. Microbial metabolism of dietary components to bioactive metabolites: opportunities for new therapeutic interventions. Genome medicine. 2016;8(1):46. doi: 10.1186/s13073-016-0296-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39(2):372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Wu D, Nishimura N, Kuo V, Fiehn O, Shahbaz S, Van Winkle L, et al. Activation of aryl hydrocarbon receptor induces vascular inflammation and promotes atherosclerosis in apolipoprotein E−/− mice. Arteriosclerosis, thrombosis, and vascular biology. 2011;31(6):1260–1267. doi: 10.1161/ATVBAHA.110.220202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bessede A, Gargaro M, Pallotta MT, Matino D, Servillo G, Brunacci C, et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 2014;511(7508):184–190. doi: 10.1038/nature13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nature medicine. 2016;22(6):586–597. doi: 10.1038/nm.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niwa T. Indoxyl sulfate is a nephro-vascular toxin. Journal of renal nutrition : the official journal of the Council on Renal Nutrition of the National Kidney Foundation. 2010;20(5 Suppl):S2–6. doi: 10.1053/j.jrn.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Adijiang A, Goto S, Uramoto S, Nishijima F, Niwa T. Indoxyl sulphate promotes aortic calcification with expression of osteoblast-specific proteins in hypertensive rats. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2008;23(6):1892–1901. doi: 10.1093/ndt/gfm861. [DOI] [PubMed] [Google Scholar]
- 47.Dawson LF, Donahue EH, Cartman ST, Barton RH, Bundy J, McNerney R, et al. The analysis of para-cresol production and tolerance in Clostridium difficile 027 and 012 strains. BMC microbiology. 2011;11:86. doi: 10.1186/1471-2180-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hida M, Aiba Y, Sawamura S, Suzuki N, Satoh T, Koga Y. Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron. 1996;74(2):349–355. doi: 10.1159/000189334. [DOI] [PubMed] [Google Scholar]
- 49.Lees HJ, Swann JR, Wilson ID, Nicholson JK, Holmes E. Hippurate: the natural history of a mammalian-microbial cometabolite. Journal of proteome research. 2013;12(4):1527–1546. doi: 10.1021/pr300900b. [DOI] [PubMed] [Google Scholar]
- 50.Zhang F, Jia Z, Gao P, Kong H, Li X, Chen J, et al. Metabonomics study of atherosclerosis rats by ultra fast liquid chromatography coupled with ion trap-time of flight mass spectrometry. Talanta. 2009;79(3):836–844. doi: 10.1016/j.talanta.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 51.Francino MP. Antibiotics and the Human Gut Microbiome: Dysbioses and Accumulation of Resistances. Frontiers in microbiology. 2015;6:1543. doi: 10.3389/fmicb.2015.01543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.