Abstract
The skull is a vertebrate novelty. Morphological adaptations of the skull are associated with major evolutionary transitions, including the shift to a predatory lifestyle and the ability to masticate while breathing. These adaptations include the chondrocranium, dermatocranium, articulated jaws, primary and secondary palates, internal choanae, the middle ear, and temporomandibular joint. The incredible adaptive diversity of the vertebrate skull indicates an underlying bauplan that promotes evolvability. Comparative studies in craniofacial development suggest that the craniofacial bauplan includes three secondary organizers, two that are bilaterally placed at the Hinge of the developing jaw, and one situated in the midline of the developing face (the FEZ). These organizers regulate tissue interactions between the cranial neural crest, the neuroepithelium, and facial and pharyngeal epithelia that regulate the development and evolvability of the craniofacial skeleton.
Keywords: cranial neural crest, morphological variation, evolution of development, Hinge, FEZ, origin of jaw
1. Introduction
The skull is a vertebrate novelty, the origin and elaboration of which is associated with major evolutionary transitions, including the shift to a predatory lifestyle, the colonization of land, and the ability to masticate while breathing. The skull is the most complex skeletal structure in the vertebrate body, and yet exhibits incredible morphological diversity (1). This diversity is remarkable given the high functional demands on the skull that have a direct impact on fitness (e.g., eating, breathing). These observations suggest the presence of an underlying bauplan that facilitates evolvability, that is, it accommodates variation while maintaining functional integration. The goal of this review is to investigate how developmental systems regulating craniofacial morphogenesis promote evolvability.
To begin, I first provide an overview of the major morphological adaptations of the craniofacial skeleton and discuss molecular, cellular, and developmental mechanisms underlying their evolution. From a morphological perspective, evolution may be reflected by either character origin or character diversification. Therefore, I aim to distinguish between mechanisms underlying character origins (novelty) and mechanisms underlying character modification (diversification) (2). A particular emphasis is placed on the origin of the gnathostome jaw. I examine several models proposed to explain jaw evolution. In considering these models, it is clear that understanding mechanisms at the origin of the jaw are also central to understanding its continued modification. Finally, I discuss the evolvability of the craniofacial skeleton. The data support a model where the craniofacial bauplan structures variation by integrating signals from three secondary organizers, bilaterally paired organizers at the midpoint of the first pharyngeal arch (the Hinge), and a midline organizer in the facial ectoderm (the FEZ).
2. Tissue contributions to the craniofacial skeleton
The vertebrate clade is characterized in part by a “new head,” which is novel in that the rostral brain is enlarged relative to other chordates and supported and protected by a cellularized endoskeleton (3). The craniofacial skeleton forms largely from cranial neural crest cells (CNC), which are a vertebrate innovation (4). CNC provided a new source of mesenchyme that, along with mesoderm, gives rise to the bone, cartilage, connective tissue, and muscle of the craniofacial skeleton. CNC migrates into the head region as three major streams, the trigeminal, hyoid, and branchial streams (5). Some authors refer to the most anterior stream as mandibular, but trigeminal seems more appropriate as this stream includes CNC that migrate rostral to the first pharyngeal arch (PA1). Further, the trigeminal CNC also migrates as three streams: the nasal (pre-optic), post-optic, and PA1 regions (6). The trigeminal CNC is Hox negative, which appears to be important for skeletogenic differentiation (7).
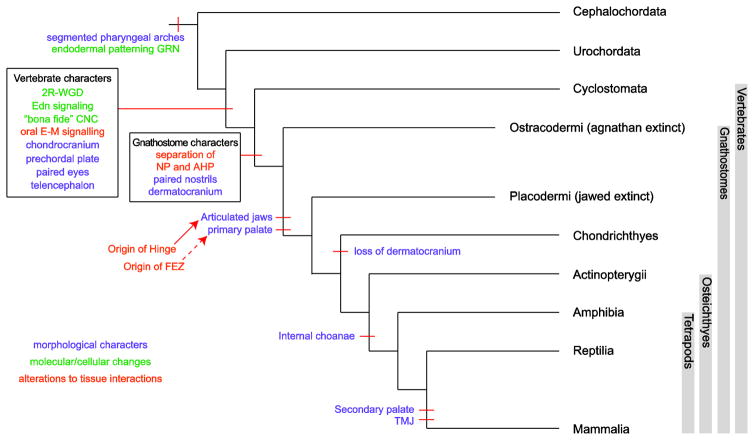
The ectoderm, which overlies the cranial mesenchyme externally, and the endoderm, which forms the internal lining of the pharyngeal arches (PAs), provide essential signals directing pattern formation and morphogenesis of craniofacial mesenchyme (8–9). The PAs form when outpocketing of the pharyngeal endoderm contacts the surface ectoderm (10). These ectodermal-endodermal points of contact demarcate the anterior and posterior boundaries of each segmented arch. CNC and cranial mesoderm migrate into these preformed arches (9). Reciprocal signaling interactions between these neighboring tissues are required for the proper patterning and growth of the craniofacial skeleton. Modifications to epithelial-mesenchymal cross-talk and tissue interactions have mediated morphological changes to the skull (1). The major morphological adaptations and mechanisms underlying their origin and diversification are summarized in Figure 1 and discussed below.
Figure 1.
Major morphological adaptations of the craniofacial skeleton. A chordate phylogeny is presented with evolutionary changes in craniofacial morphology and development mapped on. Molecular and cellular changes are shown in green, morphological novelties are shown in turquoise, and alterations to tissue interactions are shown in orange.
3. Morphological novelties of the craniofacial skeleton
3.1. Chondrocranium and dermatocranium
The vertebrate head skeleton consists of two components, the viscerocranium and the neurocranium. The viscerocranium is derived from mesenchyme within the segmented PAs, while the neurocranium, which encases and protects the brain, forms from mesenchyme lying anterior to the arches (1). In the agnathan cyclostomes, lamprey and hagfish, the head skeleton consists of only cartilage (11–12). The cartilaginous head skeleton is referred to as the chondrocranium, which includes both viscerocranial and neurocranial elements. Perichondral ossification evolved after the split of cyclostomes and gnathostomes (13–14). The advent of ossification is associated with the presence of the dermatocranium, a bony covering of the chondrocranium. With the exception of the Chondrichthyes, which have secondarily lost the dermal skeleton, gnathostomes have a head skeleton composed of cartilage and bone (15).
Most of the tissues required for chondrocranial development exist or have homologues in non-vertebrate chordates. In particular, chordates have segmented PAs and cranial placodes (16). The mechanism driving PA formation, outpocketing of pharyngeal endoderm, is a basal deuterostome character and regulatory networks mediating this process are conserved (16). The key innovations occurring at the base of the vertebrate clade contributing to the origin of the chondrocranium are: 1) the evolution of bona fide CNC, including the acquisition of a cartilage differentiation program in these cells (17–18), and 2) localization of secreted signaling factors that induce cartilage differentiation (19).
The molecular evolution of CNC has been relatively well studied (20–22). It is now apparent that tunicates (Urochordata) possess a neural-crest like cell population (23–24), suggesting that the last common ancestor of vertebrates had an intermediate cell type that might have gained multi-potency, leading to the evolution of “true” neural crest (4). Vertebrate CNC are characterized by a complex gene regulatory network (GRN), consisting of four major components (20;25). Orthologs of genes from each of the four major components of the CNC-GRN are present in the amphioxus (Cephalochordata) genome, however, most of these genes are present as single copy, whereas they have multiple paralogs in vertebrates (26–27). It is hypothesized that two rounds of whole genome duplication at the base of the vertebrate clade facilitated the co-option of the newly generated paralogs into the CNC-GRN (28–29). Gene co-option led to an expansion of function that is particularly relevant to the origin of the CNC specification GRN, which regulates delamination and migration, and is mostly absent in non-vertebrate chordates (30). The evolution of new genes appears to play a minor, but important role, in CNC function. Of particular note is the endothelin signaling system, which is a vertebrate novelty (31).
In sum, the origin of the head skeleton involved the evolution of migratory CNC initially capable of generating cartilage; acquisition of ossifying capabilities contributed to its diversification through the addition of dermal bone. Population of the PAs with CNC gave rise to the viscerocranium. The neurocranium arises from CNC migrating anterior to the PAs, into head regions that don’t exist in amphioxus. Therefore, the neurocranium evolved in association with brain expansion. In particular, the origin of the telencephalon in vertebrates is associated with overall expansion of the rostral brain and paired eyes (amphioxus has a single eye at the midline) (32).
3.2. Articulated jaws
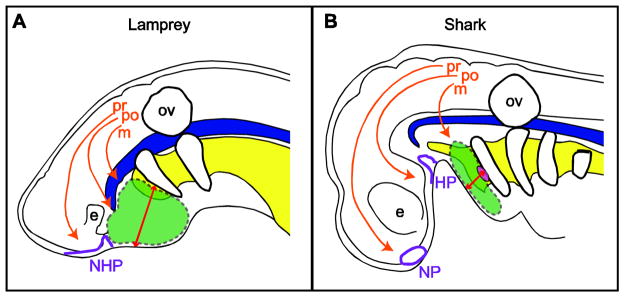
The gnathostome jaw derives predominantly from the first pharyngeal arch (PA1), which forms two cartilaginous elements, the palatoquadrate of the upper jaw and Meckel’s cartilage in the lower jaw. In the larval lamprey, PA1 also gives rise to two cartilaginous elements, the velum and the lower lip. The upper lip is formed from post-optic CNC, which migrates rostral to PA1. Thus, the oral apparatus in lamprey (upper and lower lips) is not homologous to the jaw of vertebrates (6; Fig. 2). Additionally, the pharyngeal cartilages of lamprey are not jointed dorso-ventrally as in gnathostomes, but rather are arranged in a basket-like structure (11–12). These data suggest that modifications to patterning of the trigeminal CNC, and PA1 in particular, underlie the origin of the jaw. Although the lamprey does not necessarily represent the ancestral condition, differences between cyclostomes and gnathostomes are relevant to understanding the origin of the jaw, and models of jaw evolution have contrasted these two taxa.
Figure 2.
Model for the origin of the gnathostome jaw. Comparative embryology of A) lamprey, a representative cyclostome, and B) skark, a representative basal gnathostome. In lamprey, the nasal and hypophyseal placodes (NHP) are continuous. In shark the nasal (NP) and hypophyseal (HP) placodes are distinct. The early separation of these placodes in shark allows post-optic CNC to migrate anterior to the HP. Migration of trigeminal CNC streams are modeled in with orange arrows: m, mandibular CNC stream; po, post-optic CNC stream; pr, pre-optic CNC stream. The Dlx+ CNC contributing to the oral apparatus (upper and lower lips in lamprey and the jaw in shark) is shown in green. Note that both the mandibular and post-optic CNC express Dlx in lamprey. The alteration in CNC migration patterns also reconfigures the relationship between the oral ectoderm and the first pharyngeal pouch such that the distance between them (represented by red arrows) is greatly reduced in shark relative to lamprey. This alteration to spatial relationships between the pharyngeal epithelia is hypothesized to mediate inductive interactions generating an organizer at the jaw Hinge (pink disc). e, eye; ov, otic vesicle; notochord in blue; pharynx in yellow. Morphological depictions adapted from (49).
Cyclostomes have all the cell populations, and many of the tissue interactions required to form the gnathostome jaw (6;33–35). The origin of the jaw does not appear to be associated with the evolution of new genes, as the major genes involved in jaw patterning, especially Dlx genes and endothelin signaling, are thought to be ancestral for vertebrates (35–36). The major difference between gnathostomes and cyclostomes is the spatial relationship of the expression of these key genes. Gnathostome PAs are patterned along 2 major axes, anterior-posterior and dorsal-ventral. Patterning along the proximal-distal axis within PA1, may also be critical to evolutionary diversification of the jaw. Cyclostome PAs share patterns of Hox gene expression with gnathostomes, including a Hox-negative PA1, indicating they have anterior-posterior patterning (37–38). Thus, a major difference between agnathans and gnathostomes lies in dorsal-ventral polarity of PA1.
3.2.1. Models of jaw development and evolution
Developmental models of the evolution of the gnathostome jaw fall broadly into three groups. These are: 1) the Hinge and Caps model (39–40), 2) the heterotopy model (6;41–42), and 3) the joint co-option model (36;43). Implicit to all three of these models is the premise that dorsal-ventral polarity of PA1 is necessary for the development of articulated jaws. There is also a general consensus that dorsal-ventral polarity is mediated, in part, by nested expression of Dlx genes in PA1, which is downstream of endothelin signaling (44–47). Endothelin signaling establishes expression domains of the transcription factors Bapx1 and Hand2 within PA1. Bapx1 is expressed at the midpoint of PA1 where it specifies an intermediate region, and Hand2 is expressed ventrally in PA1, where it regulates ventral identity (45). Explanation of the acquisition of dorsal-ventral polarity in PA1 is a key difference among the models.
The Hinge and Caps model holds that PA1 is partitioned into two regions, an upper (maxillary) branch and lower (mandibular) branch separated and articulated by a “Hinge” located at their junction, which by definition is the mid-point of PA1. The Hinge is proposed to derive from “factors of epithelial origin” common to the oral ectoderm overlying PA1 and the junction of the first pharyngeal pouch endoderm and cleft ectoderm. The exact factors establishing the Hinge are not specified, but are suggested to include Fgf8 among other signaling factors (39–40;48). The Hinge forms the proximal point of both the maxillary and mandibular branches of PA1, and is argued to work in concert with distal “Caps” signaling to establish pattern and polarity in the jaw. Thus, although Depew and colleagues do not explicitly use the term organizer for the Hinge (perhaps due to the emphasis on the Hinge working in concert with Caps signaling), the defined role of this region in directing both patterning and positional information for PA1 indicates it would be one. A strength of this model is the explanation for polarity of PA1, which derives from an organizer. However, no clear explanation for the origin of the Hinge is presented.
The heterotopy model focuses almost entirely on developmental mechanisms distinguishing CNC-epithelial interactions in PA1 of lamprey and gnathostomes. Kuratani and colleagues propose a hierarchical model for the origin of the jaw that involves at least 4 successive transitions (Fig. 2). First, the relative positions of the nasal and hypophyseal placodes are altered in gnathostomes such that they are farther apart and separate earlier in development relative to lamprey (42). Second, widening of the medial-lateral axis in the anterior head leads to diplorhiny. Third, migration patterns of CNC within the trigeminal stream are altered such that the post-optic CNC lies in between the nasal and hypophyseal placodes in gnathostomes rather than posterior to them as in lamprey (41). Finally, there is a heterotopic shift in oral ectoderm expression of FGF/BMP signals, which in lamprey extends anterior to PA1, but is limited to PA1 in gnathostomes (6;41).
Kuratani and colleagues distinguish three populations within the trigeminal CNC, a mandibular stream (which migrates into PA1), a post-optic stream (which migrates anterior to PA1, but posterior to the eye), and a pre-optic stream (which migrates anterior to the eye). In lamprey, both mandibular and post-optic CNC contribute to the oral apparatus, whereas in basal gnathostomes only CNC from PA1 contribute to the jaw, while the post-optic CNC contributes to the neurocranium (6;41). Importantly, in lamprey both the post-optic and mandibular CNC express Dlx genes, since both CNC populations underlie the Fgf8 expressing oral ectoderm, which induces Dlx1 (6). The heterotopic shift in FGF/BMP signaling observed between cyclostomes and gnathostomes is argued to be a consequence of altered CNC migration patterns. Due to the early separation of the nasal and hypophyseal placodes in gnathostomes, CNC (specifically post-optic CNC) are able to migrate into the midline space anterior to the eye rather than into the stomodeal region (49; Fig. 2).
The heterotopy model accounts for morphological differences in PA1 and CNC migration that generate alterations to tissue interactions distinguishing cyclostomes and gnathostomes. However, restriction of Dlx expression to PA1 via a heterotopic shift in epithelial-mesenchymal interactions does not, in itself, confer polarity, as the Fgf-Dlx induction program exists in lamprey without generating nested Dlx expression. Thus, the heterotopy model describes pre-conditions that may be necessary for PA1 polarity, but does specifically explain how polarity is achieved.
The co-option model asserts that dorsal-ventral patterning was pre-existing in vertebrate PAs, and that the origin of the jaw was simply due to the co-option by CNC of a joint forming GRN (36;43). This model is largely based on interpretations of Dlx, Hand, and Msx gene expression in the lamprey. In contrast to other reports (35;50), Cerny and colleagues (36) argue that Dlx expression is nested in lamprey PAs, and that Hand and Msx genes are ventrally restricted, which they argue is similar to the expression pattern of these genes in gnathostome PAs. The key difference they highlight between lamprey and gnathostomes is the absence of expression of genes associated with intermediate arch specification and joint formation, namely Bapx and Gdfs.
The co-option model differs from both the Hinge and Caps model and the heterotopy model in that it argues that all PAs in cyclostomes (including PA1) exhibit nested dorsal-ventral polarity. That is, it holds that polarity of PA1 is an ancestral vertebrate trait rather than derived for gnathostomes. This model also assumes that jointless pharyngeal arches are ancestral for vertebrates. However, recent fossil descriptions of stem vertebrates suggest that bipartite (dorsoventrally segmented) arches existed prior to the divergence of gnathostomes and cyclostomes (51). Co-option of a joint formation program also does not explain the extensive re-organization of PA1 relative to the rostral head or the loss of Dlx expression in CNC anterior to PA1. As an aside, the argument that the origin of the jaw was not driven by a change in patterning or morphogenesis, but by the co-option of a joint formation GRN within a pre-patterned arch, also implies that the jaw is not an evolutionary novelty, but rather a modification of the ancestral oral apparatus, with which it would be considered homologous. In contrast, the heterotopy model argues that the connection of the nasal and hypophyseal placodes in lamprey constrains CNC migration (particularly the post-optic CNC), restricting it to the oral region. In this model, the jaw is novel because it involves the breakdown of constraints, allowing novel variation to be generated (52).
3.2.2. Towards a comprehensive model of the origin of the jaw
The models discussed above each have a different point of emphasis, in part due to differences in the authors’ assumptions of the ancestral vertebrate condition. This is reflected in their interpretations of gene expression patterns used to indicate dorsal-ventral patterning, particularly Dlx gene expression. Both the heterotopy and Hinge and Caps model assume that the ancestral condition is an unpolarized PA1, typified by the absence of nested Dlx expression in lamprey. In contrast, the joint co-option model holds that the ancestral condition for all vertebrates is nested Dlx expression in polaraized pharyngeal arches. This difference could be due in part to the fact that Dlx expression is variable in gnathostomes. For example, both shark and mouse have “nested” Dlx gene expression, but the specific patterns of Dlx nesting differ between these taxa (53–54). This is likely because alterations to expression patterns of mediators of dorsal-ventral patterning may be important for diversification of the jaw. To understand the origin of the jaw, it is not the mediators, but the source, of patterning that matters. In this regard, the hypothesis that an organizer exists within the arch itself, as suggested by the Hinge and Caps model, deserves further consideration.
Several lines of evidence point to the existence of an organizer at the Hinge, or mid-point, of PA1. First, skeletal transformations resulting from alterations to mediators of jaw patterning occur as mirror-images (1;47;55). Mirror image duplications are typical of manipulations involving organizers, because the organizer establishes a reference point to initiate positional information, which establishes polarity (56–57). The adaptive benefit of reflecting upper and lower jaw derivatives around a mid-point to ensure their functional registration has previously been noted (39;48). A second line of evidence pointing to an organizer within PA1 comes from duplication of jaw elements resulting from exogenous Shh expression near the PA1 Hinge (58). Exogenous expression of Shh induces Fgf8 and Bmp4 in the caudal PA1 ectoderm similar to the endogenous Fgf8 and Bmp4 expression pattern in the rostral oral epithelium, which is induced by Shh from the foregut endoderm (58–59). As a result of this duplication of signaling interactions, the lower jaw skeleton was duplicated (58). These data led Brito and colleagues to propose that Shh/Fgf8/Bmp4 signaling in the pharyngeal arch acts as a signaling center similar to the ZPA and AER in the limb (58).
The Hinge and Caps model proposed that signaling interactions between the pharyngeal plate (the junction of the pharyngeal pouch endoderm and pharyngeal cleft ectoderm) and the oral ectoderm generate a signaling center at the mid-point of PA1. Fgfs, Bmps, and Shh are expressed in neighboring domains in these tissues. SHH has known inductive power, however tissue relationships appear to be important to this induction. If the Hinge is generated by inductive interactions, it suggests that alterations to tissue relationships may have been necessary for the origin of the Hinge. In particular, the spatial relationship between the pharyngeal plate, oral ectoderm and foregut endoderm in early development may be critical to establish signaling interactions at the Hinge.
In this regard, the heterotopy model may provide further explanatory power. In addition to the heterotopic shift in oral epithelial-mesenchymal signaling interactions emphasized by Kuratani and colleagues, alterations to CNC migration patterns in gnathostomes also reorganized the spatial relationships between the oral ectoderm and the pharyngeal endoderm. In basal gnathostomes, PA1 is spatially separated from the post-optic CNC along the oral ectoderm. In contrast, PA1 in lamprey is folded over on itself, generating a shorter, thicker arch with larger distances between epithelia. Thus, the spatial relationships of the epithelia of the first pharyngeal pouch and the oral ectoderm are shifted in gnathostomes relative to lamprey (Fig. 2). The establishment of a jaw organizer may be related to the spatial organization of PA1, which affects the distance between competent tissue and the source of SHH. Once an organizer is established, polarized gene expression (e.g., Dlx, endothelin, Bapx, Hand2) could be directed from this source.
Although the arguments presented here (that re-organization of PA1 was central to the evolutionary origin of the jaw) would necessarily reject the co-option model, some data presented in support of that hypothesis are important to consider. The co-option model relies heavily on the interpretation of gene expression in lamprey PAs, which differs from that of the other two models. The difference in these interpretations may be due in part to technical issues, as the data shown for Dlx expression is distinct in the different manuscripts, and does appear to have more restricted domains as reported by Cerny and colleagues. However, the differences in Dlx expression exist only in the posterior arches, and the data shown by Cerny and colleagues that is most convincing is shown in sections of posterior arches. However, in all reports (even those by Cerny and colleagues), Dlx expression in PA1 of lamprey is clearly different than in the other arches and does not appear nested.
Although Kuratani and colleagues do not find nested Dlx expression in the posterior arches, they note that Hand2 expression is ventrally restricted in these arches (35). Taken together, these data suggest that the posterior arches of lamprey are differently patterned relative to PA1. This, along with the fossil data, would further suggest that the ancestral vertebrate condition exhibits polarized posterior arches. If patterning information derives from interactions between the pharyngeal endoderm and ectoderm, the fact that these tissues are closer together in the posterior arches due to their smaller and more linear morphology, would provide additional evidence that spatial reorganization of PA1 could lead to polarization.
3.3. Primary palate
The primary palate forms the floor of the nasal cavities and the anterior roof of the mouth (60). Developmentally, the primary palate derives from the medial portion of the frontonasal process (FNP), which generates the premaxilla. Thus, the origin of the primary palate is also associated with a FNP contribution to the upper jaw. Among extant gnathostomes, Osteichthyes have a primary palate while the Chondrichthyes do not. In the Chondrichthyes, the upper jaw is formed only from the maxillary portion of PA1. The maxillary processes grow medially and fuse with each other at the midline, where the jaws are suspended from the neurocranium (53;61). In contrast, in Osteichthyes, the maxillary processes grow rostrally, where they meet and fuse with the FNPs in the mid-facial region.
Given the loss of the dermatocranium in the chondrichthyian lineage, the character polarity of the primary palate is not clear. Resolution of this issue depends on whether the ancestral gnathostome was chondrichthyan-like (61–62) or osteichthyan-like (15; 63–64). The chondrichthyan-like ancestor hypothesis relies on phylogenetic analyses placing acanthodians, which display chondrichthyan-like morphology, as stem osteichthyans (61–62). Other phylogenetic analyses have placed them as stem chondrichthyans (65). Several recent fossil descriptions of more basal placoderms suggest that they have osteichthyan-like morphology (63–64;66), including upper jaws with multiple dermal bones, in which homology with the premaxilla in osteichthyans has been argued (64).
Interestingly, the earliest jawed vertebrates have a posteriorly placed nasal capsule, similar to cyclostomes (65). That is, derivatives of the post-optic CNC (pre-mandibular/trabecular) reside anterior to derivatives of the pre-optic CNC (FNP). Thus, upper jaws of the earliest jawed vertebrates would not have included an FNP contribution. Nonetheless, the shift to an anteriorly placed nasal capsule occurs in derived placoderms, and mounting evidence indicates they had premaxilla-like bones in their upper jaws (63–64;66). These data suggest that the origin of the primary palate occured shortly after the origin of the jaw, in the placoderm lineage.
The frontonasal ectodermal zone (FEZ) has been identified as a signaling center regulating growth and polarity of the upper jaw in zebrafish and amniotes (19;67–69). This signaling center consists of juxtaposed domains of Shh and Fgf8 in the mid-facial ectoderm (Fig. 3; 67;70). A major role of this signaling center is to orchestrate the rate and direction of CNC proliferation, where it is especially important to regulate mid-facial width through regulation of multiple signaling factors (69; 71–72). The origin of the FEZ is unclear and will require further investigation. However, the anterior shift of the nasal capsule is associated with an increase in size of the trabecular region, and proliferation of post-optic CNC (65). Shh expression within the FEZ is localized in the roof of the stomodeum, just below the post-optic CNC. CNC migration into this region is required for Shh expression in the ectoderm (73). These data suggest that the origin of the FEZ may be associated with the expansion of the trabecular region and the anterior shift of the nasal capsule. Further elaboration of the FEZ may be related to integration of the maxillary processes with the FNP, as alterations to the FEZ are associated with differences in growth trajectory of the facial processes (74).
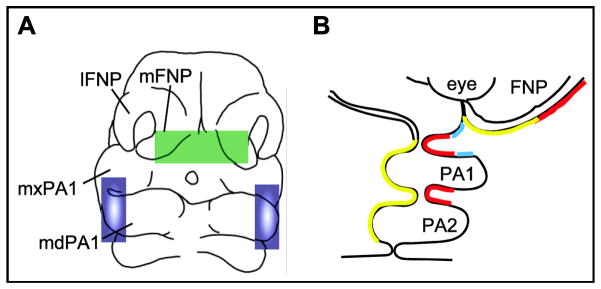
Figure 3.
Craniofacial signaling centers. A) E10.5 mouse head in frontal view. The FEZ (green oval) runs across the stomodeal and frontonasal ectoderm. Blue ovals indicate the bilateral Hinge located at the mid-point of PA1. Hinge signaling requires interactions between the stomodeal ectoderm and pharyngeal ectoderm and endoderm. B) Section through the pharyngeal arches of a generalized amniote showing juxtaposed Fgf8 (in red), Bmp (in turquoise) and Shh (in yellow) expression domains in the FEZ and Hinge. FNP, frontonasal process; md, mandibular; mx, maxillary; PA, pharyngeal arch.
3.4. Internal choanae
Most jawed fishes have two pairs of external nostrils, an anterior pair in which water enters the nasal capsule and a posterior pair where water flows out. The nostrils of fish do not open into the back of the mouth, and therefore function only in olfaction, not respiration. In contrast, tetrapods have one pair of external nostrils and one pair of internal nostrils, the choanae. The choanae open into the roof of the mouth, thereby connecting the nose to the mouth and throat. This adaptation is an essential part of the tetrapod respiratory system, as it allows breathing without opening the mouth. The choanae are homologous to the posterior external nostrils of fishes, which have been displaced internally (75).
In fishes with two external nostrils, the premaxilla arises from the FNP and simply fuses with the maxilla at its anterior edge lateral to the nasal capsule (75). In tetrapods, the FNP separates into medial and lateral processes as the nasal pits invaginate. As the facial processes fuse around the invaginating nasal pits, the choanae open between the nasal cavities and stomodeum. The morphogenetic processes generating the choanae are much more complicated than those of fish with only external nostrils, requiring coordination of cellular proliferation, migration, and apoptosis. Invagination of the nasal pits appears to involve orchestration of epithelial signals altering the rate direction of growth within the FNP, especially Bmps and Fgfs (76–78).
While the choanae provide an obvious adaptive advantage for respiration, their development generates the potential for clefting if the facial processes fail to properly fuse around the nasal opening. Because facial clefts are typically not compatible with life, morphological variation at the fusion stage of facial development is constrained (79). However, variation in this process has been observed among amniotes, notably in terms of the order of fusion of the processes and the relative growth of the different processes involved (79–80). Differences in Shh expression in the FEZ of different avian taxa and mice (74;81) are associated with differences in CNC proliferation and facial width. Therefore, evolutionary changes to patterns of facial process fusion likely involved the coordination of signaling from the FEZ (within the stomodeal ectoderm) with signaling in the nasal and cephalic ectoderm.
3.5. The middle ear and TMJ
The middle ear operates as an impedance matching system in which airborne vibrations are transferred to the cochlear fluids of the inner ear. The importance of this adaptation to terrestrial life is reflected in its independent evolution in multiple lineages (82). The amniote middle ear consists of middle ear ossicle(s) and the tympanic membrane. Reptiles and birds have only one middle ear bone, the columella, whereas mammals have three middle ear ossicles, the stapes, malleus, and incus. The stapes is homologous to the columella, while the two unique bones in the mammalian middle ear, the malleus and incus, are homologous to the quadrate and articular, which form the jaw articulation (primary jaw joint) in non-mammalian gnathostomes. The incorporation of elements forming the primary jaw joint into the mammalian middle ear was only possible after to the evolution of a novel joint, the temporomandibular joint (TMJ), forming ventral to the primary jaw articulation between the dentary and squamosal elements.
Evolution of the mammalian middle ear is associated with multiple developmental alterations. Bapx1, a marker of the intermediate PA1 region, is expressed in the primary joint mesenchyme (45; 82–83). In mice, Bapx1 is expressed dorsal to the external auditory meatus (EAM), whereas in chick, Bapx1 is expressed ventral to the EAM (82). Kitazawa and colleagues argue that this difference reflects a dorsal shift of the primary jaw joint relative to the first pharyngeal pouch in the mammalian lineage (82). Thus, in mammals, the tympanic membrane develops from the ventral (mandibular) portion of PA1. In addition to this shift in Bapx1 expression, evolution of the TMJ further involved the uncoupling of GRNs regulating joint formation, such that in mammals, Bapx1 does not regulate Gdf5 and Gdf6, two genes essential to joint formation (83).
With the establishment of the TMJ, the skeletal elements forming the primary jaw joint became incorporated into the middle ear. Initially the ectotympanic and malleus remained connected to the lower jaw by an ossified Meckel’s Cartilage. The definitive mammalian middle ear evolved after the breakdown of Meckel’s Cartilage released the ectotympanic and the malleus from the lower jaw (84). Recent reports indicate that the breakdown of Meckel’s Cartilage occurred in parallel in mammals. A heterochronic shift in the timing of osteoclast cell recruitment to Meckel’s Cartilage prior to ossification is important for breakdown in eutherians (85). In marsupials, apoptosis drives Meckel’s Cartilage breakdown (86).
3.6 Secondary palate
The secondary palate forms by fusion of the palatal processes, which are medial out-growths of the maxillary processes that undergo a complex and highly dynamic morphogenetic process that includes growth, elevation, adhesion, and fusion (87). The complete palatal skeleton, formed by fusion of the secondary palate with the primary palate and the nasal septum, separates the oral and nasal cavities. A major adaptive advantage of the secondary palate is that it allows breathing while eating or suckling. Additionally, mechanical simulations have shown that the strength and stiffness of the upper jaw increase with even incremental extension of the palatal shelves towards the midline (88). Therefore, selection for increased bite force and dietary diversification may also have played a significant role in the evolution of the secondary palate, which may have occurred incrementally.
The complex morphogenetic processes of palatogenesis involve the activity of many genes (87;89). Almost every major signaling pathway system is involved in palatal development, including Wnts, Bmps, Fgfs, Shh, Gsk-3beta, and Ephrins (90–93), and disruptions to individual genes within these pathways can induce clefts. As such, it is difficult to identify any particular signal that is more essential than the others. Instead, it appears that evolution has favored increasing the complexity of interactions among the signaling pathway families. The complexity of these genetic interactions appears to have evolved by the sequential acquisition of cis-regulatory elements directing precise patterns of temporal-spatial gene expression (94–95).
3. Organizers and craniofacial evolvability
A major goal in the field of evo-devo is to understand how the variation required for selection is generated, and what developmental processes contribute to some variation being more “evolvable,” or heritable, than others (96). I argue that the evolvability of the craniofacial skeleton is mediated by the origin and evolution of three secondary organizers, two that are bilaterally expressed at the mid-point, or Hinge, of PA1, and one at the facial midline, the FEZ (Fig. 3). A recent review of organizers in development found evidence for four “true” organizers in the embryo based on their ability to both induce and pattern neighboring tissues: the primary embryonic organizer (Spemann organizer, shield, node), the notochord, the midbrain-hindbrain boundary, and the ZPA (perhaps together with the AER) in the limb bud (57). Notably, no craniofacial organizers were even mentioned in that review. However, as discussed above, there is strong evidence that both the Hinge and FEZ have inductive and patterning functions. Further, the Hinge and FEZ are composed of neighboring SHH-FGF8 expression domains and also involve BMP signaling. SHH-FGF-BMP interactions are known components of organizers in the node, limb (ZPA/AER) and brain (MHB). Therefore, inductive and patterning information conferred by SHH-FGF-BMP gene regulatory interactions may represent a form of deep homology regulating bauplans (97).
The acquisition of novel morphological features in vertebrates (e.g., telencephalon, limbs, jaw) appears to be associated with the origin of organizing centers, suggesting this may be fundamental to their evolution. A major advantage of organizers is that gene expression changes can be integrated from a source and mediated by epithelial-mesenchymal interactions, conferring evolvability. Gene expression in vertebrates is most conserved during organogensis, arguing that evolution of body plans is mediated by subtle changes in major developmental regulators, rather than the gain or loss of genes (98).
In craniofacial development, evolutionary modification and diversification of the skull is mediated by epithelial-mesenchymal interactions downstream of organizer activity. For example, Fgf8 and Bmp4 are expressed in the epithelia overlying the proximal and distal upper and lower jaws, respectively, where they induce expression in the mesenchyme of mediators of proximal and distal jaw identity (98–99). In chick, Bmp4 expression is more distally restricted in the oral ectoderm than in mice. As a consequence, the expression of Bmp4 responsive genes, such as Satb2, is coordinately reduced in the distal domain of both upper and lower jaws (48). Satb2 regulates distal jaw size, and therefore, is hypothesized to regulate a distal jaw module. Coordination of alterations to this module between the upper and lower jaw primordia by a signaling center at their juncture, ensures maintenance of functional integration (39;48).
5. Future questions
In contrast to CNC, the role of another critical player in the origin and diversification of the craniofacial skeleton, the prechordal plate, has been underappreciated and deserves more study. The prechodal plate lies just anterior to the node. It forms the buccopharyngeal plate (foregut endoderm) and gives rise to the cranial mesoderm, which regulates endothelin signaling in PA1. Evolutionary alterations to Shh signaling in the mesendoderm of the prechordal plate may have had subsequent effects on both anterior-posterior and medial-lateral patterning of the neural plate that may be linked to multiple evolutionary transitions in the vertebrate skull, such as oral epithelial Fgf-Bmp signaling (101), the duplication (pairing) of the optic and nasal placodes, and establishment of the signaling centers in the brain (102). Interactions between the neural epithelia and CNC may have been central to the origin of novel signaling centers through inductive-responsive tissue interactions. CNC are required to mediate the induction of Shh from the brain to the FEZ (69;73). CNC also regulate Fgf and Bmp levels in the neural plate (103–104). More research on the evolution of the prechordal plate and its influence on CNC and brain patterning is needed to further elucidate these interactions.
What exactly defines the Hinge and how has it been modified during gnathostome evolution? The FEZ has been relatively well characterized and identified across a broad range of taxa (mice, avians, and zebrafish). Further, modifications to the spatial organization of Shh expression in the FEZ during development have been linked with differences in upper jaw morphology (74). In contrast, the Hinge has been less studied. In order to better understand the origin and nature of this organizer, more investigation on the spatial relationships and signaling interactions between the epithelia surrounding PA1 should be undertaken in a broad range of taxa and at multiple developmental times. This is particularly important in light of the mounting evidence that the extant taxa typically inferred to be ancestral to and/or basal gnathostomes (e.g., lamprey and shark), may in fact have rather derived morphologies (15).
The evolution of the upper jaw also requires more study, as its modification appears to be more complex than the lower jaw. This may be correlated with evolutionary changes underlying the integration of the upper jaw with the neurocranium. Some CNC lineage tracing experiments have suggested that the upper jaw derives entirely from CNC migrating anterior to PA1 (109–110). These results are controversial, as lineage tracing experiments can be difficult to control and have given inconsistent results. Genetic manipulations in mice clearly show that altering the Edn1-Dlx5/6 pathway disrupts gene expression in PA1 and morphology of the skeletal elements of the jaw, providing very strong evidence in favor of the upper jaw having a significant contribution from PA1. Nonetheless, it is possible that integration of the upper jaw with the neurocranium involved incorporation of post-optic CNC into upper jaw derivatives. This, however, would not imply a “new origin” for the maxillary jaw, but simply a modification of upper jaw development. Further research in this area, including the identification of specifiers of upper jaw identity is still needed.
6. Conclusion
The adaptive diversity of craniofacial morphology suggests the presence of a craniofacial bauplan that structures variation. Evidence from comparative vertebrate development suggests that three secondary organizers, the bilaterally paired jaw Hinge and the mid-line FEZ are central to the craniofacial bauplan. Organizers are critical mediators of development and evolution as they provide polarity, which in turn provides the potential for both integration and modularity, which are essential to evolvability. For example, the Hinge contributes to evolutionary modification and diversification of the jaw by mediating epithelial-mesenchymal interactions that generate modularity and integration of the upper and lower jaws. Histological changes to skeletal structures are also mediated by modifications downstream of this patterning system, which may result from species-specific responses of CNC to epithelial signals. Finally, cis-regulatory changes have been central to the evolution of the skull, mediating both increased complexity of genetic tool-kits via the co-option of GRNs and the evolution of novel tissue interactions (emergent properties) downstream of alterations to the expression of signaling factors.
Acknowledgments
Funding
This work was supported by the National Institutes of Health [R15 DE026611-01].
I would like to thank my collaborators and mentors, with whom I have discussed many of the ideas in this review: Claudia Compagnucci, Michael Depew, Rebecca Green, Benedikt Halgrimsson, Ralph Marcucio, Rich Schneider, and Bethan Thomas, as well as Evelyn Schwager who provided helpful feedback. I am also grateful to two anonymous reviewers for their constructive comments on an earlier version of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Depew MJ, Tucker A, Sharpe P. Craniofacial development. In: Rossant J, Tam P, editors. Mouse development: patterning, morphogenesis, and organogenesis. London: Academic Press; 2002a. pp. 421–498. [Google Scholar]
- 2.Wagner GP, Lynch VJ. Evolutionary novelties. Current Biology. 2011;20(2):R48–52. doi: 10.1016/j.cub.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Gans C, Northcutt RG. Neural crest and the origin of vertebrates: a new head. Science. 1983;220(4594):268–73. doi: 10.1126/science.220.4594.268. [DOI] [PubMed] [Google Scholar]
- 4.Bronner ME. Evolution: On the crest of becoming vertebrate. Nature. 2015;527:311–312. doi: 10.1038/nature15645. [DOI] [PubMed] [Google Scholar]
- 5.Kulesa P, Fraser S. In ovo time-lapse analysis of chick hindbrain neural crest cell migration shows cell interactions during migration to the branchial arches. Development. 2000;127(6):1161–72. doi: 10.1242/dev.127.6.1161. [DOI] [PubMed] [Google Scholar]
- 6.Shigetani Y, Sugahara F, Kawakami Y, Murakami Y, Hirano S, Kuratani S. Heterotopic shift of epithelial-mesenchymal interactions in vertebrate jaw evolution. Science. 2002;296(5571):1316–9. doi: 10.1126/science.1068310. [DOI] [PubMed] [Google Scholar]
- 7.Trainor PA, Krumlauf R. Hox genes, neural crest cells and branchial arch patterning. Curr Opin Cell Biol. 2001;13(6):698–705. doi: 10.1016/s0955-0674(00)00273-8. [DOI] [PubMed] [Google Scholar]
- 8.Trumpp A, Depew MJ, Rubenstein JL, Bishop JM, Martin GR. Cre-mediated gene inactivation demonstrates that FGF8 is required for cell survival and patterning of the first branchial arch. Genes Dev. 1999;13:3136–3148. doi: 10.1101/gad.13.23.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham A, Okabe M, Quinlan R. The role of the endoderm in the development and evolution of the pharyngeal arches. J Anat. 2005;207(5):479–87. doi: 10.1111/j.1469-7580.2005.00472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veitch E, Begbie J, Schilling TF, Smith MM, Graham AA. Pharyngeal arch patterning in the absence of neural crest. Curr Biol. 1999;9(24):1481–1484. doi: 10.1016/s0960-9822(00)80118-9. [DOI] [PubMed] [Google Scholar]
- 11.Johnels AG. On the development and morphology of the skeleton of the head of Petromyzon. Acta Zool. 1948;29:139–279. [Google Scholar]
- 12.Yao T, Ohtani K, Kuratani S, Wada H. Development of lamprey mucocartilage and its dorsal-ventral patterning by endothelin signaling, with insight into vertebrate jaw evolution. J Exp Zool B Mol Dev Evol. 2011;314B:339–346. doi: 10.1002/jez.b.21406. [DOI] [PubMed] [Google Scholar]
- 13.Hirasawa T, Kuratani S. Evolution of the vertebrate skeleton: morphology, embryology, and development. Zoological Lett. 2013;1:2. doi: 10.1186/s40851-014-0007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang NZ, Donoghue PCJ, Smith MM, Sansom IJ. Histology of the galeaspid dermoskeleton and endoskeleton, and the origin and early evolution of the vertebrate cranial endoskeleton. J Vert Paleontol. 2005;25:745–756. [Google Scholar]
- 15.Zhu M, Yu X, Ahlberg PE, Choo B, Lu J, Qiao T, Qu Q, Zhao W, Jia L, Blom H, Zhu Y. A Silurian placoderm with osteichthyan-like marginal jaw bones. Nature. 2013;502:188–193. doi: 10.1038/nature12617. [DOI] [PubMed] [Google Scholar]
- 16.Graham A, Richardson J. Developmental and evolutionary origins of the pharyngeal apparatus. EvoDevo. 2012;3:24. doi: 10.1186/2041-9139-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCauley DW, Bronner-Fraser M. Importance of SoxE in neural crest development and the evolution of the pharynx. Nature. 2006;441(7094):750–2. doi: 10.1038/nature04691. [DOI] [PubMed] [Google Scholar]
- 18.Meulemans D, Bronner-Fraser M. Insights from amphioxus into the evolution of vertebrate cartilage. PLoS One. 2007;2(8):e787. doi: 10.1371/journal.pone.0000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wada N, Javidan Y, Nelson S, Carney TJ, Kelsh RN, Schilling TF. Hedgehog signaling is required for cranial neural crest morphogenesis and chondrogenesis at the midline in the zebrafish skull. Development. 2005;132(17):3977–88. doi: 10.1242/dev.01943. [DOI] [PubMed] [Google Scholar]
- 20.Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol. 2008;9(7):557–68. doi: 10.1038/nrm2428. [DOI] [PubMed] [Google Scholar]
- 21.Munoz WA, Trainor PA. Neural crest cell evolution: how and when did a neural crest cell become a neural crest cell. Curr Top Dev Biol. 2015;111:3–26. doi: 10.1016/bs.ctdb.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Barriga EH, Trainor PA, Bronner M, Mayor R. Animal models for studying neural crest development: is the mouse different? Development. 2015;142(9):1555–60. doi: 10.1242/dev.121590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abitua PB, Gainous TB, Kaczmarczyk AN, Winchell CJ, Hudson C, Kamata K, Nakagawa M, Tsuda M, Kusakabe TG, Levine M. The pre-vertebrate origins of neurogenic placodes. Nature. 2015;524(7566):462–5. doi: 10.1038/nature14657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stolfi A, Ryan K, Meinertzhagen IA, Christiaen L. Migratory neuronal progenitors arise from the neural plate borders in tunicates. Nature. 2015;527(7578):371–4. doi: 10.1038/nature15758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Betancur P, Bronner-Fraser M, Sauka-Spengler T. Assembling neural crest regulatory circuits into a gene regulatory network. Annu Rev Cell Dev Biol. 2010;26:581–603. doi: 10.1146/annurev.cellbio.042308.113245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohno S. Gene duplication and the uniqueness of vertebrate genomes circa 1970–1999. Semin Cell Dev Biol. 1999;10(5):517–22. doi: 10.1006/scdb.1999.0332. [DOI] [PubMed] [Google Scholar]
- 27.Kuraku S. Insights into cyclostome phylogenomics: pre-2R or post-2R. Zoolog Sci. 2008;25(10):960–8. doi: 10.2108/zsj.25.960. [DOI] [PubMed] [Google Scholar]
- 28.Wada H. Origin and evolution of the neural crest: a hypothetical reconstruction of its evolutionary history. Dev Growth Differ. 2001;43(5):509–20. doi: 10.1046/j.1440-169x.2001.00600.x. [DOI] [PubMed] [Google Scholar]
- 29.Canestro CC, Albalat R, Irimia M, Garcia-Fernandez J. Impact of gene gains, losses and duplication modes on the origin and diversification of vertebrates. Sem Cell Dev Bio. 2013;24:83–94. doi: 10.1016/j.semcdb.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Yu JK, Meulemans D, McKeown SJ, Bronner-Fraser M. Insights from the amphioxus genome on the origin of vertebrate neural crest. Genome Res. 2008;18(7):1127–32. doi: 10.1101/gr.076208.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braasch II, Volff JN, Schartl M. The endothelin system: evolution of vertebrate-specific ligand-receptor interactions by three rounds of genome duplication. Mol Biol Evol. 2009;26(4):783–99. doi: 10.1093/molbev/msp015. [DOI] [PubMed] [Google Scholar]
- 32.Holland LZ. The origin and evolution of chordate nervous systems. Phil Trans R Soc B. 2015;370:20150048. doi: 10.1098/rstb.2015.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horigome N, Myoji M, Ueki T, Hirano S, Aizawa S, Kuratani S. Development of cephalic neural crest cells in embryos of Lampetra japonica, with special reference to the evolution of the jaw. Dev Biol. 1999;207(2):287–308. doi: 10.1006/dbio.1998.9175. [DOI] [PubMed] [Google Scholar]
- 34.McCauley DW, Bronner-Fraser M. Neural crest contributions to the lamprey head. Development. 2003;130(11):2317–27. doi: 10.1242/dev.00451. [DOI] [PubMed] [Google Scholar]
- 35.Kuraku S, Takio Y, Sugahara F, Takechi M, Kuratani S. Evolution of oropharyngeal patterning mechanisms involving Dlx and endothelins in vertebrates. Dev Biol. 2010;341(1):315–23. doi: 10.1016/j.ydbio.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Cerny R, Cattell M, Sauka-Spengler T, Bronner-Fraser M, Yu F, Medeiros DM. Evidence for the prepattern cooption model of vertebrate jaw evolution. Proc Natl Acad Sci USA. 2010;107:17262–17267. doi: 10.1073/pnas.1009304107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takio Y, Pasqualetti M, Kuraku S, Hirano S, Rijli FM, Kuratani S. Lamprey Hox genes and the evolution of jaws. Nature. 2004;429:1–2. doi: 10.1038/nature02616. [DOI] [PubMed] [Google Scholar]
- 38.Takio Y, Kuraku S, Murakami Y, Pasqualetti M, Rijli FM, Narita Y, Kuratani S, Kusakabe R. Hox gene expression patterns in Lethenteron japonicum embryos--insights into the evolution of the vertebrate Hox code. Dev Biol. 2007;308(2):606–20. doi: 10.1016/j.ydbio.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Depew MJ, Compagnucci C. Tweaking the hinge and caps: testing a model of the organization of jaws. J Exp Zool B Mol Dev Evol. 2008;310(4):315–35. doi: 10.1002/jez.b.21205. [DOI] [PubMed] [Google Scholar]
- 40.Depew MJ, Simpson C, Marosso M, Rubenstein JL. Reassessing the Dlx Code: The Genetic Regulation of Branchial Arch Skeletal Pattern and Development. J Anatomy. 2005;207:501–561. doi: 10.1111/j.1469-7580.2005.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shigetani Y, Sugahara F, Kuratani S. Evolutionary scenario of the vertebrate jaw: the heterotopy theory from the perspectives of comparative and molecular embryology. BioEssays. 2005;27:331–338. doi: 10.1002/bies.20182. [DOI] [PubMed] [Google Scholar]
- 42.Kuratani S, Adachi N, Wada N, Oisi Y, Sugahara F. Developmental and evolutionary significance of the mandibular arch and prechordal/premandibular cranium in vertebrates: revising the heterotopy scenario of gnathostome jaw evolution. J Anat. 2013;222(1):41–55. doi: 10.1111/j.1469-7580.2012.01505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Medeiros DM, Crump JG. New perspectives on pharyngeal dorsoventral patterning in development and evolution of the vertebrate jaw. Dev Biol. 2012;371(2):121–135. doi: 10.1016/j.ydbio.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Depew MJ, Lufkin T, Rubenstein JL. Specification of jaw subdivisions by Dlx genes. Science. 2002b;298(5592):381–5. doi: 10.1126/science.1075703. [DOI] [PubMed] [Google Scholar]
- 45.Miller CT, Yelon D, Stainier DYR, Kimmel CB. Two endothelin 1 effectors, hand2 and bapx1, pattern ventral pharyngeal cartilage and the jaw joint. Development. 2003;130(7):1353–65. doi: 10.1242/dev.00339. [DOI] [PubMed] [Google Scholar]
- 46.Depew MJ, Simpson C, Marosso M, Rubenstein JL. Reassessing the Dlx Code: The Genetic Regulation of Branchial Arch Skeletal Pattern and Development. J Anatomy. 2005;207:501–561. doi: 10.1111/j.1469-7580.2005.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato T, Kurihara Y, Asa R, Kawamura Y, Tonami K, Uchijima Y, Heude E, Ekker M, Levi G, Kurihara H. An endothelin-1 switch specifies maxillomandibular identity. Proc Natl Acad Sci USA. 2008;105(48):18806–11. doi: 10.1073/pnas.0807345105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fish JL, Villmoare B, Köbernick K, Compagnucci C, Britanova O, Tarabykin V, Depew MJ. Satb2, modularity, and the evolvability of the vertebrate jaw. Evol Dev. 2011;13(6):549–64. doi: 10.1111/j.1525-142X.2011.00511.x. [DOI] [PubMed] [Google Scholar]
- 49.Kuratani S. Developmental studies of the lamprey and hierarchical evolutionary steps towards the acquisition of the jaw. J Anat. 2005;207:489–499. doi: 10.1111/j.1469-7580.2005.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neidert AH, Virupannavar V, Hooker GW, Langeland JA. Lamprey Dlx genes and early vertebrate evolution. Proc Natl Acad Sci USA. 2001;98(4):1665–70. doi: 10.1073/pnas.98.4.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Conway Morris S, Caron J-B. A primitive fish from the Cambrian of North America. Nature. 2014;512:419–422. doi: 10.1038/nature13414. [DOI] [PubMed] [Google Scholar]
- 52.Hallgrimsson B, Jamniczky HA, Young NM, Rolian C, Schmidt-Ott U, Marcucio RS. The generation of variation and the developmental basis for evolutionary novelty. J Exp Zool B Mol Dev Evol. 2012;318(6):501–17. doi: 10.1002/jez.b.22448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Compagnucci C, Debiais-Thibaud M, Coolen M, Fish J, Griffin JN, Bertocchini F, Minoux M, Rijli FM, Borday-Birraux V, Casane D, Mazan S, Depew MJ. Pattern and polarity in the development and evolution of the gnathostome jaw: Both conservation and heterotopy in the branchial arches of the shark, Scyliorhinus canicula. Dev Biol. 2013;377(2):428–48. doi: 10.1016/j.ydbio.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 54.Gillis A, Modrell MS, Baker CV. Developmental evidence for serial homology of the vertebrate jaw and gill arch skeleton. Nat Commun. 2013;4:1436. doi: 10.1038/ncomms2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rijli FM, Mark M, Lakkaraju S, Dierich A, Dolle P, Chambon P. A homeotic transformation in generated in the rostral branchial region of the head by disruption of Hoxa-2, which acts as a selector gene. Cell. 1993;75:1333–1349. doi: 10.1016/0092-8674(93)90620-6. [DOI] [PubMed] [Google Scholar]
- 56.Spemann H. Embryonic development and induction. New Haven: Yale University Press; 1938. [Google Scholar]
- 57.Anderson C, Stern CD. Organizers in Development. Curr Top Dev Biol. 2016;117:435–54. doi: 10.1016/bs.ctdb.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 58.Brito JM, Teillet MA, Le Douarin NM. Induction of mirror-image supernumerary jaws in chicken mandibular mesenchyme by Sonic Hedgehog-producing cells. Development. 2008;135(13):2311–9. doi: 10.1242/dev.019125. [DOI] [PubMed] [Google Scholar]
- 59.Haworth KE, Wilson JM, Grevellec A, Cobourne MT, Healy C, Helms JA, Sharpe PT, Tucker AS. Sonic hedgehog in the pharyngeal endoderm controls arch pattern via regulation of Fgf8 in head ectoderm. Dev Biol. 2007;303:244–58. doi: 10.1016/j.ydbio.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 60.Janokowski R. The Evo-Devo Origin of the Nose, Anterior Skull Base and Midface. Springer; 2013. [Google Scholar]
- 61.Davis SP, Finarelli JA, Coates MI. Acanthodes and shark-like conditions in the last common ancestor of modern gnathostomes. Nature. 2012;486(7402):247–50. doi: 10.1038/nature11080. [DOI] [PubMed] [Google Scholar]
- 62.Brazeau MD. The braincase and jaws of a Devonian ‘acanthodian’ and modern gnathostome origins. Nature. 2009;457(7227):305–8. doi: 10.1038/nature07436. [DOI] [PubMed] [Google Scholar]
- 63.Giles S, Friedman M, Brazeau MD. Osteichthyan-like cranial conditions in an Early Devonian stem gnathostome. Nature. 2015;520:82–85. doi: 10.1038/nature14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu M, Ahlberg PE, Pan Z, Zhu Y, Qiao T, Zhao W, Jia L, Lu J. A Silurian maxillate placoderm illuminates jaw evolution. Science. 2016;354:334–336. doi: 10.1126/science.aah3764. [DOI] [PubMed] [Google Scholar]
- 65.Dupret V, Sanchez S, Goujet D, Tafforeau P, Ahlberg PE. A primitive placoderm sheds light on the origin of the jaw vertebrate face. Nature. 2014;507:500–503. doi: 10.1038/nature12980. [DOI] [PubMed] [Google Scholar]
- 66.Hu Y, Lu J, Young GC. New findings in a 400 million-year-old Devonian placoderm shed light on jaw structure and function in basal gnathostomes. Sci Rep. 2017;7(1):7813. doi: 10.1038/s41598-017-07674-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu D, Marcucio RS, Helms JA. A zone of frontonasal ectoderm regulates patterning and growth in the face. Development. 2003;130(9):1749–58. doi: 10.1242/dev.00397. [DOI] [PubMed] [Google Scholar]
- 68.Eberhart JK, Swartz ME, Crump JG, Kimmel CB. Early Hedgehog signaling from neural to oral epithelium organizes anterior craniofacial development. Development. 2006;133(6):1069–77. doi: 10.1242/dev.02281. [DOI] [PubMed] [Google Scholar]
- 69.Hu D, Marcucio RS. A SHH-responsive signaling center in the forebrain regulates craniofacial morphogenesis via the facial ectoderm. Development. 2009a;136(1):107–16. doi: 10.1242/dev.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abzhanov A, Tabin CJ. Shh and Fgf8 act synergistically to drive cartilage outgrowth during cranial development. Dev Biol. 2004;273(1):134–48. doi: 10.1016/j.ydbio.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 71.Abzhanov A, Protas M, Grant BR, Grant PR, Tabin CJ. Bmp4 and morphological variation of beaks in Darwin’s finches. Science. 2004;305(5689):1462–5. doi: 10.1126/science.1098095. [DOI] [PubMed] [Google Scholar]
- 72.Wu P, Jiang TX, Suksaweang S, Widelitz RB, Chuong CM. Molecular shaping of the beak. Science. 2004;305:1465–1466. doi: 10.1126/science.1098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hu D, Marcucio RS. Neural crest cells pattern the surface cephalic ectoderm during FEZ formation. Dev Dyn. 2012;241(4):732–40. doi: 10.1002/dvdy.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu D, Marcucio RS. Unique organization of the frontonasal ectodermal zone in birds and mammals. Dev Biol. 2009b;325(1):200–10. doi: 10.1016/j.ydbio.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu M, Ahlberg PE. The origin of the internal nostril of tetrapods. Nature. 2004;432:94–7. doi: 10.1038/nature02843. [DOI] [PubMed] [Google Scholar]
- 76.Asique AM, Fu K, Richman JM. Endogenous bone morphogenetic proteins regulate outgrowth and epithelial survival during avian lip fusion. Development. 2002;129(19):4647–60. doi: 10.1242/dev.129.19.4647. [DOI] [PubMed] [Google Scholar]
- 77.Szabo-Rogers HL, Geetha-Loganathan P, Nimmagadda S, Fu KK, Richman JM. FGF signals from the nasal pit are necessary for normal facial morphogenesis. Dev Biol. 2008;318:289–302. doi: 10.1016/j.ydbio.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 78.Griffin J, Compagnucci C, Hu D, Fish JL, Klein O, Marcucio R, Depew MJ. Fgf8 Dosage Determines Midfacial Integration and Polarity within the Nasal and Optic Capsules. Dev Biol. 2013;374:185–97. doi: 10.1016/j.ydbio.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Young NM, Hu D, Lainoff AJ, Smith FJ, Diaz R, Tucker AS, Trainor PA, Schneider RA, Hallgrímsson RS, Marcucio B. Embryonic bauplans and the developmental origins of facial diversity and constraint. Development. 2014;141(5):1059–63. doi: 10.1242/dev.099994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abramyan J, Thivichon-Prince B, Richman JM. Diversity in primary palate ontogeny of amniotes revealed with 3D imaging. J Anat. 2015;226:420–433. doi: 10.1111/joa.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hu D, Young NM, Xu Q, Jamniczky H, Green RM, Mio W, Marcucio RS, Hallgrímsson B. A dynamic Shh expression pattern, regulated by SHH and BMP signaling, coordinates fusion of primordia in the amniote face. Development. 2015;142(3):567–74. doi: 10.1242/dev.114835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kitazawa T, Takechi M, Hirasawa T, Adachi N, Narboux-Neme N, Kume H, Maeda K, Hirai T, Miyagawa-Tomita S, Kurihara Y, Hitomi J, Levi G, Kuratani S, Kurihara H. Developmental genetic bases behind the independent origin of the tympanic membrane in mammals and diapsids. Nat Commun. 2015;6:6853. doi: 10.1038/ncomms7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tucker AS, Watson RP, Lettice LA, Yamada G, Hill RE. Bapx1 regulates patterning in the middle ear: altered regulatory role in the transition from the proximal jaw during vertebrate evolution. Development. 2004;131(6):1235–45. doi: 10.1242/dev.01017. [DOI] [PubMed] [Google Scholar]
- 84.Anthwal N, Joshi L, Tucker AS. Evolution of the mammalian middle ear and jaw: adaptations and novel structures. J Anat. 2013;222(1):147–60. doi: 10.1111/j.1469-7580.2012.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Anthwal N, Urban DJ, Luo ZX, Sears KE, Tucker AS. Meckel’s cartilage breakdown offers clues to mammalian middle ear evolution. Nat Ecol Evol. 2017;1 doi: 10.1038/s41559-017-0093. pii: 0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Urban DJ, Anthwal N, Luo ZX, Maier JA, Sadier A, Tucker AS, Sears KE. A new developmental mechanism for the separation of the mammalian middle ear ossicles from the jaw. Proc Biol Sci. 2017;284(1848) doi: 10.1098/rspb.2016.2416. pii: 20162416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bush JO, Jiang R. Palatogenesis: morphogenetic and molecular mechanisms of secondary palate development. Development. 2012;139(2):231–43. doi: 10.1242/dev.067082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thomason JJ, Russell AP. Mechanical factors in the evolution of the mammalian secondary palate: a theoretical analysis. J Morphol. 1986;189(2):199–213. doi: 10.1002/jmor.1051890210. [DOI] [PubMed] [Google Scholar]
- 89.Dixon MJ, Marazita ML, Beaty TH, Murray JC. Cleft lip and palate: understanding genetic and environmental influences. Nat Rev Genet. 2012;(12):167–178. doi: 10.1038/nrg2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rice R, Connor E, Rice DP. Expression patterns of Hedgehog signalling pathway members during mouse palate development. Gene Expr Patterns. 2006;6:206–212. doi: 10.1016/j.modgep.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 91.Liu KJ, Arron JR, Stankunas K, Crabtree GR, Longaker MT. Chemical rescue of cleft palate and midline defects in conditional GSK-3beta mice. Nature. 2007;446:79–82. doi: 10.1038/nature05557. [DOI] [PubMed] [Google Scholar]
- 92.Brugmann SA, Goodnough LH, Gregorieff A, Leucht P, ten Berge D, Fuerer C, Clevers H, Nusse R, Helms JA. Wnt signaling mediates regional specification in the vertebrate face. Development. 2007;134:3283–3295. doi: 10.1242/dev.005132. [DOI] [PubMed] [Google Scholar]
- 93.Smith TM, Lozanoff S, Iyyanar PP, Nazarali AJ. Molecular signaling along the anterior-posterior axis of early palate development. Front Physiol. 2013;3:488. doi: 10.3389/fphys.2012.00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ferretti E, Li B, Zewdu R, Wells V, Hebert JM, Karner C, Anderson MJ, Williams T, Dixon J, Dixon MJ, Depew MJ, Selleri L. A conserved Pbx-Wnt-p63-Irf6 regulatory module controls face morphogenesis by promoting epithelial apoptosis. Dev Cell. 2011;21(4):627–41. doi: 10.1016/j.devcel.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nishihara H, Kobayashi N, Kimura-Yoshida C, Yan K, Bormuth O, Ding Q, Nakanishi A, Sasaki T, Hirakawa M, Sumiyama K, Furuta Y, Tarabykin V, Matsuo I, Okada N. Coordinately Co-opted Multiple Transposable Elements Constitute an Enhancer for wnt5a Expression in the Mammalian Secondary Palate. PLoS Genet. 2016;12(10):e1006380. doi: 10.1371/journal.pgen.1006380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hendrikse JL, Parsons TE, Hallgr msson B. Evolvability as the proper focus of evolutionary developmental biology. Evol Dev. 2007;9:393–401. doi: 10.1111/j.1525-142X.2007.00176.x. [DOI] [PubMed] [Google Scholar]
- 97.Shubin N, Tabin C, Carroll S. Deep homology and the origins of novelty. Nature. 2009;457:818–23. doi: 10.1038/nature07891. [DOI] [PubMed] [Google Scholar]
- 98.Irie N, Kuratani S. Comparative transcriptome analysis reveals vertebrate phylotypic period during organogenesis. Nat Commun. 2011;2:248. doi: 10.1038/ncomms1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ferguson CA, Tucker AS, Sharpe PT. Temporospatial cell interactions regulating mandibular and maxillary arch patterning. Development. 2000;127:403–412. doi: 10.1242/dev.127.2.403. [DOI] [PubMed] [Google Scholar]
- 100.Wilson J, Tucker AS. Fgf and Bmp signals repress the expression of Bapx1 in the mandibular mesenchyme and control the position of the developing jaw joint. Dev Biol. 2004;266(1):138–150. doi: 10.1016/j.ydbio.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 101.Kuratani S. Modularity, comparative embryology and evo-devo: developmental dissection of evolving body plans. Dev Biol. 2009;332(1):61–9. doi: 10.1016/j.ydbio.2009.05.564. [DOI] [PubMed] [Google Scholar]
- 102.Retaux S, Kano S. Midline signaling and evolution of the forebrain in chordates: a focus on the lamprey Hedgehog case. Integr Comp Biol. 2010;50(1):98–109. doi: 10.1093/icb/icq032. [DOI] [PubMed] [Google Scholar]
- 103.Creuzet SE. Regulation of pre-otic brain development by the cephalic neural crest. Proc Natl Acad Sci USA. 2009;106(37):15774–9. doi: 10.1073/pnas.0906072106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Le Douarin NM, Couly G, Creuzet SE. The neural crest is a powerful regulator of pre-otic brain development. Dev Biol. 2012;366(1):74–82. doi: 10.1016/j.ydbio.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 105.Lee SH, Bédard O, Buchtová M, Fu K, Richman JM. A new origin for the maxillary jaw. Dev Biol. 2004;276(1):207–24. doi: 10.1016/j.ydbio.2004.08.045. [DOI] [PubMed] [Google Scholar]
- 106.Cerny R, Lwigale P, Ericsson R, Meulemans D, Epperlein HH, Bronner-Fraser M. Developmental origins and evolution of jaws: new interpretation of “maxillary” and “mandibular”. Dev Biol. 2004;276(1):225–36. doi: 10.1016/j.ydbio.2004.08.046. [DOI] [PubMed] [Google Scholar]