Abstract
Aim
To investigate the physiological mechanisms leading to rapid improvement in diabetes after Roux-en-Y Gastric Bypass (RYGB). In particular, the roles of concurrent peri-operative dietary restrictions, which may also alter glucose metabolism, have not been completely elucidated.
Materials and Methods
To assess the differential contributions of diet and surgery to the mechanisms leading to the rapid improvement in diabetes after RYGB we enrolled ten patients with type 2 diabetes scheduled to undergo RYGB. All patients underwent a 10-day inpatient supervised dietary intervention equivalent with the peri-operative diet (Diet only Period), followed by, after a re-equilibration (washout) period, an identical period of pair matched-diet in conjunction with RYGB (Diet and RYGB Period). We conducted extensive metabolic assessments during a 6 hours Mixed Meal Challenge Test with stable isotope glucose tracer infusion performed before and after each intervention.
Results
Similar improvements in glucose levels, β-cell function, insulin sensitivity, and post-meal hepatic insulin resistance were observed with both interventions. Both interventions led to significant reductions in fasting and postprandial acyl-ghrelin. The Diet only intervention induced greater improvements in basal hepatic glucose output and post-meal GIP secretion. The Diet and RYGB intervention induced significantly greater increases in post-meal GLP-1, PYY, and glucagon.
Conclusions
The strict peri-operative dietary restriction is a main contributor to the rapid improvement in glucose metabolism following RYGB. The RYGB-induced changes in the incretin hormones (GLP-1 and PYY) likely exert a major role in the long-term compliance with such major dietary restrictions through central and peripheral mechanisms.
Keywords: Gastric bypass surgery, diet restriction, diabetes remission, glycemic control
1. INTRODUCTION
Roux-en-Y gastric bypass (RYGB) surgery is one of the most effective surgical approaches to alleviate obesity and its major accompanying morbidity, type 2 diabetes mellitus (T2DM) (1, 2). Glucose levels improve within few days post-RYGB, preceding substantial weight loss (3–5). Much work is under way attempting to elucidate the weight loss-independent beneficial mechanisms of RYGB. One key topic of contention is whether the radical improvement observed in glucose homeostasis immediately after RYGB is determined by surgery-mediated alterations in glucose metabolism or by the severe diet restrictions enforced in the peri-surgical period.
The biologic mechanisms by which RYGB induces such a profound impact on glycemia are far from understood. The role of ghrelin, the “hunger hormone”, the effect of incretins like glucose-dependent insulinotropic peptide (GIP) and glucagon-like peptide 1 (GLP-1), the alterations in bile acid recirculation and changes in gut microbiota have all been implicated in the rapid glycemic improvement after gastric bypass surgery (3, 6, 7). On the other hand, it has been long know that a very low calorie diet (<800-1,000 kcal/day) in patients with T2DM has significant positive impact on fasting and postprandial glucose levels as well as β-cell function, insulin sensitivity, hepatic glucose output, and ectopic fat deposition (8–12). The immediate post-RYGB diet is very restrictive, starting with few calories in the first postoperative day and gradually increasing over the next few days. By the end of the first week post-surgery, most patients still consume less than 500 kcal/day. Therefore, it is reasonable to consider that at least some of the improvement in glycemia observed in the days following RYGB is due to diet restriction. Studies that evaluated the contribution of diet restriction in the immediate post-operative period had contradictory results, some concluding that the restricted diet mediates the noted effects (13–16), while others conclude that surgery-induced changes are driving this process (17, 18).
To further explore this controversy, we enrolled obese patients with T2DM in a study comparing the effects of a typical peri-operative diet in the absence of surgery with the effects of a matched diet administered in conjunction with RYGB in the same patients after a several months-long wash-out period (5). Our goal was to contrast the metabolic signature of the two interventions in order to expand our understanding of the mechanisms leading to glycemic improvement following RYGB, as to better understand the differential roles of diet vs surgically induced metabolic changes in T2DM resolution.
2. MATERIALS AND METHODS
2.1. Study population
We recruited adult patients diagnosed with T2DM and meeting criteria for RYGB from the Bariatric Surgery Clinic at University of Texas Southwestern (UTSW) Medical Center. Exclusion criteria included severe anemia, chronic renal disease, problematic peripheral intravenous access, and treatment with incretin mimetics or dipeptidyl peptidase-4 inhibitors within 3 months before recruitment. UTSW Institutional Review Board approved the study, and all patients signed informed consent before enrollment.
2.2. Study design and protocol
The study design was previously published (5). The protocol comprised two identical 10-day rigorously overseen inpatient periods, separated by an average length of 3.3 months washout interval sufficient for the metabolic parameters to return to the baseline values. During the first study period (Diet only Period) patients followed the strict post-RYGB recommended diet, were treated with the same intravenous fluids, and observed the same activity restrictions recommended for patients who undergo the procedure. In the morning of days 2 and 10, after a 12 hours overnight fasting, a 6 hours (6-h) mixed meal challenge test (MMCT) was performed, with blood sampling at 22 time points. The total caloric intake (oral or intravenous; including calorie-containing liquids, intravenous fluids, and medications) for the period spanning between the two MMCTs was ≈1,700 kcal (total for 8 days), which is customary intake for the first week post-RYGB. Total volume intake (oral plus intravenous) was approximately 18 liters for the entire period. During the washout period patients were advised to return to their usual lifestyle. The second study period (Diet and RYGB Period) was identical with the first study period, with the exception of day 3 when RYGB surgery was performed. During this period patients were pair-fed to their own daily oral intake during the first study period, were administered the same amount and type of intravenous fluids, and followed the same activity restrictions. The RYGB procedure was performed laparoscopically using a 25-mm EEA stapler to create a gastro-jejunal anastomosis and a linear 60-mm stapler to create a jejuno-jejunal anastomosis (19). The length of the Roux limb was 100 cm. No surgery-related complications were observed. The 6-h MMCT was repeated on the same days 2 and 10 as in the preceding period (overall a total of four tests per subject). All oral anti-diabetic medications were held starting 3 days before and all subcutaneous insulins 24 hours before each study period. During the active study periods patients were treated only with intravenous boluses of regular insulin if capillary glucose readings were over 200 mg/dL. No insulin was administered within 10 hours of each MMCT. No other glucose lowering agents were administered during the active study periods.
2.3. Measurements
A 6-h MMCT combined with a deuterated glucose (6,6-2H2-G) (Cambridge Isotope Laboratories Inc., Andover, MA) tracer investigation was performed on days 2 and 10 of each active study period. A bolus infusion of 3.5 mg 6,6-2H2-G/kg was started at −4 hours and continued at a dose of 0.04 mg 6,6-2H2-G/kg/minute through the end of the MMCT (total 10 hours). Four baseline blood samples (−30, −20, −10, and −5 minutes) were collected. At time “0” the patient ingested (over 5 minutes) 240 mL of chocolate Boost Plus Nestle (Healthcare Nutrition, Florham Park, NJ) containing 45 g carbohydrates, 14 g protein and 14 g fat (total 360 kcal). Blood samples were collected at 5, 10, 15, 20, 25, 30, 40, 50, 60, 80, 100, 120, 150, 180, 210, 240, 300, and 360 minutes post-ingestion. Samples for the hormone measurements were collected according to the specifications for each analyte (in chilled tubes) and processed according to manufacturer instructions.
We report the fasting, mean and area under the curve (AUC) values. Total AUC was computed using the trapezoidal rule.
We estimated insulin resistance/sensitivity using the corrected homeostasis model assessment of insulin resistance (HOMA2-IR) (20) and Matsuda index [10,000/(FPG × FPI × Glucosemean0-360 × Insulinmean0-360)0.5, where FPI=fasting plasma insulin and FPG=fasting plasma glucose] (21). Two additional insulin sensitivity indexes were quantified using the oral glucose insulin sensitivity (OGIS) (22) and Stumvoll’s index (23).
β-cell function estimates were calculated using mathematical modeling analysis based on C-peptide deconvolution (22, 24). This model provides calculation of β-cell glucose sensitivity (the slope of the insulin secretion-glucose concentration relationship), rate sensitivity (marker of early insulin release), and potentiation factor (described in detail by Mari et al) (24). Basal and stimulated insulin clearance were calculated from the ratio of insulin secretion AUC/insulin AUC (25).
Insulin secretion at 9 mmol/L glucose, and integral of total insulin secretion during the MMCT, were determined from the model-estimated β-cell dose-response as previously described (26).
An empirical additional β-cell function index was estimated by the ratio between AUC for C-peptide and glucose. Disposition index (DI) was used to account for the correlation between insulin secretion and insulin sensitivity (27) and was determined by multiplying the insulin secretion (AUCC-peptide/AUCGlucose) by the Matsuda index (28).
Fasting hepatic glucose production, glucose clearance, and the total rate of glucose appearance during the 6-h MMCT were estimated using a previously described glucose kinetics model (29), based on the deuterated glucose tracer method (30). Labeled glucose was measured by isotope dilution with mass spectrometry as previously described (31). Calibration curves were calculated from gravimetric mixtures of 2H-isotype labeled and unlabeled glucose. The final glucose concentrations were corrected for the 2H-isotopic isomer abundance of [M+1] to [M+6] glucose (30). The hepatic insulin resistance index was determined by multiplication of hepatic glucose production to basal insulin (32).
2.4. Analytes measurements
Complete blood count, comprehensive metabolic and lipid panel were measured in a commercial laboratory (Quest Diagnostics, Irving, TX). Whole-blood glucose was measured using YSI 2300 STAT Plus Glucose analyzer (Yellow Spring Instruments Life Sciences, Yellow Spring, OH). HbA1c was measured by high-performance liquid chromatography. Plasma insulin and C-peptide were quantified by enzyme-linked immunosorbent assay (ELISA) (Mercodia, Uppsala, Sweden; Millipore, Billerica, MA, respectively). Glucagon was determined by radioimmunoassay using commercial kits (Millipore, Billerica, MA). Serum FGF19 and FGF21 were measured using a quantitative ELISA (BioVendor, LLC, and Asheville, NC). Plasma FFAs were quantified using an acyl–coenzyme A oxidase-based colorimetric kit (Wako Diagnostics, Richmond, VA). GLP-1, total GIP and total PYY plasma levels were determined by electrochemiluminescence-based immunoassays (Meso Scale Discovery, Gaithersburg, MD). Acyl ghrelin and des-acyl ghrelin were quantified using an enzyme immunoassay (Cayman Chemical, Ann Arbor, MI). Plasma adiponectin and leptin were measured using sandwich ELISA kits (Millipore).
2.5. Statistical analysis
Mixed-effects linear models with the study participant modeled as a random effect were constructed to compare baseline and end values within each period of the study and between the corresponding values of the two periods. This model had doubly repeated factors, day and period. The net difference in response between the two periods was tested with the day × period interaction term and the within period comparisons were made from the model least square means pairwise differences. Results are reported as mean ± standard deviation (SD) or 95% confidence interval (CI). A two-sided P-value < 0.05 was considered statistically significant. Relationships between the measured parameters were evaluated using Spearman correlation analysis. Analysis was performed with SAS9.4 (SAS Institute, Cary, NC).
3. RESULTS
3.1. Baseline characteristics and weight change
As reported previously (5), the average age of the 10 subjects who completed both study periods was 53.2 years (95%CI, 48.0–58.4), they had a body mass index (BMI) of 51.14 (95%CI, 45.96-56.32), and were diagnosed with T2DM for 7.4 years (95%CI, 4.8–10.0). Seven patients were using insulin (average dose 1.16 units/kg/day). Patients lost an average of 8.71 kg (95%CI, 4.69-12.73) during the first study period (from 143.19 kg to 134.48 kg; P=0.0009) and 5.05 kg (95%CI, 0.67-9.43) during the second study period (from 138.65 to 133.60 kg; P=0.03) (P=0.0008 between periods).
3.2. Glycemic parameters
We previously reported that all measured glycemic parameters (daily CBG readings, HbA1c, FPG, maximum glucose, and glucose AUC) improved numerically in both study periods, but only the improvement during the Diet Period reached statistical significance (Table 1, Supplemental Table 1, and Figure 1A) (5).
Table 1.
Changes in glucose metabolism following the period of caloric restriction alone (Diet only Period) versus identical caloric restriction along with gastric bypass surgery (Diet and RYGB Period)
| Measurement | Diet only Period | Diet and RYGB Period | Between periods | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | End | Change | P | Baseline | End | Change | P | P | |
| Fasting Glucose (mmol/L) |
9.22 (7.23 to 11.21) | 7.25 (5.65 to 8.84) | −1.97 (−3.82 to −0.12) | 0.04 | 8.53 (6.55 to 10.51) | 7.53 (5.95 to 9.12) | −0.99 (−2.83 to 0.85) | 0.25 | 0.19 |
| Glucose AUC (mmol L−1min) |
4,221.3 (3,232.4 to 5,210.2) | 3,566.6 (2,811.0 to 4,322.2) | −654.7 (−1,279.2 to −30.2) | 0.04 | 3,942.8 (3,013.3 to 4,872.3) | 3,399.4 (2,689.2 to 4,099.7) | −543.3 (−1,130.3 to 43.7) | 0.07 | 0.60 |
| Fasting C-peptide (ng/mL) | 2.72 (1.91 to 3.54) | 3.26 (1.90 to 4.61) | 0.54 (−0.84 to 1.91) | 0.40 | 2.83 (1.94 to 3.72) | 2.63 (1.15 to 4.11) | −0.20 (−1.71 to 1.30) | 0.77 | 0.10 |
| C-peptide AUC (ng mL−1min) |
1,701.7 (1,102.0 to 2,301.4) | 2,061.7 (1,416.0 to 2,707.3) | 360.0 (−286.9 to 1,006.8) | 0.24 | 1,613.3 (1,122.5 to 2,103.7) | 1,556.0 (1,027.8 to 2,084.2) | −57.1 (−586.3 to 472.1) | 0.81 | 0.06 |
| Ratio (C-peptide AUC/Glucose AUC) | 0.02 (0.01 to 0.04) | 0.04 (0.03 to 0.05) | 0.02 (0.004 to 0.03) | 0.01 | 0.03 (0.02 to 0.03) | 0.03 (0.02 to 0.04) | 0.004 (−0.005 to 0.01) | 0.35 | 0.02 |
| Ratio (Δ0-30 min C-peptide/Δ0-30 Glucose) | 0.06 (0.03 to 0.09) | 0.07 (0.03 to 0.11) | 0.01 (−0.04 to 0.06) | 0.74 | 0.06 (0.03 to 0.09) | 0.05 (0.02 to 0.08) | −0.01 (−0.05 to 0.04) | 0.76 | 0.66 |
| Fasting Insulin (pmol/L) |
70.76 (44.95 to 96.58) | 51.94 (38.17 to 65.70) | −18.83 (−42.63 to 4.98) | 0.11 | 64.63 (37.61 to 91.65) | 34.65 (20.24 to 49.06) | −29.98 (−54.89 to −5.06) | 0.02 | 0.41 |
| Insulin AUC (pmol L−1min) |
52,260 (33,946 to 70,575) | 54,786 (39,789 to 69,783) | 2,527 (−17,141 to 22,195) | 0.78 | 48,116 (32,435 to 63,796) | 29,581 (16,740 to 42,421) | −18,535 (−35,375 to −1,695) | 0.03 | 0.51 |
| Fasting Insulin Secretion (pmol min−1m−2) |
102.3 (72.0 to 132.6) | 120.7 (67.8 to 173.6) | 18.4 (−34.7 to 71.5) | 0.45 | 105.4 (73.0 to 137.8) | 99.1 (42.5 to 155.6) | −6.3 (−63.01 to 50.4) | 0.81 | 0.14 |
| ISR at 9 mmol/L (pmol min−1m−2) |
172.0 (48.4 to 295.6) | 287.9 (116.4 to 459.4) | 115.9 (−27.6 to 259.4) | 0.10 | 160.5 (80.8 to 240.2) | 183.6 (73.0 to 294.2) | 23.1 (−69.4 to 115.6) | 0.59 | 0.054 |
| Glucose Sensitivity (pmol min−1m−2mmol−1L) |
29.31 (6.22 to 52.40) | 53.63 (14.86 to 92.40) | 24.32 (−20.90 to 69.54) | 0.25 | 26.28 (10.64 to 41.93) | 32.65 (6.38 to 58.92) | 6.36 (−24.28 to 37.00) | 0.65 | 0.12 |
| Rate Sensitivity (pmol m−2mmol−1L)† |
711.3 (217.0 to 1,666.0) | 1,458.0 (881.6 to 1,880.6) | 526.5 (−523.3 to 1,402.5) | 0.15 | 1,213.7 (784.5 to 2,131.7) | 844.93 (249.5 to 1,860.3) | −199.7 (−1,539.8 to 733.3) | 0.34 | 0.07 |
| 3-h Potentiation Ratio (dimensionless) | 1.22 (0.90 to 1.53) | 1.19 (0.78 to 1.59) | −0.03 (−0.47 to 0.41) | 0.88 | 1.28 (0.96 to 1.60) | 1.28 (0.87 to 1.69) | −0.003 (−0.44 to 0.43) | 0.99 | 0.92 |
| Total ISR (nmol m−2) |
66.15 (42.06 to 90.24) | 81.37 (55.67 to 107.07) | 15.22 (−10.40 to 40.84) | 0.21 | 61.93 (42.17 to 81.69) | 60.87 (39.78 to 81.95) | −1.06 (−22.08 to 19.95) | 0.91 | 0.07 |
| Clinsb (L min−1m−2) |
1.91 (1.08 to 2.74) | 2.80 (1.12 to 4.47) | 0.89 (−0.54 to 2.31) | 0.19 | 2.03 (1.17 to 2.90) | 3.55 (1.81 to 5.29) | 1.52 (0.04 to 3.00) | 0.046 | 0.43 |
| Clins (L min−1m−2) |
1.77 (0.89 to 2.66) | 1.97 (1.12 to 2.82) | 0.2 (−0.59 to 0.98) | 0.59 | 1.68 (0.99 to 2.38) | 2.55 (1.88 to 3.22) | 0.87 (0.24 to 1.49) | 0.01 | 0.03 |
| Disposition Index | 0.14 (0.06 to 0.22) | 0.27 (0.07 to 0.46) | 0.13 (−0.02 to 0.28) | 0.09 | 0.13 (0.06 to 0.21) | 0.29 (0.11 to 0.48) | 0.16 (0.02 to 0.31) | 0.03 | 0.59 |
| 2-h OGIS (mL min−1m−2) |
251.1 (198.5 to 303.6) | 306.6 (260.6 to 352.5) | 55.5 (2.2 to 108.7) | 0.04 | 298.6 (201.3 to 395.9) | 309.0 (223.9 to 394.0) | 10.4 (−88.2 to 109.0) | 0.82 | 0.25 |
| HOMA2-IR | 1.73 (1.12 to 2.35) | 1.20 (0.82 to 1.58) | −0.53 (−1.06 to 0.004) | 0.051 | 1.47 (0.94 to 2.00) | 0.82 (0.49 to 1.15) | −0.65 (−1.11 to −0.19) | 0.01 | 0.64 |
| Matsuda Index | 4.60 (2.94 to 6.27) | 6.73 (4.20 to 9.27) | 2.13 (0.28 to 3.98) | 0.03 | 5.24 (2.36 to 8.11) | 9.16 (4.78 to 13.53) | 3.92 (0.73 to 7.11) | 0.02 | 0.29 |
| Stumvoll Index (μmol min−1kg−1) |
0.75 (−0.22 to 1.71) | 1.83 (0.84 to 2.83) | 1.08 (0.41 to 1.76) | 0.006 | 1.26 (0.02 to 2.49) | 2.65 (1.37 to 3.93) | 1.39 (0.52 to 2.26) | 0.006 | 0.27 |
Data represents Least Squares Means (95% CI). The significance of the change within each period under the Diet only Period and Diet and RYGB Period, respectively, whereas the significance of the change between periods is shown in the Between periods column.
AUC – area under the curve, ISR - Insulin secretion at 9.0 mmoL/L glucose from the dose-response, Glucose Sensitivity - mean slope of the dose-response in the observed glucose range, Rate Sensitivity – derivate component evaluating the dynamic dependence of insulin secretion on the rate of change of glucose concentration, 3-h Potentiation Ratio - ratio between the mean value in the intervals [160-180] and [0-20] min. Total ISR - integral of total insulin secretion, Clinsb - basal insulin clearance (basal insulin secretion/basal insulin), Clins - insulin clearance (insulin secretion AUC/insulin AUC), 2-h OGIS - oral glucose insulin sensitivity index (2-h equation), HOMA2-IR – homeostasis model of assessment of insulin resistance.
Rate Sensitivity was rank transformed prior to analysis; data reported as median and 95%CI.
Figure 1.
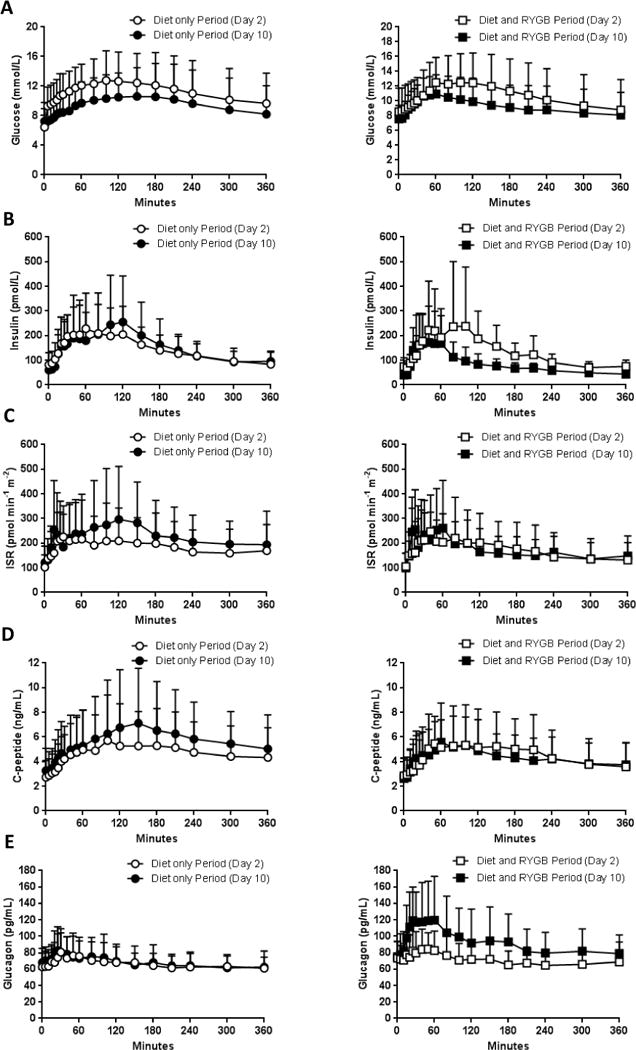
Results of glucose (A), insulin (B), ISR (C), C-peptide (D), and glucagon (F) measured during the 6-h MMCT following the period of caloric restriction alone (left column) vs identical caloric restriction alongside with RYGB (right column); open circles (○) indicates day 2 in the Diet only Period, filled circles (●) indicates day 10 in Diet only Period, open squares (□) indicates day 2 in Diet and RYGB Period, filled squares (■) indicates day 10 in Diet and RYGB Period. Plotted values are presented as mean ± SD
ISR – total insulin secretion rate, MMCT – mixed-meal challenge test, RYGB - Roux-en-Y gastric bypass
3.3. β-cell function
All parameter estimates of β-cell function and insulin sensitivity are presented in Table 1. Fasting (basal) insulin, and insulin AUC during the 6-h MMCT did not change significantly in the Diet only Period, but decreased in the Diet and RYGB Period (Table 1 and Figure 1B). C-peptide AUC/Glucose AUC increased during the Diet only Period (P=0.01) and did not change during the Diet and RYGB Period (P=0.35) (P=0.02 between periods) (Table 1 and Figure 1D).
Glucose-stimulated insulin secretion was increased in the post-Diet only Period and was unchanged after the Diet and RYGB Period (Table 1 and Supplemental Figure 1).
β-cell glucose sensitivity (slope of β-cell dose–response) improved numerically more during the Diet only Period, from 29.31 to 53.63 pmol min−1m−2mmol−1L, vs the Diet and RYGB Period, from 26.28 to 32.65 pmol min−1m−2mmol−1L (Table 1). A similar pattern was observed for insulin secretion at 9 mmol/L glucose.
Insulin clearance improved during both periods, but was statistically significant only after the Diet and RYGB Period, from 1.68 to 2.55 L min−1m−2 (P=0.01), with a significant difference between periods (P=0.03) (Table 1).
3.4. Insulin sensitivity
All indices of insulin resistance (HOMA2-IR) and sensitivity (Matsuda, Stumvoll, and 2-h OGIS) improved comparably during both study periods (Table 1), as well as the disposition index (Table 1).
3.5. Hepatic glucose production and glucose appearance rate
Fasting (basal) hepatic glucose production decreased significantly after the Diet only Period (P=0.007), while it increased slightly after Diet and RYGB Period (P=0.55; P=0.03 between periods) (Table 2).
Table 2.
Results of the glucose tracer investigations following the period of caloric restriction alone (Diet only Period) versus identical caloric restriction along with gastric bypass surgery (Diet and RYGB Period)
| Measurement | Diet only Period | Diet and RYGB Period | Between periods | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | End | Change | P | Baseline | End | Change | P | P | |
| Basal Glucose Clearance (mL min−1kg−1) |
0.90 (0.70 to 1.10) | 0.82 (0.59 to 1.05) | −0.08 (−0.30 to 0.15) | 0.46 | 0.92 (0.53 to 1.32) | 1.03 (0.58 to 1.47) | 0.10 (−0.34 to 0.55) | 0.61 | 0.37 |
| Mean MMCT Glucose Clearance (mL min−1kg−1) |
1.00 (0.78 to 1.21) | 1.04 (0.74 to 1.34) | 0.04 (−0.20 to 0.28) | 0.72 | 1.04 (0.86 to 1.22) | 1.05 (0.78 to 1.31) | 0.01 (−0.20 to 0.22) | 0.91 | 0.81 |
| Basal Glucose Production (mg min−1kg−1) |
1.56 (1.23 to 1.88) | 1.07 (0.78 to 1.35) | −0.49 (−0.81 to −0.17) | 0.007 | 1.36 (0.83 to 1.89) | 1.5 (1.03 to 1.97) | 0.14 (−0.38 to 0.67) | 0.55 | 0.03 |
| Mean MMCT Glucose Appearance (mL min−1kg−1) |
2.09 (1.54 to 2.65) | 1.72 (1.28 to 2.16) | −0.37 (−0.72 to −0.03) | 0.04 | 1.87 (1.55 to 2.19) | 1.65 (1.39 to 1.91) | −0.21 (−0.41 to −0.02) | 0.03 | 0.39 |
| 3-h Mean Glucose Appearance Time (min) | 78.80 (71.84 to 85.75) | 83.97 (77.52 to 90.42) | 5.17 (−0.72 to 11.07) | 0.08 | 75.97 (67.58 to 84.36) | 65.36 (57.35 to 75.37) | −10.61 (−17.64 to −3.59) | 0.008 | 0.008 |
| EGP-IR (mL min−1kg− pmol L−1) |
118.13 (44.72 to 191.55) | 55.28 (25.05 to 85.50) | −62.86 (−123.92 to −1.79) | 0.045 | 92.99 (14.07 to 171.90) | 57.63 (25.15 to 90.12) | −35.35 (−100.99 to −30.29) | 0.25 | 0.36 |
Data represents Least Squares Means (95% CI). The significance of the change within each period under the Diet only Period and Diet and RYGB Period, respectively, whereas the significance of the change between periods is shown in the Between periods column.
MMCT - mixed meal challenge test, 3-h Mean Glucose Appearance Time - integral of time × rate of appearance in the first 3 hours of the MMCT divided by the integral of the rate of appearance over the same interval, EGP-IR - endogenous glucose production × basal insulin (hepatic insulin resistance index).
Appearance of oral glucose was delayed after the Diet only Period by 5.17 minutes (P=0.08) and faster after Diet and RYGB Period by 10.61 minutes (P=0.008; between periods, P=0.008) (Table 2).
Hepatic insulin resistance index improved numerically only and comparably in both periods (Table 2).
3.6. Hormonal changes
Table 3 summarizes the results of the hormone measurements (other than insulin and C-peptide, which are presented in table 1), both in fasting state as well as AUC over the 6 hours MMCT. The dynamic profiles of insulin, C-peptide and glucagon over the duration of each MMCT are presented in Figure 1(B, D and E), while those of acyl ghrelin, des-acyl ghrelin, total GIP, GLP-1, PYY, FGF19 and FGF21 are presented in Figure 2(A-G).
Table 3.
Hormonal changes following the period of caloric restriction alone (Diet only Period) versus identical caloric restriction along with gastric bypass surgery (Diet and RYGB Period)
| Measurement | Diet only Period | Diet and RYGB Period | Between periods | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | End | Change | P | Baseline | End | Change | P | P | |
| Fasting Glucagon (pg/mL) |
62.55 (50.67 to 74.43) | 68.00 (56.45 to 79.55) | 5.45 (−2.59 to 13.49) | 0.16 | 73.15 (58.63 to 87.70) | 75.15 (61.03 to 89.27) | 2.00 (−7.83 to 11.83) | 0.66 | 0.49 |
| Glucagon AUC (pg mL−1min) |
23,890 (20,020 to 27,760) | 24,333 (19,420 to 29,240) | 440 (−2,925 to 3,810) | 0.78 | 25,530 (19,430 to 31,170) | 32,930 (25,480 to 40,370) | 7,630 (2,520 to 12,740) | 0.008 | 0.02 |
| Fasting Acyl Ghrelin (pg/mL) |
12.49 (4.44 to 20.54) | 5.40 (1.39 to 9.42) | −7.09 (−11.83 to −2.34) | 0.008 | 17.66 (3.69 to 31.64) | 6.86 (−0.11 to 13.82) | −10.81 (−19.05 to −2.57) | 0.02 | 0.28 |
| Acyl Ghrelin AUC (pg mL−1min) |
4,660.1 (2,034.0 to 7,286.3) | 1,613.3 (274.6 to 2,952.0) | −3,046.8 (−4,579.4 to −1,514.3) | 0.002 | 5,409.3 (116.6 to 10,702.0) | 2,803.5 (105.6 to 5,501.4) | −2,605.8 (−5,694.6 to 482.9) | 0.09 | 0.72 |
| Fasting Des-acyl Ghrelin (pg/mL) |
43.29 (31.91 to 54.66) | 40.20 (22.53 to 57.88) | −3.08 (−17.92 to 11.75) | 0.65 | 39.64 (30.92 to 48.37) | 38.40 (24.84 to 51.96) | −1.24 (−12.62 to 10.14) | 0.81 | 0.59 |
| Des-acyl Ghrelin AUC (pg mL−1min) | 14,305 (10,728 to 17,882) | 10,954 (7,294 to 14,614) | −3,352 (−6,606 to −98) | 0.045 | 13,111 (9,661 to 16,561) | 11,509 (7,979 to 15,040) | −1602 (−4,741 to 1,537) | 0.28 | 0.16 |
| Fasting GIP (pg/mL) |
87.04 (49.86 to 124.22) | 100.87 (51.16 to 150.58) | 13.83 (−6.61 to 34.27) | 0.16 | 114.74 (47.05 to 182.42) | 121.02 (30.53 to 211.51) | 6.28 (−30.93 to 43.50) | 0.71 | 0.69 |
| GIP AUC (pg mL−1min) |
92,811 (31,114 to 154,508) | 154,041 (68,484 to 239,599) | 61,230 (19,690 to 102,770) | 0.009 | 92,583 (49,798 to 135,369) | 85,919 (26,587 to 145,251) | −6,664 (−35,472 to 22,143) | 0.61 | 0.002 |
| Fasting GLP-1 (pg/mL) |
26.28 (11.41 to 41.14) | 31.26 (14.39 to 48.12) | 4.98 (−2.91 to 12.87) | 0.19 | 23.90 (13.35 to 34.45) | 30.41 (18.44 to 42.37) | 6.51 (0.90 to 12.11) | 0.03 | 0.51 |
| GLP-1 AUC (pg mL−1min) |
12,253 (4,451 to 20,056) | 14,028 (6,136 to 21,921) | 1,775 (−3,342 to 6,893) | 0.45 | 10,448 (1,389 to 19,507) | 25,071 (15,908 to 34,235) | 14,623 (8,682 to 20,565) | 0.0003 | 0.0001 |
| Fasting PYY (pg/mL) |
39.65 (28.61 to 50.69) | 30.36 (17.62 to 43.09) | −9.29 (−18.47 to −0.11) | 0.048 | 36.29 (25.36 to 47.22) | 34.92 (22.31 to 47.53) | −1.37 (−10.46 to 7.71) | 0.74 | 0.051 |
| PYY AUC (pg mL−1min) |
21,798 (15,581 to 28,016) | 21,795 (13,718 to 29,872) | −3.7 (−9,407 to 9,401) | 1.00 | 19,139 (9,411 to 28,867) | 48,198 (35,561 to 60,836) | 29,060 (14,345 to 43,774) | 0.002 | 0.001 |
| Fasting FGF19 (pg/mL) |
196.1 (115.7 to 276.5) | 262.5 (183.4 to 341.6) | 66.4 (−27.2 to 160.0) | 0.14 | 222.8 (142.6 to 303.0) | 214.6 (135.7 to 293.5) | −8.2 (−101.6 to 85.2) | 0.85 | 0.18 |
| FGF19 AUC (pg mL−1min) |
111,180 (73,316 to 149,044) | 146,946 (103,317 to 190,575) | 35,766 (−15,517 to 87,049) | 0.15 | 142,971 (107,761 to 178,181) | 73,434 (32,863 to 114,005) | −69,537 (−117,226 to −21,848) | 0.009 | 0.01 |
| Fasting FGF21 (pg/mL) |
548.9 (247.7 to 850.1) | 578.9 (270.5 to 887.3) | 30.0 (−145.9 to 205.9) | 0.71 | 451.9 (249.3 to 654.5) | 413.0 (205.6 to 620.4) | −38.9 (−157.2 to 79.4) | 0.48 | 0.17 |
| FGF21 AUC (pg mL−1min) |
154,400 (70,640 to 238,160) | 175,540 (−73,994 to 277,090) | 21,144 (−29,890 to 72,178) | 0.37 | 130,180 (69,479 to 190,880) | 132,130 (58,538 to 205,730) | 1,952 (−35,033 to 38,936) | 0.91 | 0.16 |
| Adiponectin (μg/mL) |
5.29 (3.47 to 7.12) | 3.80 (2.51 to 5.09) | −1.50 (−2.44 to −0.54) | 0.006 | 4.76 (3.29 to 6.23) | 3.87 (2.83 to 4.91) | −0.89 (−1.66 to −0.13) | 0.03 | 0.04 |
| Leptin (ng/mL) |
76.44 (43.61 to 109.26) | 67.83 (38.72 to 96.93) | −8.61 (−28.92 to 11.70) | 0.36 | 79.74 (50.33 to 109.15) | 37.68 (11.61 to 63.76) | −42.06 (−60.25 to −23.86) | 0.0005 | 0.02 |
| Cortisol (μg/dL) |
11.09 (8.77 to 13.41) | 11.57 (9.64 to 13.51) | 0.48 (−2.2 to 3.17) | 0.70 | 10.47 (7.30 to 13.65) | 12.33 (9.69 to 14.97) | 1.86 (−1.82 to 5.53) | 0.28 | 0.29 |
Data represents Least Squares Means (95% CI). The significance of the change within each period under the Diet only Period and Diet and RYGB Period, respectively, whereas the significance of the change between periods is shown in the Between periods column.
AUC – area under the curve; GIP – gastric inhibitory polypeptide; GLP-1 – glucagon like peptide 1; PYY – peptide YY; FGF – fibroblast growth factor.
Figure 2.
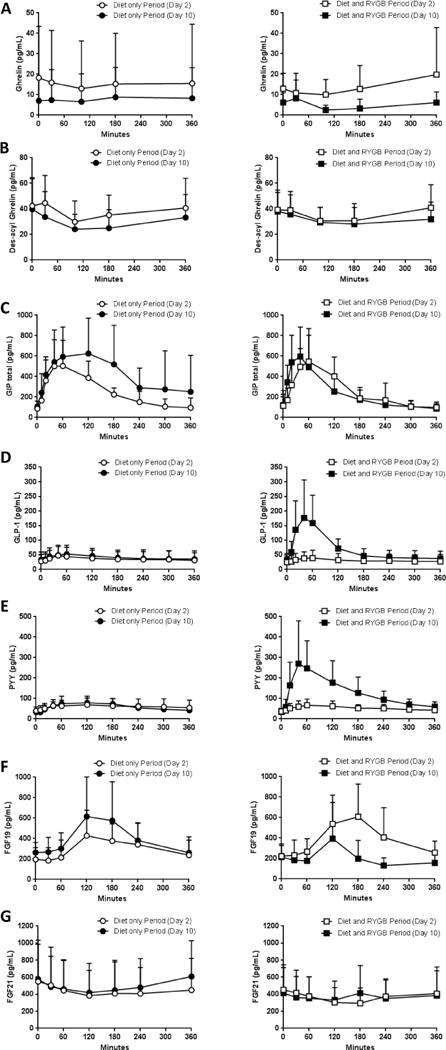
Results of acyl ghrelin (A), des-acyl ghrelin (B), GIP (C), GLP-1 (D), PYY (F), FGF19 (G), and FGF21 (H) measured during the 6-h MMCT following the period of caloric restriction alone (left column, Diet only Period) vs identical caloric restriction alongside with RYGB (right column, Diet and RYGB Period); open circles (○) indicates day 2 in Diet only Period, filled circles (●) indicates day 10 in Diet only Period, open squares (□) indicates day 2 in Diet and RYGB Period, filled squares (■) indicates day 10 in Diet and RYGB Period. Plotted values are presented as mean ± SD
FGF – fibroblast growth factor, GIP – gastric inhibitory polypeptide, GLP-1 – glucagon like peptide 1, MMCT – mixed meal challenge test, PYY – peptide YY, RYGB - Roux-en-Y gastric bypass
In the Diet only Period, the change in GIP AUC had a significant direct correlation with the change in total insulin secretion rate (ISR) (r=0.927, P=0.0002, Supplemental Figure 2A); this association was not observed in the Diet and RYGB Period.
Neither GLP-1 nor PYY AUC correlates with the change in total ISR in either period (Supplemental Figures 2B-C).
3.7. Lipid metabolism
Changes in total cholesterol, LDL, and HDL were not different between periods (Supplemental Table 1). Triglycerides decreased during both study periods, but significantly more during the Diet only Period (P=0.04 between periods), while fasting and AUC for FFA increased significantly only during the Diet only Period (P=0.01 between study periods) (Supplemental Table 1).
4. DISCUSSION
In this study we performed a very tightly controlled intervention to precisely evaluate and contrast the acute metabolic and hormonal changes which occur with a bariatric-like diet (Diet only Period) vs the same diet in the context of RYGB (Diet and RYGB Period). The goal was to evaluate the contribution of the severe dietary restriction imposed in the immediate post-operative period to the rapid improvement in glycemia in obese patients with T2DM undergoing RYGB. The main conclusion of our study is that the austere caloric restriction employed immediately post-gastric bypass surgery is a main mediator of the acute improvement in glycemia observed after RYGB. Both interventions improved β-cell function, insulin resistance, and hepatic glucose output, but interestingly several parameters of β-cell function/insulin secretion, as well as fasting hepatic glucose production, improved significantly more during the Diet only Period compared with the Diet and RYGB Period.
Several other studies also evaluated the contribution of dietary restriction to the overall glycemic effects of RYGB surgery and many (14–16, 33) but not all (17, 18, 34, 35) concluded that dietary restriction exerts similar short-term effects to surgery. All these studies varied considerably in regards to study population, study design and type of measurements, therefore interpretation of these results should take these differences in consideration. First, these studies employed different degrees of caloric restriction, ranging from less than 300 kcal/day (14) to as much as 1,000 kcal/day (16, 17). All studies that noted a differential effect of RYGB vs diet employed a higher caloric intake (800-1,000 kcal/day) in the diet groups leading to a slower weigh loss rate, which could explain the smaller noted effect on the measured metabolic parameters. We employed a strictly supervised (inpatient) pair-feeding strategy where each patient consumed the same amount of calories each corresponding study day, ensuring a complete match between the two interventions, a unique feature amongst these studies. Second, most studies used matched comparator groups (14–17, 33, 35) which ensured matching of key baseline parameters like age, BMI and diabetes duration. We employed the two interventions to the same group of patients (with a long washout period) to ensure that all measured and unmeasured subject-related confounders are accounted for. Our patients did not regain all the weight lost during the first intervention despite the long washout period. We unfortunately could not overcome this limitation, yet it is notable that large differences in weight were present in all studies despite matching the two groups. For example, the baseline weights in the Jackness et al study were 121.4 vs 114.2 kg (15), in the Isbell at all study 153.2 vs 127.0 kg (14), and in the Plum et al study 132 vs 122 kg (35), a difference which could have contributed to the observed outcome. Other studies evaluated the same patients sequentially with a diet intervention followed by diet plus RYGB (18, 34), but none of these studies employed a wash-out period, thus the baseline metabolic parameters were significantly different at the start of each intervention. Third, the population evaluated in these studies varies in regards to the degree of metabolic abnormalities present at baseline, which could influence the degree of metabolic improvement attainable. Most studies enrolled patients with more recent diagnosis of T2DM (14, 17, 18) and lower baseline HbA1c (14), while Jorgensen et al only enrolled patients after having already lost 8% of their initial body weight (34). Our patient population was most similar to that studied by Jackness et al (15) and Vetter et al (16), as were our reported results and conclusions. Lastly, the type of metabolic assessments employed can also influence the outcomes. We chose a standardized oral mixed-meal stimulus over an intravenous stimuli or glucose-only stimuli as it most closely mimics the real-life physiologic stimuli for these patients and thus allowed us to evaluate their metabolism in a close to real-life setting. We also extended the MMCT to 6 hours, unique amongst all these studies, to capture the full postprandial dynamic profile of glucose and hormonal excursions. This long post-prandial assessment is especially important in a population with T2DM known to have a distorted and delayed meal-response. Berggen et al employed a MMCT with only 90 minutes of follow-up (18), which would preferentially capture the acute post-prandial changes, which are known to be exaggerated by the rapid postprandial glucose absorption induced by RYGB, but would not capture the full postprandial response, which is delayed in patients with T2DM.
We, and many other investigators (36, 37), noted significant increases in the peak postprandial GLP-1 and PYY after surgery, but not after the Diet only Period. The postoperative surge in these hormones is thought to be due to the delivery of undigested, highly concentrated food content directly to the hindgut, where it stimulates the secretion of these hormones with known anti-diabetes effect (38). While it is very tempting to claim a causative effect between the rapid improvement in glycemia and the significant postoperative rise of these known anti-diabetogenic hormones, our findings suggest that this relationship may just be an association. Other investigators have made similar observations when they reported that GLP-1 deficient mice retain the positive effects of RYGB in glycemia improvement (39). Furthermore, Jimenez et al demonstrated that blocking endogenous GLP-1 with exendin(9–39) does not impair the glucose response to meal after RYGB (40), a similar finding also reported by Vetter et al (16). However, Jorgensen concluded differently by showing that blockade with exendin(9–39) abolished the improved β-cell function and glucose tolerance observed after RYGB (34). Surgery-induced changes in GLP-1 and PYY, while not directly causing the dramatic and rapid changes in glycemia, may have a critical role in maintaining these improvements long-term through central effects on the control of appetite and satiety. This hypothesis is supported by findings from human studies performed by le Roux (41) and Svane (42) which showed that blocking the GLP-1 and PYY receptors after RYGB reverse the satiety induced by surgery and increases appetite. On the other hand, Ye et al (43) found that while blocking the central GLP-1 and PYY receptors led to an increase in food intake and weight in both RYGB and sham operated animals, GLP-1 receptor deficient animals still lost weight after RYGB. Their observations suggest that central GLP-1 and PYY signaling is important for weight regulation, but it may not be the critical mechanism uniquely responsible for the RYGB-induced weight loss.
Several limitations are noteworthy. We used a single tracer to avoid further complicating an already complex protocol. The sample size of 10 completers is relatively small, though comparable to other similarly detailed mechanistic studies. Lastly, it was not possible to randomize the order of the two interventions and therefore we cannot exclude a possible carryover effect from the first intervention to the second. Despite a long wash-out period (average 101.6 days), weight did not return to baseline (143.2 kg vs 138.6 kg) and some metabolic parameters were numerically (but not statistically) different (Supplemental Table 2). While this difference between the baseline metabolic status might have minimized the effect of the second intervention, it is notable that all measured glycemic indices post-RYGB were still above normal and as such opportunity for further improvement was present.
In conclusion, our study has shown that the improvements in the glycemic parameters, insulin sensitivity, β-cell function, and hepatic glucose production (well described after RYGB) are primarily mediated by the very restricted peri-operative diet as these changes were also observed (some with even greater magnitude) when a matched peri-operative diet was consumed in the absence of surgery. Surgery induced dramatic increases in post-prandial GLP-1 and PYY, but these do not appear to improveglycemia, insulin resistance, or β-cell function beyond the effect observed with the equivalent dietary restriction alone.
Supplementary Material
Supplemental Figure 1. Glucose-stimulated insulin secretion measured during 6-h MMCT following the period of caloric restriction alone (left graph) vs identical caloric restriction alongside with RYGB (right graph); open circles (○) indicates day 2 in Diet only Period, filled circles (●) indicates day 10 in Diet only Period, open squares (□) indicates day 2 in Diet and RYGB Period, filled squares (■) indicates day 10 in Diet and RYGB Period. Plotted values are mean ± SD.
MMCT – mixed-meal challenge test, RYGB - Roux-en-Y gastric bypass.
Supplemental Figure 2. Correlations between changes in GIP AUC (A), GLP-1 AUC (B), and PYY AUC (C) during a 6-h MMCT and ISR following a period of caloric restriction alone [open circles (○)] vs identical caloric restriction alongside with RYGB [filled circles (●)].
AUC – area under the curve, GIP – gastric inhibitory polypeptide, GLP-1 – glucagon like peptide 1, ISR – total insulin secretion, MMCT – mixed meal challenge test, PYY – peptide YY, RYGB - Roux-en-Y gastric bypass.
Acknowledgments
This study was supported by a Pilot Award to I.L. from NIH 3UL1RR024982-05S1. I.L. was supported by NIH K23RR024470. The authors extend their deepest appreciation to Michael Brown, MD (Department of Molecular Genetics), for assistance in planning the study and editing the manuscript and to Laura Golici, BA, and Madhuri Poduri, MS (Division of Endocrinology, Department of Internal Medicine) for their expert help in protocol implementation, patient care, and data collection. The authors would also like to thank all the study volunteers, the Clinical and Translational Research Center staff, Bariatric Clinic staff, University Hospital Staff, Adam Coster, and Nic Burtea.
References
- 1.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 2.Sjostrom L, Peltonen M, Jacobson P, Ahlin S, Andersson-Assarsson J, Anveden A, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311(22):2297–304. doi: 10.1001/jama.2014.5988. [DOI] [PubMed] [Google Scholar]
- 3.Cummings DE. Endocrine mechanisms mediating remission of diabetes after gastric bypass surgery. Int J Obes (Lond) 2009;33(Suppl 1):S33–40. doi: 10.1038/ijo.2009.15. [DOI] [PubMed] [Google Scholar]
- 4.Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240(2):236–42. doi: 10.1097/01.sla.0000133117.12646.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lingvay I, Guth E, Islam A, Livingston E. Rapid improvement in diabetes after gastric bypass surgery: is it the diet or surgery? Diabetes Care. 2013;36(9):2741–7. doi: 10.2337/dc12-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dirksen C, Jorgensen NB, Bojsen-Moller KN, Jacobsen SH, Hansen DL, Worm D, et al. Mechanisms of improved glycaemic control after Roux-en-Y gastric bypass. Diabetologia. 2012;55(7):1890–901. doi: 10.1007/s00125-012-2556-7. [DOI] [PubMed] [Google Scholar]
- 7.Sweeney TE, Morton JM. Metabolic surgery: action via hormonal milieu changes, changes in bile acids or gut microbiota? A summary of the literature. Best Pract Res Clin Gastroenterol. 2014;28(4):727–40. doi: 10.1016/j.bpg.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelley DE, Wing R, Buonocore C, Sturis J, Polonsky K, Fitzsimmons M. Relative effects of calorie restriction and weight loss in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1993;77(5):1287–93. doi: 10.1210/jcem.77.5.8077323. [DOI] [PubMed] [Google Scholar]
- 9.Sathananthan M, Shah M, Edens KL, Grothe KB, Piccinini F, Farrugia LP, et al. Six and 12 Weeks of Caloric Restriction Increases beta Cell Function and Lowers Fasting and Postprandial Glucose Concentrations in People with Type 2 Diabetes. J Nutr. 2015;145(9):2046–51. doi: 10.3945/jn.115.210617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steven S, Hollingsworth KG, Al-Mrabeh A, Avery L, Aribisala B, Caslake M, et al. Very Low-Calorie Diet and 6 Months of Weight Stability in Type 2 Diabetes: Pathophysiological Changes in Responders and Nonresponders. Diabetes Care. 2016;39(5):808–15. doi: 10.2337/dc15-1942. [DOI] [PubMed] [Google Scholar]
- 11.Amatruda JM, Richeson JF, Welle SL, Brodows RG, Lockwood DH. The safety and efficacy of a controlled low-energy (‘very-low-calorie’) diet in the treatment of non-insulin-dependent diabetes and obesity. Arch Intern Med. 1988;148(4):873–7. [PubMed] [Google Scholar]
- 12.Malandrucco I, Pasqualetti P, Giordani I, Manfellotto D, De Marco F, Alegiani F, et al. Very-low-calorie diet: a quick therapeutic tool to improve beta cell function in morbidly obese patients with type 2 diabetes. Am J Clin Nutr. 2012;95(3):609–13. doi: 10.3945/ajcn.111.023697. [DOI] [PubMed] [Google Scholar]
- 13.Campos GM, Rabl C, Peeva S, Ciovica R, Rao M, Schwarz JM, et al. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg. 2010;14(1):15–23. doi: 10.1007/s11605-009-1060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isbell JM, Tamboli RA, Hansen EN, Saliba J, Dunn JP, Phillips SE, et al. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care. 2010;33(7):1438–42. doi: 10.2337/dc09-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackness C, Karmally W, Febres G, Conwell IM, Ahmed L, Bessler M, et al. Very low-calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and beta-cell Function in type 2 diabetic patients. Diabetes. 2013;62(9):3027–32. doi: 10.2337/db12-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vetter ML, Wadden TA, Teff KL, Khan ZF, Carvajal R, Ritter S, et al. GLP-1 plays a limited role in improved glycemia shortly after Roux-en-Y gastric bypass: a comparison with intensive lifestyle modification. Diabetes. 2015;64(2):434–46. doi: 10.2337/db14-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laferrere B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93(7):2479–85. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berggren J, Lindqvist A, Hedenbro J, Groop L, Wierup N. Roux-en-Y gastric bypass versus calorie restriction: support for surgery per se as the direct contributor to altered responses of insulin and incretins to a mixed meal. Surg Obes Relat Dis. 2017;13(2):234–42. doi: 10.1016/j.soard.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton EC, Sims TL, Hamilton TT, Mullican MA, Jones DB, Provost DA. Clinical predictors of leak after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Surg Endosc. 2003;17(5):679–84. doi: 10.1007/s00464-002-8819-5. [DOI] [PubMed] [Google Scholar]
- 20.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21(12):2191–2. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–70. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 22.Mari A, Pacini G, Murphy E, Ludvik B, Nolan JJ. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care. 2001;24(3):539–48. doi: 10.2337/diacare.24.3.539. [DOI] [PubMed] [Google Scholar]
- 23.Stumvoll M, Mitrakou A, Pimenta W, Jenssen T, Yki-Jarvinen H, Van Haeften T, et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care. 2000;23(3):295–301. doi: 10.2337/diacare.23.3.295. [DOI] [PubMed] [Google Scholar]
- 24.Mari A, Schmitz O, Gastaldelli A, Oestergaard T, Nyholm B, Ferrannini E. Meal and oral glucose tests for assessment of beta -cell function: modeling analysis in normal subjects. Am J Physiol Endocrinol Metab. 2002;283(6):E1159–66. doi: 10.1152/ajpendo.00093.2002. [DOI] [PubMed] [Google Scholar]
- 25.Natali A, Gastaldelli A, Camastra S, Sironi AM, Toschi E, Masoni A, et al. Dose-response characteristics of insulin action on glucose metabolism: a non-steady-state approach. Am J Physiol Endocrinol Metab. 2000;278(5):E794–801. doi: 10.1152/ajpendo.2000.278.5.E794. [DOI] [PubMed] [Google Scholar]
- 26.Muscelli E, Casolaro A, Gastaldelli A, Mari A, Seghieri G, Astiarraga B, et al. Mechanisms for the antihyperglycemic effect of sitagliptin in patients with type 2 diabetes. J Clin Endocrinol Metab. 2012;97(8):2818–26. doi: 10.1210/jc.2012-1205. [DOI] [PubMed] [Google Scholar]
- 27.Bergman RN, Ader M, Huecking K, Van Citters G. Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes. 2002;51(Suppl 1):S212–20. doi: 10.2337/diabetes.51.2007.s212. [DOI] [PubMed] [Google Scholar]
- 28.Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42(11):1663–72. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]
- 29.Mari A, Stojanovska L, Proietto J, Thorburn AW. A circulatory model for calculating non-steady-state glucose fluxes. Validation and comparison with compartmental models. Comput Methods Programs Biomed. 2003;71(3):269–81. doi: 10.1016/s0169-2607(02)00097-4. [DOI] [PubMed] [Google Scholar]
- 30.Sunny NE, Parks EJ, Browning JD, Burgess SC. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab. 2011;14(6):804–10. doi: 10.1016/j.cmet.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bequette BJ, Sunny NE, El-Kadi SW, Owens SL. Application of stable isotopes and mass isotopomer distribution analysis to the study of intermediary metabolism of nutrients. J Anim Sci. 2006;84(Suppl):E50–9. doi: 10.2527/2006.8413_supple50x. [DOI] [PubMed] [Google Scholar]
- 32.Gastaldelli A, Casolaro A, Pettiti M, Nannipieri M, Ciociaro D, Frascerra S, et al. Effect of pioglitazone on the metabolic and hormonal response to a mixed meal in type II diabetes. Clin Pharmacol Ther. 2007;81(2):205–12. doi: 10.1038/sj.clpt.6100034. [DOI] [PubMed] [Google Scholar]
- 33.Lips MA, de Groot GH, van Klinken JB, Aarts E, Berends FJ, Janssen IM, et al. Calorie restriction is a major determinant of the short-term metabolic effects of gastric bypass surgery in obese type 2 diabetic patients. Clin Endocrinol (Oxf) 2014;80(6):834–42. doi: 10.1111/cen.12254. [DOI] [PubMed] [Google Scholar]
- 34.Jorgensen NB, Dirksen C, Bojsen-Moller KN, Jacobsen SH, Worm D, Hansen DL, et al. Exaggerated glucagon-like peptide 1 response is important for improved beta-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes. 2013;62(9):3044–52. doi: 10.2337/db13-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plum L, Ahmed L, Febres G, Bessler M, Inabnet W, Kunreuther E, et al. Comparison of glucostatic parameters after hypocaloric diet or bariatric surgery and equivalent weight loss. Obesity (Silver Spring) 2011;19(11):2149–57. doi: 10.1038/oby.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beckman LM, Beckman TR, Sibley SD, Thomas W, Ikramuddin S, Kellogg TA, et al. Changes in gastrointestinal hormones and leptin after Roux-en-Y gastric bypass surgery. JPEN J Parenter Enteral Nutr. 2011;35(2):169–80. doi: 10.1177/0148607110381403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobsen SH, Olesen SC, Dirksen C, Jorgensen NB, Bojsen-Moller KN, Kielgast U, et al. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes Surg. 2012;22(7):1084–96. doi: 10.1007/s11695-012-0621-4. [DOI] [PubMed] [Google Scholar]
- 38.Cummings DE, Overduin J, Foster-Schubert KE, Carlson MJ. Role of the bypassed proximal intestine in the anti-diabetic effects of bariatric surgery. Surg Obes Relat Dis. 2007;3(2):109–15. doi: 10.1016/j.soard.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mokadem M, Zechner JF, Margolskee RF, Drucker DJ, Aguirre V. Effects of Roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency. Mol Metab. 2014;3(2):191–201. doi: 10.1016/j.molmet.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jimenez A, Casamitjana R, Viaplana-Masclans J, Lacy A, Vidal J. GLP-1 action and glucose tolerance in subjects with remission of type 2 diabetes after gastric bypass surgery. Diabetes Care. 2013;36(7):2062–9. doi: 10.2337/dc12-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246(5):780–5. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 42.Svane MS, Jorgensen NB, Bojsen-Moller KN, Dirksen C, Nielsen S, Kristiansen VB, et al. Peptide YY and glucagon-like peptide-1 contribute to decreased food intake after Roux-en-Y gastric bypass surgery. Int J Obes (Lond) 2016;40(11):1699–706. doi: 10.1038/ijo.2016.121. [DOI] [PubMed] [Google Scholar]
- 43.Ye J, Hao Z, Mumphrey MB, Townsend RL, Patterson LM, Stylopoulos N, et al. GLP-1 receptor signaling is not required for reduced body weight after RYGB in rodents. Am J Physiol Regul Integr Comp Physiol. 2014;306(5):R352–62. doi: 10.1152/ajpregu.00491.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Glucose-stimulated insulin secretion measured during 6-h MMCT following the period of caloric restriction alone (left graph) vs identical caloric restriction alongside with RYGB (right graph); open circles (○) indicates day 2 in Diet only Period, filled circles (●) indicates day 10 in Diet only Period, open squares (□) indicates day 2 in Diet and RYGB Period, filled squares (■) indicates day 10 in Diet and RYGB Period. Plotted values are mean ± SD.
MMCT – mixed-meal challenge test, RYGB - Roux-en-Y gastric bypass.
Supplemental Figure 2. Correlations between changes in GIP AUC (A), GLP-1 AUC (B), and PYY AUC (C) during a 6-h MMCT and ISR following a period of caloric restriction alone [open circles (○)] vs identical caloric restriction alongside with RYGB [filled circles (●)].
AUC – area under the curve, GIP – gastric inhibitory polypeptide, GLP-1 – glucagon like peptide 1, ISR – total insulin secretion, MMCT – mixed meal challenge test, PYY – peptide YY, RYGB - Roux-en-Y gastric bypass.


