Abstract
Background and Purpose
Circulating lymphocytes are exquisitely sensitive to radiation exposure, even to low scattered doses which can vary drastically between radiation modalities. We compared the relative risk of radiation-induced lymphopenia between Intensity Modulated Radiation Therapy (IMRT) or Proton Beam Therapy (PBT) in esophageal cancer (EC) patients undergoing neoadjuvant chemoradiation therapy (nCRT).
Material and Methods
EC patients treated with IMRT and PBT were propensity matched based on key clinical variables. Treatment-associated lymphopenia was graded using CTCAE v.4.0. Using matched cohorts, univariate and multivariable multiple logistic regression was used to identify factors associated with increased risk of grade 4 lymphopenia as well as characterize their relative contributions.
Results
Among the 480 patients treated with nCRT, 136 IMRT patients were propensity score matched with 136 PBT patients. In the matched groups, a greater proportion of the IMRT patients (55/136, 40.4 %) developed grade 4 lymphopenia during nCRT compared with the PBT patients (24/136, 17.6 %, P < 0.0001). On multivariable analysis, PBT was significantly associated with a reduction in grade 4 lymphopenia risk (odds ratio, 0.29; 95% confidence interval, 0.16 to 0.52; P < 0.0001).
Conclusion
PBT is associated with significant risk reduction in grade 4 lymphopenia during nCRT in esophageal cancer.
Keywords: Lymphopenia, Proton beam therapy, Intensity Modulated Radiation Therapy, chemoradiation, esophageal cancer
Introduction
A number of studies have associated treatment-induced lymphopenia with worse clinical outcomes in cancer patients[1–4]. Radiation therapy (RT) is an important contributor to treatment-induced lymphopenia as lymphocytes and their precursors are very sensitive to ionizing radiation[5]. Although lymphocytes are known to play a critical role in promoting systemic anti-tumor responses, examination of possible mitigating treatment factors on treatment-associated lymphopenia and correlation with clinical outcomes in esophageal cancer patients is lacking.
RT-induced lymphopenia can likely be mitigated by modifying RT technique, fractionation, and possibly, modality. For instance, altered RT fractionation using shorter courses with stereotactic body radiation therapy (SBRT) for pancreatic cancer over 2 weeks has been associated with significantly less radiation-induced lymphopenia than standard chemoradiation therapy (CRT) over 5 weeks[6]. Radiation target volume has also been identified as an important factor with greater treated volumes associated with lower posttreatment lymphocyte counts in non-small cell lung cancer[7]. Radiation-induced lymphopenia could be further reduced by the volume of radiation exposure, which is known to be substantially different comparing photon therapy to charged particles like proton beam therapy (PBT)[8]. However, there is a paucity of evidence that this difference has clinical impact on lymphopenia. We therefore conducted this propensity matched analysis with the hypothesis that PBT compared to photon RT could result in a lower risk of clinically significant lymphopenia with treatment.
Materials and Methods
Patients
This was an Institutional Review Board approved retrospective analysis of 480 patients with esophageal cancer treated with surgical resection after CRT at our institution between March 2005 and March 2016. Patients were included in the analyses if they had no distant metastases at presentation and were treated with preoperative concurrent CRT using PBT or IMRT with or without induction chemotherapy followed by surgery.
Treatment
Patients were typically treated with neoadjuvant CRT with or without induction chemotherapy to a median dose of 50.4 Gy at 1.8 Gy per fraction. Patients were simulated supine in an upper body cradle with their arms abducted overhead. Four-dimensional (4D) computed tomography (CT) simulation was used to track tumor motion throughout the respiratory cycle, as patients were treated in free-breathing. IMRT plans were generated using the Pinnacle treatment planning system (version 9.0, Philips, Andover, MA). Proton plans were generated using the Eclipse treatment planning system (Varian medical systems, Liverpool, NY).
Chemotherapy agents consisted of fluoropyrimidine, were typically given alone or in combination with either a platinum compound (classified as FP) or a taxane (classified as FT). Types of surgical procedures included Ivor-Lewis esophagectomy (with proximal gastrectomy and mediastinal + abdominal lymph node dissection), transthoracic esophagectomy, transhiatal esophagectomy, three-field esophagectomy, and minimally invasive esophagectomy.
Propensity matched analysis
To control for potential imbalances in prognostic risk factors for lymphopenia arising from differences in patient selection, we conducted a propensity matched analysis that used key clinical factors to match each PBT patient with an IMRT patient exhibiting similar demographic and clinical characteristics. An appropriate prognostic model was identified through multivariable logistic regression with backward elimination based on Akaike information criterion[9]. The initial model included gender, clinical stage, KPS, tumor location, induction chemotherapy, histology, age and PTV. Age, PTV, and histology remained as contributors to a final model attaining the highest degree of goodness-of-fit. Propensity scores were estimated based on these factors as well as, tumor location which exhibited a marginally significant association with grade 4 lymphopenia.
Statistical Analysis
The analysis plan endeavored to identify risk factors associated with an extent of lymphopenia that was considered clinically significant as well as ascertain the relative partial contribution of RT modality after adjusting for baseline clinical risk factors. Lymphopenia during radiation therapy was dichotomized to grade 4 lymphopenia versus those with grade 0–3 lymphopenia. Clinical and treatment factors were tested for significant association with presence/absence of grade 4 lymphopenia in univariate analysis. Adhering to conventional assumptions, the univariate hypothesis tests utilized two-sample t-test or Wilcoxon tests for continuous variables as well as chi-square test or Fisher’s exact test for categorical variables. Thereafter, univariate and multivariable logistic regression models were used to identify factors associated with an increased risk of grade 4 lymphopenia as well as estimate the impact of RT modality. The estimated odds ratios (ORs) and their 95% confidence intervals (CIs) are reported. Overall survival (OS) and distant metastasis-free survival (DMFS) were estimated using the Kaplan-Meier method. Event times were calculated from surgery date to the first occurrence of death or distant progression. Multivariable Cox proportional hazards regression was used to characterize the independent partial effects of patient, disease, and treatment factors associated with OS and DMFS. The estimated hazard ratios (HRs) and their 95% CIs are reported. All statistical tests were two-sided with P < 0.05 used to confer statistical significance. All analyses were conducted using SAS 9.4 (SAS Institute INC, Cary, NC).
Results
Patient characteristics and propensity score matching
Table 1 summarizes baseline characteristics for the entire patient cohort (N = 480) by RT modality. From the initial dataset of 480 patients, a subset of 272 patients were chosen consisting of 136 matched pairs, with equal numbers treated with PBT and IMRT by propensity score matching, which formed a commensurate subset of patients exhibiting similar baseline clinical and demographic characteristics based on our propensity score model (Supplementary Figure S1). Table 2 summarizes baseline characteristics for the two matched groups.
Table 1.
Distribution of patient, tumor, and treatment factors for entire cohort (N = 480).
| Characteristic | Parameter | IMRT (N = 334) | PBT (N = 146) | P value |
|---|---|---|---|---|
| Age (years) | Mean, Median (Minimum-Maximum) | 59.0, 60 (26 – 82) | 61.3, 64 (26 – 76) | 0.018 |
| PTV (cc) | Mean, Median (Minimum-Maximum) | 707, 653 (112 – 2554) | 552, 506 (174 – 1727) | <0.001 |
| Gender, n (%) | Female | 40 (12) | 15 (10) | 0.59 |
| Male | 294 (88) | 131 (90) | ||
| Stage, n (%) | I | 1 (0) | 4 (3) | 0.09 |
| IIA | 115 (34) | 46 (32) | ||
| IIB | 10 (3) | 8 (5) | ||
| III | 196 (59) | 82 (56) | ||
| IVA | 12 (4) | 6 (4) | ||
| KPS, n (%) | 70 | 13 (4) | 2 (1) | 0.25 |
| 80–100 | 321 (96) | 144 (99) | ||
| Tumor location, n (%) | Upper-Middle | 19 (6) | 6 (4) | 0.47 |
| Lower | 315 (94) | 140 (96) | ||
| Induction chemotherapy, n (%) | No | 205 (61) | 96 (66) | 0.36 |
| Yes | 129 (39) | 50 (34) | ||
| Histology, n (%) | Adenocarcinoma | 313 (94) | 140 (96) | 0.14 |
| SCC | 21 (6) | 5 (3) | ||
| Other | 0 | 1 (1) |
Abbreviations: IMRT = intensity-modulated radiation therapy, PBT = proton beam therapy, PTV = planning target volume, KPS = Karnofsky performance status, SCC = Squamous cell carcinoma, RT = radiation therapy.
Table 2.
Distribution of patient, tumor, and treatment factors for IMRT/PBT propensity matched patients (N = 272).
| Characteristic | Parameter | IMRT (N = 136) | PBT (N = 136) | Range of standardized difference or proportion of discordant pairs |
|---|---|---|---|---|
| Age (years) | Mean, Median (Minimum-Maximum) | 59.8, 60 (26 – 82) | 60.9, 63 (26 – 76) | (−3.2 – 3.1) |
| PTV (cc) | Mean, Median (Minimum-Maximum) | 565, 531 (112 – 1094) | 562, 520 (174 – 1727) | (−3.6 – 2.8) |
| Gender, N (%) | Female | 18 (13) | 14 (10) | 0.22 |
| Male | 118 (87) | 122 (90) | ||
| Stage, N (%) | I | 1 (1) | 3 (2) | 0.56 |
| IIA | 49 (36) | 38 (28) | ||
| IIB | 4 (3) | 7 (5) | ||
| III | 79 (58) | 82 (60) | ||
| IVA | 3 (2) | 6 (4) | ||
| KPS, N (%) | 70 | 6 (4) | 2 (2) | 0.06 |
| 80–100 | 130 (96) | 134 (98) | ||
| Tumor location, N (%) | Upper-Middle | 4 (3) | 6 (4) | 0.07 |
| Lower | 132 (97) | 130 (96) | ||
| Induction chemotherapy, N (%) | No | 85 (63) | 89 (65) | 0.49 |
| Yes | 51 (37) | 47 (35) | ||
| Histology, N (%) | Adenocarcinoma | 134 (98) | 131 (96) | 0.05 |
| SCC | 2 (2) | 5 (4) |
Abbreviations: IMRT = intensity-modulated radiation therapy, PBT = proton beam therapy, PTV = planning target volume, KPS = Karnofsky performance status, SCC = Squamous cell carcinoma, RT = radiation therapy.
Radiation-induced lymphopenia for the entire cohort
For the entire study cohort (N = 480), the incidence of grade 1, 2, 3, and 4 lymphopenia nadir during CRT was seen in 9 (1.9%), 43 (9.0%), 266 (55.4%), and 159 (33.1%) patients, respectively. Since nearly 90% of patients developed grade 3–4 lymphopenia during CRT, we focused subsequent analysis on grade 4 lymphopenia. During the same period, comparable rate of other severe grade 4 hematologic toxicities for leukopenia, neutropenia, and thrombocytopenia was rather low, occurring in only 2 (0.4%), 1 (0.2%), and 2 (0.4%) patients, respectively. For the patients who received induction chemotherapy (N = 179), grade 4 hematologic toxicities were not common during the induction chemotherapy phase, as only 0 (0%), 2 (1.1%), 1 (0.6%) and 0 (0%) patients experienced grade 4 leukopenia, neutropenia, lymphopenia and thrombocytopenia prior to CRT.
We compared patients with or without grade 4 lymphopenia during CRT in the entire study cohort (N = 480). Age (P = 0.02), PTV (P < 0.0001) and RT modality (P < 0.0001) were significantly different between patients with grade 4 lymphopenia vs. grade 0–3 lymphopenia. Distal tumor location was borderline significantly associated with grade 4 lymphopenia (P = 0.06) (Supplementary Table S1). Univariate logistic regression identified older age (P = 0.07), larger PTV (P = 0.0002), lower tumor location (P = 0.07) and IMRT relative to PBT (P < 0.0001) as factors potentially associated with increased risk of grade 4 lymphopenia (Supplementary Table S2). A multivariable logistic regression model, including four variables with univariate P-value < 0.15, is presented in Supplementary Table S2. PBT compared to IMRT, adjusting for age, PTV and tumor location, was significantly associated with lower risk of grade 4 lymphopenia.
Figure 1 depicts curves that represent the model estimated risk of grade 4 lymphopenia as a function of log PTV for both IMRT and PBT. While PTV is positively associated with increasing risk of lymphopenia for both modalities, the magnitude of risk-inflation was substantially higher for IMRT at equivalent levels of PTV.
Figure 1.
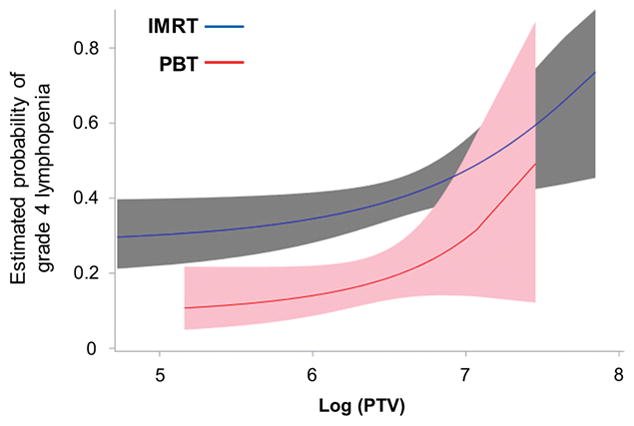
Model to represent the estimated risk of grade 4 lymphopenia as a function of the Planning Target Volume (PTV) for the two radiation modalities. Blue line represents intensity-modulated radiation therapy (IMRT) and red line represents proton beam therapy (PBT), along with 95% point-wise confidence intervals (gray and pink shaded regions, respectively).
Lymphopenia and clinical outcomes for the propensity matched patient cohort
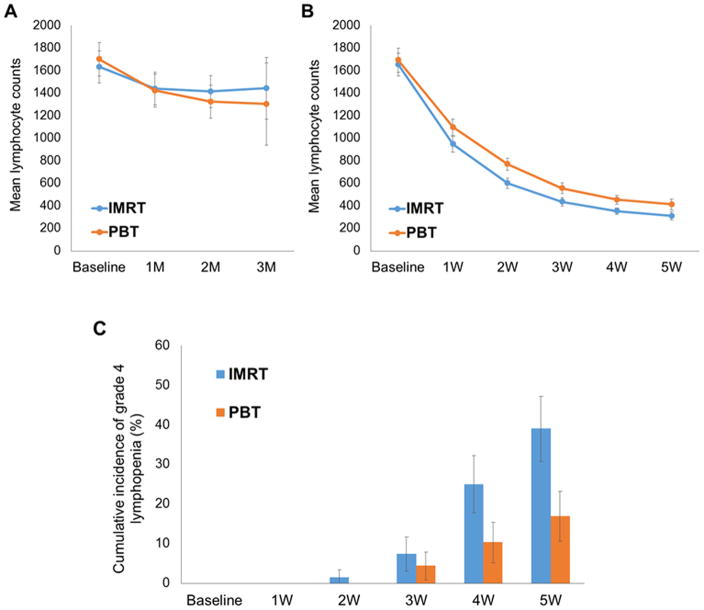
In the matched cohort, over a third of patients had induction chemotherapy prior to starting CRT. Figure 2 shows the fluctuation of mean lymphocyte counts during induction chemotherapy (A) and CRT (B). While lymphocyte counts declined slightly after the first cycle of chemotherapy, it remained stable and within normal limits throughout the subsequent 1–2 additional chemotherapy cycles without significant differences between the two radiation cohorts. In contrast, after the first week of the start of radiation therapy, a significant decline was observed in lymphocyte count, by nearly a third from baseline levels. The decline continued exponentially, with the nadir beginning the 3rd week of therapy. Grade 4 lymphopenia (< 200 cells/microliter) emerged starting the 3rd week, reaching by the 5th week 40.4 % (55/136) in the IMRT group and 17.6 % (24/136) in the PBT group (P < 0.0001) (Figure 2C).
Figure 2.
Lymphocyte count and risk of grade 4 lymphopenia during the course of preoperative chemoradiation therapy. Mean estimated lymphocyte counts during induction chemotherapy (A) and chemoradiation therapy (B) for the propensity matched patient cohort. Error bars depict 95% confidence intervals. M represents month from the beginning of induction chemotherapy and W represents week from the beginning of chemoradiation therapy. (C) Cumulative incidence of grade 4 lymphopenia during chemoradiation therapy for the propensity matched patient cohort. Error bars depict 95% confidence intervals. Abbreviations: IMRT = intensity-modulated radiation therapy; PBT = proton beam therapy
Age (P = 0.02) and RT modality (P < 0.0001) were significantly different among patients in the matched cohort with grade 4 lymphopenia vs. grade 0–3 lymphopenia (Table 3). PTV was of borderline significant (P = 0.05). Univariate logistic regression analyses identified older age (P = 0.03), larger PTV (P = 0.08) and IMRT modality relative to PBT (P < 0.0001) as factors associated with increased risk of grade 4 lymphopenia. A multivariable logistic regression model, including three variables with univariate p-value < 0.15, is presented in Table 4. Adjusting for partial effects of age and PTV, a significant reduction in the risk of grade 4 lymphopenia was seen among patients who received PBT compared to IMRT (OR, 0.290; 95% CI, 0.164 to 0.515; P < 0.0001), which was especially pronounced in patients with stage I–II disease (OR, 0.093; 95% CI, 0.028 to 0.313; P < 0.0001).
Table 3.
Comparisons of patients with or without grade 4 lymphopenia in propensity score matched cohort (N = 272).
| Characteristic | Parameter | Grade 0–3 Lymphopenia (N = 193) | Grade 4 Lymphopenia (N = 79) | P value |
|---|---|---|---|---|
| Age | Mean | 59.5 | 62.5 | 0.02 |
| PTV (cc) | Mean | 548 | 601 | 0.05 |
| Gender, N (%) | Female | 21 (66) | 11 (34) | 0.48 |
| Male | 172 (72) | 68 (28) | ||
| Stage, N (%) | I | 3 (75) | 1 (25) | 0.95 |
| IIA | 63 (72) | 24 (28) | ||
| IIB | 7 (64) | 4 (36) | ||
| III | 114 (71) | 47 (29) | ||
| IVA | 6 (67) | 3 (33) | ||
| KPS, N (%) | 70 | 5 (63) | 3 (38) | 0.69 |
| 80–100 | 188 (71) | 76 (29) | ||
| Tumor location, N (%) | Upper-Middle | 8 (80) | 2 (20) | 0.73 |
| Lower | 185 (71) | 77 (29) | ||
| Induction chemotherapy, N (%) | No | 126 (72) | 48 (28) | 0.48 |
| Yes | 67 (68) | 31 (32) | ||
| Histology, N (%) | Adenocarcinoma | 188 (71) | 77 (29) | 1.00 |
| SCC | 5 (71) | 2 (29) | ||
| RT Modality, N (%) | IMRT | 81 (60) | 55 (40) | <0.0001 |
| PBT | 112 (82) | 24 (18) |
Abbreviations: PTV = planning target volume, KPS = Karnofsky performance status, SCC = Squamous cell carcinoma, RT = radiation therapy, IMRT = intensity-modulated radiation therapy, PBT = proton beam therapy.
Table 4.
Univariate and multivariate logistic regression analysis for grade 4 lymphopenia in propensity score matched cohort (N = 272).
| Characteristic | Univariate | Multivariable | ||
|---|---|---|---|---|
|
| ||||
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age | 1.032 (1.003–1.061) | 0.03 | 1.038 (1.007–1.069) | 0.02 |
| PTV (per cc increase) | 1.001 (1.000–1.002) | 0.08 | 1.000 (1.000–1.002) | 0.09 |
| Gender | ||||
| Female | 1.000 | |||
| Male | 0.755 (0.345–1.649) | 0.48 | ||
| Stage | ||||
| I–IIB | 1.000 | |||
| III–IVA | 1.049 (0.610–1.803) | 0.86 | ||
| KPS | ||||
| 70 | 1.000 | |||
| 80–100 | 0.674 (0.157–2.889) | 0.59 | ||
| Tumor location | ||||
| Upper-middle | 1.000 | |||
| Lower | 1.663 (0.345–8.009) | 0.53 | ||
| Induction chemotherapy | ||||
| No | 1.000 | |||
| Yes | 1.215 (0.708–2.085) | 0.48 | ||
| Histology | ||||
| Adenocarcinoma | 1.000 | |||
| SCC | 0.977 (0.186–5.143) | 0.98 | ||
| RT Modality | ||||
| IMRT | 1.000 | 1.000 | ||
| PBT | 0.316 (0.181–0.552) | 0.0001 | 0.290 (0.164–0.515) | <0.0001 |
Abbreviations: OR = odds ratio, CI = confidence interval, PTV = planning target volume, KPS = Karnofsky performance status, SCC = Squamous cell carcinoma, RT = radiation therapy, IMRT = intensity-modulated radiation therapy, PBT = proton beam therapy.
To account for the potential confounding effects conferred by chemotherapy, we performed additional analysis stratifying for the types of chemotherapy used. Among the 272 propensity-matched patients, 146 (53.7%) and 72 (26.5%) patients were given taxane/5FU and platinum/5FU as concurrent chemotherapeutic regimen, respectively (Supplementary Table S3). In order to further test the association of radiation modality with grade 4 lymphopenia while accounting for the conjoint effects of chemotherapy, we evaluated the relative incidence of grade 4 lymphopenia between modality within each of the six predominant induction and concurrent chemotherapeutic regimens observed in our study population (Supplementary Table S4). The estimated relative incidence was consistently lower for proton patients within each of the six subtypes (OR: PBT/IMRT, 0.148–0.682).
In the entire surgical cohort, grade 4 lymphopenia during CRT was significantly associated with poorer OS on both univariable and multivariable analysis, along with gender and clinical stage (Supplementary Table S5). However, for the matched patients, multivariable Cox regression including clinical stage and grade 4 lymphopenia showed grade 4 lymphopenia only trended towards significance for poorer OS (P = 0.09) (Supplementary Figure S2A, Supplementary Table S6). However, grade 4 lymphopenia was significantly associated with reduced PFS (Supplementary Figure S2B) and DMFS (Supplementary Figure S2C) in both univariable as well as multivariable regression analyses after adjusting for gender and clinical stage (Supplementary Tables S7–8).
Discussion
In this study examining the role of RT treatment factors on lymphopenia risk, we found that treatment with IMRT compared to PBT, irradiating greater target volumes, and older age were associated with greater grade 4 lymphopenia risk. In a propensity-matched patient cohort, PBT compared to IMRT, adjusting for age and PTV, was associated with a clinically meaningful 71% risk reduction in grade 4 lymphopenia.
The use of more conformal radiation modalities (such as PBT) or minimizing the duration of exposure (such as using short course radiation like stereotactic body radiation therapy (SBRT)) can minimize normal tissue exposure. Previous studies suggest that neoadjuvant PBT compared with photon RT may reduce the incidence and severity of postoperative complications[10] and reduce treatment-related toxicities during or shortly after CRT for esophageal cancer[11]. One proposed mechanism for radiation-induced lymphopenia is via radiation exposure of the circulating blood pool, as lymphopenia occurs even after irradiation of tissues such as breast and brain, which contain little bone marrow or lymphatic tissues, respectively[12, 13]. Peripheral blood lymphocytes are known to be extremely sensitive to radiation despite mitotic inactivity[5]. Yovino et al[14] modeled radiation treatment for glioblastoma (60 Gy in 30 fractions) and found that a single fraction of RT resulted in 5% of all circulating cells being exposed to 0.5 Gy. Over the course of 30 fractions, 99% of circulating cells would receive at least 0.5 Gy (with mean dose 2.2 Gy). This model suggests that decreasing target volume and fraction number could significantly reduce circulating blood dose. This would also suggest that irradiating large pools of blood, such as the heart, would also lead to enhanced risk of lymphopenia. This would explain why the distal tumor location, and the large dosimetric differences of the heart comparing PBT and IMRT for mid to distal tumors[8] could lead to such big differences in lymphopenia risk between these two radiation modalities. Taken together, our results suggest that differences in the dosimetric scatter of radiation between the two RT modalities and the volume of radiation exposure were the key radiation factors that influenced lymphopenia risk.
We also found that patients without grade 4 lymphopenia demonstrated evidence of prolonged DFS and DMFS, and marginally also for better OS. This relationship remained intact on multivariable analysis, adjusting for key patient demographic, treatment, and tumor characteristics, including PTV. Although few studies have evaluated the association between lymphopenia and patient outcomes in patients with esophageal cancer[15], several studies have also demonstrated a link between treatment-induced lymphopenia and inferior survival in glioblastoma[3], NSCLC[2, 7] and pancreatic cancer[1, 4]. Our study does also indicate an association between higher lymphocyte levels during treatment and better clinical outcomes in esophageal cancer. Maintaining an intact adaptive immune system during cancer therapy may be important for enhancing the effectiveness of cytotoxic therapies and improving cancer control. This may impact cancer recurrence by affecting the numbers of tumor-infiltrating lymphocytes which correlates with prognosis for various cancers[16, 17]. This is consistent with our observation that severe lymphopenia was associated with worse PFS and DMFS. The relative lymphopenia sparing effects of PBT and implications for long-term endpoints through mediation of lymphocyte levels warrants prospective assessment in ongoing and planned randomized trials comparing PBT vs. IMRT.
Limitations to our study are the caveats of being a retrospective analysis, which, despite robust propensity-matching, may not be able to correct other potential (and unknown) external confounding factors. Since all patients were treated within the same period of time in the modern era (2005–2016), the delivery of radiotherapy was done based on uniform target definitions by nearly the same group of radiation oncologists at our institution, and any selection bias that could explain our results would likely be at the level of patient selection for either PBT or IMRT. Because of Medicare insurance coverage of PBT, PBT treated patients tend to be older than IMRT patients, but this negatively confounds PBT patients, in that older patients tend to also have higher risk of developing lymphopenia, both during induction chemotherapy and during CRT. Despite this negative selection, the protective effect of PBT on severe lymphopenia was still highly significant, indicating strongly that it is the low dose “bath” of IMRT, rather than other unknown patient, tumor, chemotherapy, or other treatment characteristics, that comparatively accounts for the dramatic reduction in lymphopenia risk in the PBT patients.
Conclusion
PBT substantially reduces the risk of grade 4 lymphopenia during CRT in esophageal cancer. These results warrant further confirmation in the ongoing and planned randomized trials comparing PBT vs IMRT in esophageal cancer, particularly proving how the distinct dosimetric differences between these two modalities may have long term impact on both toxicity and disease-specific outcomes.
Supplementary Material
Acknowledgments
Funding: This work was supported in part by the Cancer Center Support Grant (NCI Grant P30 CA016672).
References
- 1.Balmanoukian A, Ye X, Herman J, Laheru D, Grossman SA. The association between treatment-related lymphopenia and survival in newly diagnosed patients with resected adenocarcinoma of the pancreas. Cancer Invest. 2012;30:571–6. doi: 10.3109/07357907.2012.700987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campian JL, Ye X, Brock M, Grossman SA. Treatment-related lymphopenia in patients with stage III non-small-cell lung cancer. Cancer Invest. 2013;31:183–8. doi: 10.3109/07357907.2013.767342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17:5473–80. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wild AT, Ye X, Ellsworth SG, Smith JA, Narang AK, Garg T, et al. The Association Between Chemoradiation-related Lymphopenia and Clinical Outcomes in Patients With Locally Advanced Pancreatic Adenocarcinoma. Am J Clin Oncol. 2015;38:259–65. doi: 10.1097/COC.0b013e3182940ff9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sellins KS, Cohen JJ. Gene induction by gamma-irradiation leads to DNA fragmentation in lymphocytes. Journal of immunology (Baltimore, Md: 1950) 1987;139:3199–206. [PubMed] [Google Scholar]
- 6.Wild AT, Herman JM, Dholakia AS, Moningi S, Lu Y, Rosati LM, et al. Lymphocyte-Sparing Effect of Stereotactic Body Radiation Therapy in Patients With Unresectable Pancreatic Cancer. Int J Radiat Oncol Biol Phys. 2016;94:571–9. doi: 10.1016/j.ijrobp.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang C, Liao Z, Gomez D, Levy L, Zhuang Y, Gebremichael RA, et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys. 2014;89:1084–91. doi: 10.1016/j.ijrobp.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Palmer M, Bilton SD, Vu KN, Greer S, Frame R, et al. Comparing Proton Beam to Intensity Modulated Radiation Therapy Planning in Esophageal Cancer. Int J Particle Ther. 2015;1:866–77. [Google Scholar]
- 9.Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–23. [Google Scholar]
- 10.Wang J, Wei C, Tucker SL, Myles B, Palmer M, Hofstetter WL, et al. Predictors of postoperative complications after trimodality therapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2013;86:885–91. doi: 10.1016/j.ijrobp.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gajjar SR, Allen PK, He L, Gomez DR, Komaki R, Liao Z, et al. Reduced Incidence of Severe Acute Toxicities in Esophageal Cancer Treated with Proton Therapy. International Journal of Particle Therapy. 2015 [Google Scholar]
- 12.MacLennan IC, Kay HE. Analysis of treatment in childhood leukemia. IV. The critical association between dose fractionation and immunosuppression induced by cranial irradiation. Cancer. 1978;41:108–11. doi: 10.1002/1097-0142(197801)41:1<108::aid-cncr2820410116>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 13.Meyer KK. Radiation-induced lymphocyte-immune deficiency. A factor in the increased visceral metastases and decreased hormonal responsiveness of breast cancer. Archives of surgery (Chicago, Ill: 1960) 1970;101:114–21. doi: 10.1001/archsurg.1970.01340260018003. [DOI] [PubMed] [Google Scholar]
- 14.Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 2013;31:140–4. doi: 10.3109/07357907.2012.762780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng JF, Liu JS, Huang Y. Lymphopenia predicts poor prognosis in patients with esophageal squamous cell carcinoma. Medicine (Baltimore) 2014;93:e257. doi: 10.1097/MD.0000000000000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31:860–7. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.