Abstract
While the effects of weather variability on cardio-respiratory mortality are well described, research examining the effects on morbidity, especially for vulnerable populations, is warranted. We investigated the associations between lung function and outdoor temperature (T in Celsius degrees (C°)) and relative humidity (RH), in a cohort of elderly men, the Normative Aging Study.
Our study included 1103 participants whose forced vital capacity (FVC), forced expiratory volume in one second (FEV1), and weather exposures were assessed one to five times during the period 1995–2011 (i.e. 3162 observations). Temperature and relative humidity were measured at one location 4 hr to 7 days before lung function tests. We used linear mixed-effects models to examine the associations with outdoor T and RH.
A 5-degree increase in the 3-day moving average T was associated with a significant 0.7% decrease (95%CI: −1.24, −0.20) in FVC and a 5 % increase in the 7-day moving average RH was associated with a significant 0.2% decrease (95%CI: −0.40, −0.02) in FVC and FEV1. The associations with T were greater when combined with higher exposures of black carbon with a 1.6% decrease (95%CI −2.2; −0.9) in FVC and a 1% decrease (95%CI −1.7; −0.4) in FEV1. The relationships between T and RH and lung function were linear. No synergistic effect of T and RH was found.
Heat and lung function are two predictors of mortality. Our findings suggest that increases in temperature and relative humidity are related to decreases in lung function, and such observations might be amplified by high black carbon levels.
Keywords: respiratory health, adult, cohort, climate, air pollution, black carbon
1. Introduction
Extreme weather conditions (hot and cold) as well as variations across the common range of temperatures have been associated with increased morbidity and mortality (Basu and Samet, 2002; McGeehin and Mirabelli, 2001; Ye et al., 2012). Interestingly, a recent multi-country study, reported that most of the effects of temperature on mortality was due to moderately non-optimum temperatures rather than extreme heat or cold events (Gasparrini et al., 2015). Such issues have received more attention in the light of climate change (i.e. long-term trends in climate) and its expected global warming effect, which will likely increase the occurrence of diseases especially in populations with limited adaptive capacities.
Of importance, much of the excess weather-related mortality is from pulmonary and cardiovascular diseases (Basu and Samet, 2002). However, most of the research on pulmonary effects of weather variations (i.e. short-term variations) remains limited to time series studies on respiratory hospital admissions (Ye et al., 2012). Such studies have reported associations for respiratory diseases admissions and increase in ambient temperature, particularly in the elderly (Michelozzi et al., 2009), who are less able to cope with environmental stressors due to age-related decline in physiological capacity and co-morbidities. The elderly population (>65 years old) is expected to rise up to 19% of the US population by 2030 (http://www.aoa.gov/aoaroot/aging_statistics/index.aspx), which will likely increase impact of climate changes on public health. While research examining the associations of weather and morbidity in vulnerable populations is warranted (Basu and Samet, 2002; McGeehin and Mirabelli, 2001), to our knowledge, no such study has been conducted yet on lung function in the elderly.
We investigated the short-term associations between lung function and ambient temperature and relative humidity, in a cohort of elderly men, the Normative Aging Study (NAS). We adjusted our results for potential confounding by air pollution and investigated effect modification by particulate air pollution and ozone. We further examined susceptibility factors such as respiratory diseases and diabetes.
2. Methods
2.1. Study population and lung function assessment
The Normative Aging Study is a longitudinal closed cohort established by the U.S. Veterans Administration in 1963 (Bell et al., 1966). It enrolled 2280 male volunteers living in the Boston area, aged between 21–80 years at entry, who underwent an initial health screening that determined they were then free of known chronic medical conditions. Participants returned every 3–5 years for clinical examinations, which took place in the morning after an overnight fast and smoking abstinence. During these visits, height, weight, and medication use were assessed. Pulmonary disorders confirmed by a physician (asthma, chronic bronchitis, emphysema) and smoking history were collected using the American Thoracic Society (ATS) questionnaire (Ferris, 1978). Spirometric tests were performed following the ATS guidelines, as previously reported (Sparrow et al., 1987). Spirometry was assessed in the standing position with a noseclip using a 10-litre water-filled survey-recording spirometer and an Eagle II minicomputer (Warren E. Collins, Braintree, Massachusetts). Values were adjusted by body temperature and pressure. A minimum of three acceptable spirograms was obtained, of which at least two were reproducible within 5% for both forced vital capacity (FVC) and forced expiratory volume in one second (FEV1); the highest of the reproducible trials was selected from a given encounter. Each technician underwent training prior to taking measurements for this study. Chronic obstructive pulmonary disease (COPD) was defined as meeting the Global Initiative for Chronic Obstructive Lung Diseases (GOLD) stage II (or higher) criteria (FEV1/FVC<70% and FEV1<80% predicted, using the Crapo et al. equations (Crapo et al., 1981)) at least twice consecutively for participants with two or more visits and once for participants having only one visit. Methacholine challenge tests were conducted between 1984–2000 using procedures adapted from Chatham and colleagues (Chatham et al., 1982). We used data from the most recent test available for each subject. Participants having ischemic heart disease or baseline FEV1 at < 60% of the predicted value were excluded from the test, and some subjects elected not to participate. Methacholine inhalations were administered at incremental doses corresponding to 0, 0.330, 1.98, 8.58, 16.8, and 49.8 μmol (Izbicki and Bar-Yishay, 2001; Jayet et al., 2005). Participants whose FEV1 declined by 20% in response to any of the doses at or before 8.58 μmol were classified as having airway hyperresponsiveness. Participants whose FEV1 did not decline by 20% in response to any of the administered doses and those who demonstrated a 20% decline in FEV1 only from a higher methacholine dosage (16.8 or 49.8 μmol) were categorized as having no airway hyperresponsiveness. Our study included 1103 participants whose lung function, temperature and relative humidity were assessed one to five times between 1995 and 2011 (i.e. 3162 observations). Participants provided written informed consent and the study was approved by the institutional review boards of all participating institutions.
2.2. Environmental data
We obtained hourly data of ambient temperature (°C) and relative humidity (%) collected at the Boston Logan airport. Exposure to particulate black carbon and ozone was estimated using fixed monitoring stations. Black carbon concentrations were measured using an aethalometer (Magee Scientific Inc., Berkeley, CA, USA) positioned at the top of a building located < 1 km from the medical examination center, where the participants’ visits took place. Ozone was assessed by the average of the 4 local state monitors operated by the Massachusetts Department of Environmental Protection (Mass DEP). We considered a range of short-term exposure windows for temperature, humidity and air pollution indices preceding each subject’s examination, including: 4 hours, 24 hours (ie. lag 0), previous day (lag 1), previous 2 to 6 days (lag 2–lag 6) and 2 to 7-day moving averages. Since all visits were scheduled during the morning, exposure windows were calculated from 8am the day of the visit (e.g.: 24 hours average is the average from 8am the day of the visit to 8am the previous day).
2.3. Statistical analysis
We used the natural logarithms of FVC and FEV1 to stabilize the variance and increase the normality of the residuals. Separate models were run for each lung function measurement. The 4-hour time-window, 0–6 day distributed lag and moving averages were investigated in separate models. We applied a mixed linear model to examine the associations of ambient temperature and relative humidity with lung function:
| (1) |
with ε~N (0,σ2) and u~N (0,σu2)
where: Yi(t) is the log-transformed lung function measurement for participant i at visit t, β0 is the intercept, ui is the random effect for participant i, f1 and f2 are the distributed-lag functions with sets of coefficients β1 and β2 constrained by a second degree polynomial structure that corresponds to the temperature and relative humidity associations with lung function at lags 0 to 6 days, β3 is the vector of unknown parameters of confounders for participant i at visit t. Potential confounders were selected a priori, and we added a quadratic term whenever it was significant: age (linear and quadratic), height (linear and quadratic) and standardized weight (weight-average weight) (linear), race (white/black), education level (<12, 12, 13–15, >15 years), smoking status (former/current/never), cumulative smoking in pack-years, season of the medical exam (using sine and cosine of time), day of the week, doctor’s diagnosis of chronic lung conditions (asthma, emphysema, chronic bronchitis) (yes/no), methacholine responsiveness (yes/no), medication use (sympathomimetic α and β, anticholinergics) (yes/no).
2.3.1. Primary analyses
We investigated simultaneously ambient temperature and relative humidity and examined separately the 4-hour time-window and the polynomial distributed lag (PDL). We then investigated cumulative exposures (i.e. 2- to 7-day moving averages) and examined the shape of their relationship with lung function using penalized spline models in the generalized additive mixed models framework. The number of degrees of freedom for ambient temperature and relative humidity were chosen by cross-validation.
In our sensitivity analyses, since traffic-related air pollutants concentrations have been associated with lung function and are influenced by the prevailing meteorological conditions, we studied possible confounding by further adjusting for the 7-day moving averages of black carbon and ozone concentrations. We also adjusted for potential selection bias by applying inverse probability weighting (Hernán et al., 2006). We used logistic regression to calculate the probability of having a subsequent visit given age, education level, body mass index, smoking status, pack-years, hypertension, cholesterol, diabetes, FEV1, asthma, emphysema, chronic bronchitis, methacholine responsiveness and air pollutant exposure at previous visit. Finally, because ambient temperature and relative humidity may be better surrogates of real exposures during transitional seasons (spring (March–May) and fall (September–November)) compared to summer and winter (Nguyen et al., 2013), we repeated our analysis on visits performed during transitional seasons.
2.3.2. Secondary analyses
We tested interactions between ambient temperature and relative humidity, categorized using the tertiles of their distribution, in order to investigate whether combined exposure to ambient temperature and humidity would have a stronger/weaker association with lung function than the individual exposures; Such interactions were tested for 4-hour time-windows and cumulative exposures in two separate regressions. We then investigated whether the associations of ambient temperature or relative humidity with lung function were modified by season (dichotomized into warm (May–September) and cold (October–April)), pollutants exposure (black carbon and ozone categorized using the tertiles of the distribution), age >65 years, asthma, emphysema, chronic bronchitis, methacholine responsiveness, COPD, and obesity; such modifications were examined by adding an interaction term between the aforementioned variables and temperature and relative humidity during 4-hour time-window and cumulative exposures in separate regressions. P-values < 0.05 were considered statistically significant. Statistical analyses were conducted with SAS version 9.2 (SAS Institute, Cary, NC) and R version 2.15.1 (R Development Core Team, 2011).
3. Results
3.1. Descriptive results
Participants were aged from 49 to 97 years over the course of the follow-up; at first visit 72% of them were elderly (65 years old or above). Among the 3162 visits, 85% were conducted on elderly. They were mainly white, well educated, and former smokers (Table 1). Mean ± SD forced expiratory volume in one second (FEV1) was 2.5 ± 0.6 liters. Spearman correlation between FVC and FEV1 was 0.90. Among participants, 24.7% had one visit, 22.4% had two visits, 15.5% had three visits, 16.5% had four visits, and 20.9% had five visits.
Table 1.
Characteristics of the 1103 men participating in 3162 visits in the context of the Normative Aging Study, 1995–2011.
| Participant characteristics at 1st visit (n=1103) | Statistics |
|---|---|
| Age, mean ± SD, yr | 69.1 ± 7.2 |
| <65 yrs, n (%) | 309 (28.0) |
| Race, n (%) | |
| Black | 19 (1.7) |
| White | 1069 (96.9) |
| missing | 15 (1.4) |
| Height, mean ± SD, cm | 173.7 ± 6.7 |
| Weight, mean ± SD, kg | 84.4 ± 13.5 |
| Education, n (%), yr | |
| <12 | 47 (4.3) |
| 12 | 272 (24.7) |
| 13–15 | 307 (27.8) |
| >15 | 429 (38.9) |
| missing | 48 (4.4) |
| Smoking status, n (%) | |
| Never | 303 (27.5) |
| Current | 69 (6.3) |
| Former | 731 (66.3) |
| Pack-years* ± SD | 23.0 ± 28.1 |
| Diabetes, n (%) | 110 (10.0) |
| Obesity, n (%) | 280 (25.4) |
| Asthma, n (%) | 65 (5.9) |
| Chronic bronchitis, n (%) | 70 (6.3) |
| Emphysema, n (%) | 35 (3.2) |
| Methacholine responsiveness, n (%) | 115 (10.4) |
| Missing | 207 (18.8) |
| Corticosteroids, n (%) | 44 (4.0) |
| Sympathomimetic (α, β), n (%) | 106 (9.6) |
| Anticholinergic, n (%) | 12 (1.1) |
| FVC, mean ± SD, L | 3.5 ± 0.7 |
| FEV1, mean ± SD, L 1st sec | 2.6 ± 0.6 |
| Visit characteristics (n=3162) | |
| Season, n (%) | |
| Spring (March–May) | 795 (25.1) |
| Summer (June–Aug) | 926 (29.3) |
| Fall (Sept–Nov) | 914 (28.9) |
| Winter (Dec–Feb) | 527 (16.7) |
| Day of the week, n (%) | |
| Tuesday | 462 (14.6) |
| Wednesday | 456 (14.4) |
| Thursday | 1559 (49.3) |
| Friday | 685 (21.7) |
forced vital capacity= FVC, forced expiratory volume in 1 second= FEV1
Among current or former smokers
Average 3-day temperature was 13 °C for the whole year with a mean of 6°C and 19°C during the cold and warms seasons, respectively (Table 2). Average 7-day relative humidity was 68% for the whole year and slightly higher in the warm season. The correlation between daily ambient temperature and relative humidity was low (r=0.06). Ozone was negatively correlated with black carbon (r=−0.14) and relative humidity (r=−0.19), and positively correlated with ambient temperature (r=0.43). Black carbon was positively correlated with relative humidity (r=0.29) and ambient temperature (r=0.25). Correlation between lag days 0 to 6 was between 0.82–0.93 for ambient temperature and 0.03–0.50 for relative humidity.
Table 2.
Weather characteristics and pollutant concentrations 24h before lung function assessment for 3162 visits undergone by 1103 men, the Normative Aging Study, 1995–2011.
| Environmental factor | Statistics |
|---|---|
| mean ± SD (percentiles 5, 33, 66, 95) | |
| Temperature 4 hours (°C) | 11 ± 9 (−4, 7, 16, 23) |
| Temperature 3-days MA (°C) | 13 ± 8 (−1, 8, 18, 25) |
| Warm | 19 ± 5 (11, 18, 21, 26) |
| Cold | 6 ± 6 (−3, 4, 9, 16) |
| Relative humidity 4 hours (%) | 74 ± 16 (47, 65, 83, 97) |
| Relative humidity 7-days MA (%) | 68 ± 9 (53, 64, 72, 83) |
| Warm | 70 ± 9 (56, 66, 74, 84) |
| Cold | 67 ± 9 (51, 63, 71, 82) |
| Black carbon 4 hours (μg/m3) | 1.5 ± 1.1 (0.4, 0.9, 1.6, 3.4) |
| Black carbon 7-days MA (μg/m3) | 1.0 ± 0.4 (0.4, 0.7, 1.1, 1.8) |
| Warm | 1.0 ± 0.4 (0.5, 0.8, 1.2, 1.7) |
| Cold | 0.9 ± 0.4 (0.4, 0.7, 1.0, 1.9) |
| Ozone 4 hours (μg/m3) | 31 ± 20 (4, 19, 38, 68) |
| Ozone 7-days MA (μg/m3) | 50 ± 17 (22, 40, 59, 76) |
| Warm | 59 ± 14 (36, 53, 66, 82) |
| Cold | 41 ± 15 (20, 32, 44, 68) |
MA=Moving average 7-days (lag 0–lag 6)
3.2. Weather parameters and lung function
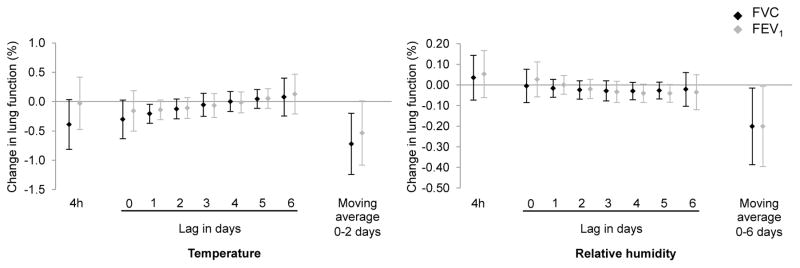
In our primary analyses, we observed a trend for a negative association between 4-hour mean ambient temperature and FVC (Figure 1). Ambient temperature increase on lags 0 to 2 was negatively associated with both FVC and FEV1; the associations were significant or borderline significant, for example a 0.2% decrease (95%CI: −0.37, −0.05) in FVC for a 5 °C increase in ambient temperature at lag 1 (p-value<0.05, Figure 1). As for relative humidity, the pattern of the plot indicated lags 4 and 5 as potential sensitive windows, but the associations between individual lags and lung function did not reach statistical significance (Figure 1). When we examined the shape of the association using penalized splines, cross-validation chose one degree of freedom for all cumulated exposure time-windows (3- to 7-day moving averages) to ambient temperature and relative humidity, suggesting linear relationships with both FVC and FEV1. For both outcomes, the most significant time-windows were the 3-day (i.e. 0 to 2 day) moving average for temperature and the 7-day (i.e. 0 to 6 day) moving average for relative humidity. We observed a significant 0.7% decrease (95%CI: −1.24, −0.20) in FVC for a 5 °C increase in the 3-day moving average ambient temperature. For relative humidity, we saw a 0.2% decrease (95%CI: −0.40, −0.02) in FVC and FEV1 for a 5% increase in the 7-day moving average relative humidity (Figure 1).
Figure 1.
Percent change in forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) (and 95% confidence intervals) for an increase of 5°C in ambient temperature or 5% in relative humidity (%), the Normative Aging Study, 1995–2011.
Temperature and relative humidity exposures were investigated simultaneously in 3 models depending on the time-windows of exposure: 4 hours, polynomial distributed lag from 0 to 6 days, moving averages, respectively. Results were adjusted for age, race, height, weight, education level, smoking status, cumulative smoking, season of the medical exam, day of the week, asthma, chronic bronchitis, emphysema, methacholine responsiveness, corticosteroids, sympathomimetic alpha and beta, anticholinergics.
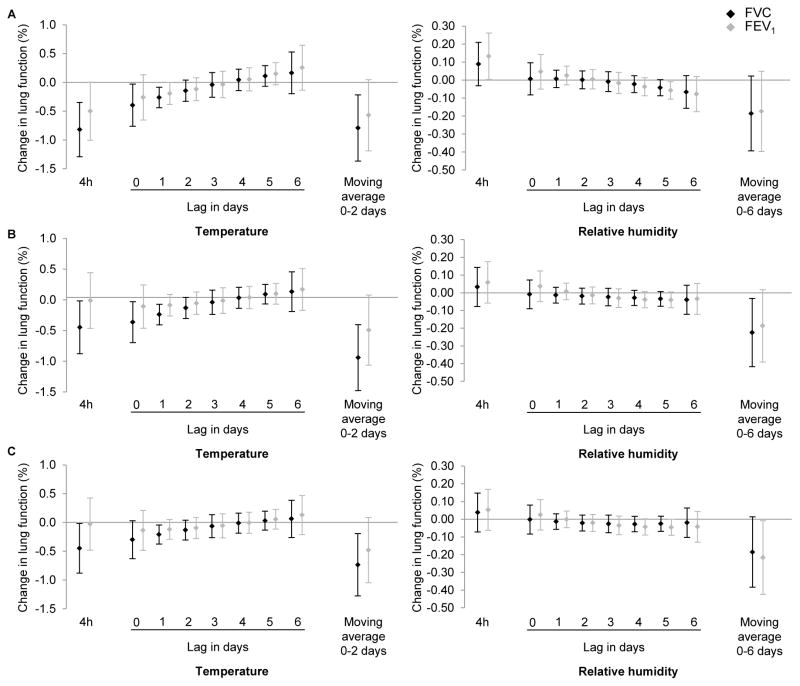
Sensitivity analyses indicated that further adjustment for black carbon exposure, ozone exposure or potential survival bias did not substantially change the results (Figure 2A, B, C, respectively); Associations with ambient temperature were generally slightly stronger, especially for the 4-hour time-window, and associations with relative humidity moving averages were slightly weaker. Results of analyses performed on visits undergone during the intermediate seasons were consistent with those of the main analysis (not shown).
Figure 2.
Percent change in forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) (and 95% confidence intervals) for a 5°C increase in temperature or 5% in relative humidity (%) adjusting for potential survival bias using inverse probability weighting (A), or adjusting for black carbon exposure (B), or adjusting for ozone exposure (C), the Normative Aging Study, 1995–2011.
Temperature and relative humidity exposures were investigated simultaneously in 3 models depending on the time-windows of exposure: 4 hours, polynomial distributed lag from 0 to 6 days, moving averages, respectively. Results were adjusted for age, race, height, weight, education level, smoking status, cumulative smoking, season of the medical exam, day of the week, asthma, chronic bronchitis, emphysema, methacholine responsiveness, corticosteroids, sympathomimetic alpha and beta, anticholinergics.
We focused our secondary analyses on 4-hour and 3-day moving averages for ambient temperature and on 4-hour and 7-day moving averages for relative humidity. Interactions between ambient temperature and relative humidity were not significant for both the 4-hour time-window and the cumulative exposure, suggesting there was no synergistic effect of these meteorological parameters on lung function. We found no significant interaction of ambient temperature and relative humidity with season or ozone exposure, but we saw significant interactions of both ambient temperature and relative humidity with black carbon exposure (Table 3). Participants in the highest tertile for black carbon exposure experienced stronger association between lung function and ambient temperature and relative humidity. We observed a 1.6% decrease (95%CI −2.2; −0.9) in FVC and 1.0% decrease (95%CI −1.7; −0.4) in FEV1 for a 5 °C increase in 3-day average ambient temperature in subjects in the highest tertile of exposure to black carbon, while we did not observe any significant association of temperature and lung function in subjects in the lowest tertile of exposure. We observed a 0.3% decrease (95%CI −0.5; −0.1) in FVC and 0.2% decrease (95%CI −0.4; 0.0) in FEV1 for a 5 % increase in relative humidity 4-hour before examination in subjects in the highest tertile of exposure to black carbon, while we observed a significant positive association in subjects in the lowest tertile of exposure.
Table 3.
Percent change (and 95% confidence intervals) in forced vital capacity and forced expiratory volume in 1 second for a 5°C increase in ambient temperature or 5 % in relative humidity (%) according to the concentration of black carbon in tertiles and to chronic diseases, the Normative Aging Study, 1995–2011.
| Exposure | Timing of exposure | Interaction factor | FVC | FEV1 | ||
|---|---|---|---|---|---|---|
| Beta (CI95%) | p-value of interaction | beta (CI95%) | p-value of interaction | |||
| Temperature | 4h | Black carbon | ns | 0.01 | ||
| 1st tertile | −0.07 (−0.62, 0.48) | |||||
| 2nd tertile | 0.38 (−0.17, 0.92) | |||||
| 3rd tertile | −0.41 (−0.95, 0.14) | |||||
| MA 0–2 days | Black carbon | 10−4 | 0.01 | |||
| 1st tertile | −0.45 (−1.05, 0.15) | −0.30 (−0.93, 0.34) | ||||
| 2nd tertile | −0.79 (−1.42, − 0.16) | −0.48 (−1.15, 0.19) | ||||
| 3rd tertile | −1.56 (−2.17, − 0.95) | −1.06 (−1.71, −0.41) | ||||
| MA 0–2 days | Obesity | 0.02 | ns | |||
| No | −0.86 (−1.40, − 0.33) | |||||
| Yes | −0.31 (−0.94, 0.32) | |||||
| Relative humidity | 4h | Black carbon | 10−4 | 10−3 | ||
| 1st tertile | 0.30 (0.12, 0.48) | 0.35 (0.17, 0.54) | ||||
| 2nd tertile | 0.05 (−0.14, 0.24) | −0.06 (−0.26, 0.15) | ||||
| 3rd tertile | −0.28 (−0.47, − 0.08) | −0.17 (−0.38, 0.04) | ||||
| 4h | Emphysema | 10−2 | 0.02 | |||
| No | 0.01 (−0.10, 0.12) | 0.03 (−0.08, 0.15) | ||||
| Yes | 1.04 (0.40, 1.68) | 0.83 (0.16, 1.51) | ||||
| 4h | COPD | 0.03 | 0.03 | |||
| No | −0.01 (−0.12, 0.11) | 0.01 (−0.11, 0.14) | ||||
| Yes | 0.31 (0.03, 0.58) | 0.36 (0.08, 0.65) | ||||
| MA 0–6 days | Hyper-responsiveness | ns | 0.02 | |||
| No | −0.86 (−1.40, −0.33) | |||||
| Yes | −0.31 (−0.94, 0.32) | |||||
MA: Moving average, ns: not significant. Results adjusted for age, race, height, weight, education level, smoking status, cumulative smoking, season of the medical exam, day of the week, visit number, temperature, relative humidity, asthma, chronic bronchitis, emphysema, methacholine responsiveness, corticosteroids, sympathomimetic alpha and beta, and anticholinergic
Regarding personal characteristics, we observed significant modification of association with temperature by obesity and of association with relative humidity by emphysema status, COPD and airways hyperresponsiveness (Table 3).
Participants suffering from emphysema or COPD experienced stronger increase on FVC and FEV1 associated with 4-hour mean relative humidity than participants with no such diseases. Participants with airway hyperresponsiveness experienced smaller decrease on FEV1 associated with a 6-day cumulated increase in relative humidity compared to participants with no such disease.
4. Discussion
In the elderly population of males examined in the present study, we found significant linear associations between short-term increase in ambient temperature or relative humidity and both decreased FVC and FEV1. The lag structure indicated days 0 to 2 for temperature and days 0 to 6 for relative humidity as the most significant time windows associated with both FVC and FEV1. Associations were of similar magnitude as those usually observed for ambient pollutants at moderate concentrations. We generally observed stronger associations with FVC than with FEV1. We also found larger adverse associations of temperature and relative humidity with lung function in those with the highest exposure to black carbon. Our findings suggest that increases in temperature and relative humidity are related to decreases in lung function, and such observations might be amplified by high black carbon levels.
To our knowledge, our study is the first to report lung function responses to short-term variations in ambient temperature and relative humidity in an elderly population. A previous study of 21 healthy university students (>6000 measurements), found a significant inverse association between temperature and morning peak expiratory flow, but no significant association with FEV1 (Wu et al., 2014). Other studies focusing on populations with preexisting diseases have reported associations between increases in ambient temperature and reduced lung function in subjects with chronic lung diseases (Mann et al., 1993), in cystic fibrosis patients (Collaco et al., 2011) and in children with asthma (Li et al., 2014).
The current study showed that associations of temperature and relative humidity with lung function last several days. For temperature, we observed an association with the cumulative exposure (days 0–2) and with the following individual exposure windows: 4 h, day 0 and day 1 preceding the pulmonary exam. The associations with temperature were more pronounced on FVC than on FEV1. For relative humidity, we mainly observed an association with a cumulative exposure (days 0–6). Individual exposure during days 4 and 5 before pulmonary exam were borderline significant. A short lag period, about a week or less, for the association with temperature and lung function, is consistent with results reported in studies investigating temperature associations with morbidity (Li et al., 2014; Ye et al., 2012) and mortality from all- and from respiratory-causes (Braga et al., 2002; Curriero et al., 2002; Kunst et al., 1993).
Exposure to high and low temperatures over a few days have been linked to increases in morbidity (Ye et al., 2012) and mortality (Basu and Samet, 2002), particularly in vulnerable populations such as the elderly; However, the biological mechanisms responsible for the observed association are unclear. High humidity conditions limit the evaporation by perspiration, but their general effects on lung function have rarely been studied. Physiologic efforts to thermoregulate involves increases in cardiac output, which in turn increases cutaneous blood flow and breathing rate. In the elderly, this ability to thermoregulate is limited by their cardiac capacity. Impaired thermoregulatory mechanisms might also be due to higher sweating thresholds in the elderly (Foster et al., 1976) and to the thermal perception, which is compromised in old age (Guergova and Dufour, 2011). Higher temperatures and humidity rates in elderly can create a physiological stress state and lead to dysfunctions on various systems, such as respiratory function. Thermoregulation may also play a role in our finding that non obese participants had stronger responses to temperature. Our results call for more research into the mechanisms responsible for the associations of ambient temperature and relative humidity rates with lung function.
Several studies identified a U-, V- or J-shape relationship between temperature and morbidity, with the highest morbidity rates observed below and above a certain threshold (Ye et al., 2012). Thresholds can vary by disease and geographic location as people tend to adapt to local climatic conditions. Evaluating the shape of the relationship between weather and morbidity requires data spanning the entire range of ambient temperature throughout a year, and not only during summer and winter seasons. In our study, the ambient temperature and relative humidity ranged between −15C° and 32C° and 23% and 100%, respectively. We observed linear relationships of temperature and relative humidity with lung function, which is consistent with those reported in university students in China (Wu et al., 2014). In children with asthma, Li et al. (2014) also found a linear temperature-lung function relationship across the yearly temperature range in Australia. Furthermore, our findings indicated that there was no synergistic effect of temperature and relative humidity on lung function and the temperature- and relative humidity-lung function relationships did not exhibit seasonal variability.
Air pollution is a well-known risk factor of adverse respiratory outcomes. Both sources and transport of air pollutants are influenced by the prevailing meteorological conditions. For instance, the amount of fossil fuels consumed for heating and cooling homes and commercial buildings is directly related to ambient temperature and to a lesser extent to relative humidity. The accumulation of air pollutants in urban areas is governed by the thermal stability and water content of air masses. Finally, the rates of atmospheric reactions responsible for the formation of secondary pollutants including ozone are controlled by ambient temperature and relative humidity conditions. Therefore, the air pollution and meteorology are intertwined and multiple relationships exist between the different parameters. Results of our analyses adjusted for black carbon and ozone concentrations were similar to our main results, thus suggesting a low potential for confounding by these pollutants. Although many studies on the association of temperature and morbidity have adjusted for air pollutant concentrations, few of them have investigated the potential synergistic effect of temperature and air pollutants. Our study found evidence for interaction of temperature and relative humidity with black carbon but not with ozone. For participants with higher exposure to black carbon, the adverse relationships of temperature (moving average 0–2 days) and relative humidity (4 hours) with lung function were stronger, while associations of relative humidity and lung function were positive for the lowest exposed participants. Our results agree with those of Wu et al. (2014) in Chinese students, where adverse associations of temperature and peak expiratory flow were stronger for participants exposed to highest levels of PM2.5 compared to those exposed to lower levels. Ren et al. (2006) reported that associations of temperature and respiratory admissions were stronger when PM10 levels were higher. In Boston, black carbon is a marker of traffic particles influenced by both local traffic, with a morning peak, and regional sources. In a previous study on the NAS cohort, we reported adverse associations of black carbon exposure with lung function (Lepeule et al., 2014b). Ozone is a strong oxidant that has been associated with decreased lung function in some studies (Chang et al., 2012), although others failed to demonstrate such an effect (Barraza-Villarreal et al., 2008) including one performed on the present cohort (Lepeule et al., 2014a).
As for personal characteristics, we did not observe effect modification by age, asthma, or chronic bronchitis in this study. However, we found a protective association of relative humidity within a 4-hour exposure window preceding lung function measurement, which was stronger in participants suffering from emphysema or COPD. There has been limited research regarding medical conditions that may confer greater susceptibility to weather conditions, especially for humidity. Further studies are needed to explore these differences according to medical conditions.
Investigating the health effects of ambient temperature and relative humidity is challenging and we acknowledge several limitations of our study. We were unable to account for indoor temperature and relative humidity, which introduces measurement error into personal exposure assessments. It has been reported that air conditioning and behavior modify the adverse effects of temperature (Braga et al., 2002). Assuming that elderly have reduced outdoor activities, indoor exposures might be a larger contributor to personal exposure than in other populations. Furthermore, Boston homes are heated for most of the winter season and are air-conditioned during hot summer days. Therefore, it is possible that ambient temperature and relative humidity are poor surrogates of real exposures. However, a recent study conducted in Boston reported a correlation of 0.87 between indoor and ambient air temperature with a piecewise linear relationship with a threshold of 12.7°C (Nguyen et al., 2013). In that same study, indoor and ambient relative humidity had a linear relationship with a correlation of 0.55. These results suggest that in Boston, ambient relative humidity is not a good surrogate of that measured indoors whereas ambient temperature can be used as a reasonable proxy for indoor temperature exposure, especially during days with moderate temperatures (>12.7°C). In our study, human exposures to weather and air pollution are measured using the same monitoring station for all participants, thus measurement error is likely to be mainly of Berkson type. In this case, the power of the study would be reduced and the CIs widened, but no bias in the regression coefficients would be expected (Armstrong 2008; Zeger et al., 2000). Furthermore short-term effects studies tend to focus on temporal variation rather than spatial variation. Assuming that daily variation in temperature and relative humidity is homogeneous across the study area might have introduced random noise in exposure estimates, which would tend to underestimate the association with lung function. Therefore it is unlikely that any exposure measurement error would bias the effect away from the null. Our sensitivity analyses performed during the transition seasons of spring and fall, when the temperature and relative humidity indoor-outdoor relationships are stronger as compared to those for winter and summer, showed results consistent with those of our main analysis. Our results were observed in a cohort of elderly men living in the Greater Boston area, which may limit their generalizability to other populations and locations. Our study also has a number of strengths. We used a well-characterized cohort of community-dwelling men whose lung function was defined using standardized procedures. Data were collected through validated questionnaires. The repeated measures study design provided gain in statistical efficiency. The results were adjusted for a wide range of potential confounders including smoking history, co-morbidities, lung-related drugs, and we performed a number of sensitivity analyses that showed the robustness of our findings.
5. Conclusion
This study adds to the growing body of literature on health effects of short- and long-term weather variations. Our research findings suggest that short-term increases in ambient temperature and relative humidity have substantial adverse relationships with lung function in the elderly, a susceptible population so far rarely investigated. These effects relationships stronger when combined with higher exposures to black carbon.
Global climate models predict not only warmer temperatures on average, but more frequent extreme weather events (McMichael and Lindgren, 2011). This will increased the threat of weather-related morbidity, especially for individual with impaired adaptation to weather variability, such as the elderly. Therefore, identifying subpopulations that are more susceptible to weather variability is of paramount importance for the development of public health policies that protect them.
Highlights.
We studied pulmonary effects of temperature and relative humidity in elderly
Temperature and relative humidity were associated with decreased FEV1 and FVC
The lag structure suggested that acute effects may last several days
Effects of temperature and relative humidity were stronger on FVC than on FEV1
Effects of temperature and relative humidity were amplified by black carbon exposure
Acknowledgments
Source of support
This work was supported by the US Environmental Protection Agency grants R832416 and RD 83479801, by the National Institute of Environmental Health Sciences grants ES015172-01 and ES000002, and by a VA Research Career Scientist award to David Sparrow. AG was supported by the Medical Research Council-UK (Grant IDs: G1002296 and MR/M022625/1) The VA Normative Aging Study is supported by the Cooperative Studies Program/Epidemiology Research and Information Center of the U.S. Department of Veterans Affairs and is a component of the Massachusetts Veterans Epidemiology Research and Information Center, Boston, Massachusetts.
We thank Tania Kotlov, data programmer, from the Harvard School of Public Health, and the NAS subjects for their participation.
Footnotes
Conflict of interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armstrong B. Measurement_error: consequences_and_design_issues. In: Baker D, Nieuwenhuijsen M, editors. Environmental Epidemiology: Study Methods and Application. New York: Oxford University Press; 2008. pp. 93–112. [Google Scholar]
- Barraza-Villarreal A, Sunyer J, Hernandez-Cadena L, Escamilla-Nuñez MC, Sienra-Monge JJ, Ramírez-Aguilar M, Cortez-Lugo M, Holguin F, Diaz-Sánchez D, Olin AC, Romieu I. Air pollution, airway inflammation, and lung function in a cohort study of Mexico City schoolchildren. Environ Health Perspect. 2008;116:832–838. doi: 10.1289/ehp.10926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Samet JM. Relation between elevated ambient temperature and mortality: a review of the epidemiologic evidence. Epidemiol Rev. 2002;24:190–202. doi: 10.1093/epirev/mxf007. [DOI] [PubMed] [Google Scholar]
- Bell B, Rose CL, Damon A. The Veterans Administration longitudinal study of healthy aging. Gerontologist. 1966;6:179–184. doi: 10.1093/geront/6.4.179. [DOI] [PubMed] [Google Scholar]
- Braga ALF, Zanobetti A, Schwartz J. The effect of weather on respiratory and cardiovascular deaths in 12 U.S. cities. Environ Health Perspect. 2002;110:859–863. doi: 10.1289/ehp.02110859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YK, Wu CC, Lee LT, Lin RS, Yu YH, Chen YC. The short-term effects of air pollution on adolescent lung function in Taiwan. Chemosphere. 2012;87:26–30. doi: 10.1016/j.chemosphere.2011.11.048. [DOI] [PubMed] [Google Scholar]
- Chatham M, Bleecker ER, Norman P, Smith PL, Mason P. A screening test for airways reactivity. An abbreviated methacholine inhalation challenge. Chest. 1982;82:15–18. doi: 10.1378/chest.82.1.15. [DOI] [PubMed] [Google Scholar]
- Collaco JM, McGready J, Green DM, Naughton KM, Watson CP, Shields T, Bell SC, Wainwright CE, Cutting GR ACFBAL Study Group. Effect of temperature on cystic fibrosis lung disease and infections: a replicated cohort study. PLoS ONE. 2011;6:e27784. doi: 10.1371/journal.pone.0027784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981;123:659–664. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- Curriero FC, Heiner KS, Samet JM, Zeger SL, Strug L, Patz JA. Temperature and mortality in 11 cities of the eastern United States. Am J Epidemiol. 2002;155:80–87. doi: 10.1093/aje/155.1.80. [DOI] [PubMed] [Google Scholar]
- Ferris BG. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978;118:1–120. [PubMed] [Google Scholar]
- Foster KG, Ellis FP, Doré C, Exton-Smith AN, Weiner JS. Sweat responses in the aged. Age Ageing. 1976;5:91–101. doi: 10.1093/ageing/5.2.91. [DOI] [PubMed] [Google Scholar]
- Gasparrini A, Guo Y, Hashizume M, Lavigne E, Zanobetti A, Schwartz J, Tobias A, Tong S, Rocklöv J, Forsberg B, Leone M, De Sario M, Bell ML, Guo YLL, Wu CF, Kan H, Yi SM, de Sousa Zanotti Stagliorio Coelho M, Saldiva PHN, Honda Y, Kim H, Armstrong B. Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet. 2015;386:369–375. doi: 10.1016/S0140-6736(14)62114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guergova S, Dufour A. Thermal sensitivity in the elderly: a review. Ageing Res Rev. 2011;10:80–92. doi: 10.1016/j.arr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Hernán MA, Lanoy E, Costagliola D, Robins JM. Comparison of dynamic treatment regimes via inverse probability weighting. Basic Clin Pharmacol Toxicol. 2006;98:237–242. doi: 10.1111/j.1742-7843.2006.pto_329.x. [DOI] [PubMed] [Google Scholar]
- Izbicki G, Bar-Yishay E. Methacholine inhalation challenge: a shorter, cheaper and safe approach. Eur Respir J. 2001;17:46–51. doi: 10.1183/09031936.01.17100460. [DOI] [PubMed] [Google Scholar]
- Jayet P-Y, Schindler C, Künzli N, Zellweger J-P, Brändli O, Perruchoud AP, Keller R, Schwartz J, Ackermann-Liebrich U, Leuenberger P SAPALDIA team. Reference values for methacholine reactivity (SAPALDIA study) Respir Res. 2005;6:131. doi: 10.1186/1465-9921-6-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunst AE, Looman CW, Mackenbach JP. Outdoor air temperature and mortality in The Netherlands: a time-series analysis. Am J Epidemiol. 1993;137:331–341. doi: 10.1093/oxfordjournals.aje.a116680. [DOI] [PubMed] [Google Scholar]
- Lepeule J, Bind M-AC, Baccarelli AA, Koutrakis P, Tarantini L, Litonjua A, Sparrow D, Vokonas P, Schwartz JD. Epigenetic influences on associations between air pollutants and lung function in elderly men: the normative aging study. Environ Health Perspect. 2014a;122:566–572. doi: 10.1289/ehp.1206458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepeule J, Litonjua AA, Coull B, Koutrakis P, Sparrow D, Vokonas PS, Schwartz J. Long-term effects of traffic particles on lung function decline in the elderly. Am J Respir Crit Care Med. 2014b;190:542–548. doi: 10.1164/rccm.201402-0350OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Baker PJ, Jalaludin BB, Marks GB, Denison LS, Williams GM. Ambient temperature and lung function in children with asthma in Australia. European Respiratory Journal. 2014;43:1059–1066. doi: 10.1183/09031936.00079313. [DOI] [PubMed] [Google Scholar]
- Mann M, Patel K, Reardon JZ, Goldstein M, Godar TJ, ZuWallack RL. The influence of spring and summer New England meteorologic conditions on the respiratory status of patients with chronic lung disease. Chest. 1993;103:1369–1374. doi: 10.1378/chest.103.5.1369. [DOI] [PubMed] [Google Scholar]
- McGeehin MA, Mirabelli M. The potential impacts of climate variability and change on temperature-related morbidity and mortality in the United States. Environ Health Perspect. 2001;109(Suppl 2):185–189. doi: 10.1289/ehp.109-1240665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael AJ, Lindgren E. Climate change: present and future risks to health, and necessary responses. Journal of Internal Medicine. 2011;270:401–413. doi: 10.1111/j.1365-2796.2011.02415.x. [DOI] [PubMed] [Google Scholar]
- Michelozzi P, Accetta G, De Sario M, D’Ippoliti D, Marino C, Baccini M, Biggeri A, Anderson HR, Katsouyanni K, Ballester F, Bisanti L, Cadum E, Forsberg B, Forastiere F, Goodman PG, Hojs A, Kirchmayer U, Medina S, Paldy A, Schindler C, Sunyer J, Perucci CA PHEWE Collaborative Group. High temperature and hospitalizations for cardiovascular and respiratory causes in 12 European cities. Am J Respir Crit Care Med. 2009;179:383–389. doi: 10.1164/rccm.200802-217OC. [DOI] [PubMed] [Google Scholar]
- Nguyen JL, Schwartz J, Dockery DW. The relationship between indoor and outdoor temperature, apparent temperature, relative humidity, and absolute humidity. Indoor Air. 2013;24:103–112. doi: 10.1111/ina.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C, Williams GM, Tong S. Does particulate matter modify the association between temperature and cardiorespiratory diseases? Environ Health Perspect. 2006;114:1690–1696. doi: 10.1289/ehp.9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow D, O’Connor G, Colton T, Barry CL, Weiss ST. The relationship of nonspecific bronchial responsiveness to the occurrence of respiratory symptoms and decreased levels of pulmonary function. The Normative Aging Study. Am Rev Respir Dis. 1987;135:1255–1260. doi: 10.1164/arrd.1987.135.6.1255. [DOI] [PubMed] [Google Scholar]
- Wu S, Deng F, Hao Y, Wang X, Zheng C, Lv H, Lu X, Wei H, Huang J, Qin Y, Shima M, Guo X. Fine particulate matter, temperature, and lung function in healthy adults: findings from the HVNR study. Chemosphere. 2014;108:168–174. doi: 10.1016/j.chemosphere.2014.01.032. [DOI] [PubMed] [Google Scholar]
- Ye X, Wolff R, Yu W, Vaneckova P, Pan X, Tong S. Ambient temperature and morbidity: a review of epidemiological evidence. Environ Health Perspect. 2012;120:19–28. doi: 10.1289/ehp.1003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Thomas D, Dominici F, Samet JM, Schwartz J, Dockery D, Cohen A. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect. 2000;108:419–426. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]