Abstract
Background
The histamine H3 receptor is regarded as a drug target for cognitive impairments in psychiatric disorders. H3 receptors are expressed in neocortical areas, including the prefrontal cortex, the key region of cognitive functions such as working memory. However, the role of prefrontal H3 receptors in working memory has not yet been clarified. Therefore, using functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) techniques, we aimed to investigate the association between the neural activity of working memory and the density of H3 receptors in the prefrontal cortex.
Findings
Ten healthy volunteers underwent both fMRI and PET scans. The N-back task was used to assess the neural activities related to working memory. H3 receptor density was measured with the selective PET radioligand [11C] TASP457. The neural activity of the right dorsolateral prefrontal cortex during the performance of the N-back task was negatively correlated with the density of H3 receptors in this region.
Conclusions
Higher neural activity of working memory was associated with lower H3 receptor density in the right dorsolateral prefrontal cortex. This finding elucidates the role of H3 receptors in working memory and indicates the potential of H3 receptors as a therapeutic target for the cognitive impairments associated with neuropsychiatric disorders.
Keywords: Histamine H3 receptor, Working memory, PET, fMRI
Findings
Background
Working memory, the ability to retain information for a short period of time [1], is regarded as a core cognitive function that underpins a wide range of complex behaviours such as problem solving, decision-making and reasoning. A substantial number of neuroimaging studies using fMRI have shown that the dorsolateral prefrontal cortex (DLPFC) is the key cortical region involved in working memory [2]. Moreover, several neurotransmitters are known to be involved in this process.
Among the multiple neurotransmitter systems, components of histaminergic neurotransmission, particularly H3 receptors, are known to modulate working memory in animals [3]. The H3 receptor is a presynaptic receptor that regulates the release of histamine (as an autoreceptor) as well as other neurotransmitters such as dopamine, norepinephrine and acetylcholine (as a heteroreceptor), which are involved in cognitive function [3]. More specifically, increased release of histamine via H3 receptor antagonists has been shown to improve working memory in rats under various maze tasks and the delayed match-to-sample test [3]. Thus, extensive preclinical studies have assessed the role of H3 receptors in working memory. However, very few such studies have been conducted in humans, and the published clinical studies of H3 receptor antagonist/inverse agonists have shown the mixed results of cognitive improvements in neuropsychiatric diseases ([4, 5] for review). For example, some studies reported the positive effects of H3 receptor drugs in episodic memory in Alzheimer’s disease, while others found no beneficial effects examined by various drugs in different diseases [5]. These unclear therapeutic effects may come from the complex biology and pharmacology of H3 receptor, such as the heterogeneity of isoforms and the different profile of drug activity (full agonists, partial agonists, neutral antagonists, inverse agonists and protean ligands) [5].
Nevertheless, H3 receptors are highly expressed in the human cerebral cortex and basal ganglia, as revealed by autoradiographic studies of post-mortem brain tissue and by recent PET studies using radioligands targeting H3 receptors [3, 6]. Furthermore, a post-mortem brain sample study revealed that the prefrontal cortex of schizophrenia patients with cognitive impairments showed increased H3 receptor binding [7].
Thus, because the prefrontal cortex is the key region for working memory and H3 receptors are highly expressed in this region, the present study aimed to clarify whether brain activity related to working memory was associated with H3 receptor density in the prefrontal cortex. To accomplish this, we used fMRI as well as PET with the radioligand [11C] TASP457, which has high affinity and selectivity for H3 receptors [6, 8].
Methods
Participants
Ten right-handed (self-reported) healthy male volunteers (mean age ± standard deviation, 25 ± 4 years) participated in the study. All participants had no history of neurological and psychiatric disorders and were not taking any medications. The subjects underwent an fMRI with N-back task followed by a PET scan at rest, with the mean interval of 18.8 ± 20.6 days (mean ± standard deviation).
Radioligand preparation
The [11C] TASP457 precursor and standard used in this study were provided by Taisho Pharmaceutical Co., Ltd. [11C] TASP457 was radiosynthesised by O-alkylation of the 2-pirydone-containing precursor (desmethyl TASP457) with [11C] methyl triflate [4]. At a time of administration, the radiochemical purity of [11C] TASP457 was > 95%, and its specific activity was > 37 GBq/μmol.
PET procedures
After intravenous injection of [11C] TASP457 (390 ± 6 MBq with molar activity of 92 ± 32 GBq/μmol), we acquired three-dimensional dynamic images with a PET camera (Eminence SET-3000GCT/X, Shimadzu, Kyoto, Japan) for 120 min in 39 frames of increasing duration (from 10 s to 5 min). All PET images were reconstructed using the filtered back-projection method (Gaussian filter, kernel 5 mm; the reconstructed in-plane resolution was 7.5 mm in full width at half maximum (FWHM); voxel size 2 × 2 × 2.6 mm) corrected for attenuation, randoms, scatter and head motion. Plasma input functions were measured with arterial blood sampling and metabolite analyses using a radio high-performance liquid chromatography, as described previously [6].
fMRI procedures
We designed a modified version of the N-back task used in a previous study [2]. Participants responded to the numeric characters of previously seen stimuli according to three conditions, 0-, 1- and 2-backs.
A Siemens Verio 3 T MRI system (Siemens, Erlangen, Germany) was used to obtain T2*-weighted echo-planar imaging (repetition time [TR] = 2000 ms, echo time [TE] = 25 ms, slice number = 33 (interleaved), slice thickness = 3.8 mm, matrixes = 64 × 64, 345 volumes) with bold oxygen level-dependent (BOLD) contrasts and structural T1 image (TR = 2300 ms, TE = 1.95 ms, slice number = 176, slice thickness = 1 mm, matrixes = 256 × 256).
Preprocessing analysis with SPM8 software (Wellcome Department of Cognitive Neurology, London, UK) included slice time correction, realignment, DARTEL normalisation and smoothing with a 6-mm FWHM Gaussian kernel. First-level analysis modelled the task as a block design, with working memory load as a linear regressor (0-back = − 1, 1-back = 0, 2-back = 1). Six realignment parameters and two derivatives were used as covariates. Artefacts in fMRI time series data were detected and corrected using robust weighted least squares [9]. A mask image of the prefrontal cortex (including Brodmann areas 8, 9, 10, 11, 12, 13, 14, 24, 25, 32, 44, 45, 46 and 47) was created using WFU PickAtlas 3.0.5 software (Wake Forest University, Winston-Salem, NC). Group-level random effect analysis was performed to identify the activity corresponding to increased working memory load within the prefrontal cortex, using a threshold of P < 0.001 (uncorrected) with a minimum cluster size of 20 voxels [10]. Age was included as a nuisance covariate.
PET and fMRI analyses
The contrast coefficients (ß value) were extracted from the cluster images of increased working memory load. Time–activity curves were generated using data extracted from the PET images by applying the spherical (radius 4 mm) regions of interest images centred on the peak coordinates of each cluster. Total distribution volume (VT), which reflects the H3 receptor density in the brain, was calculated using Ichise’s multilinear analysis (MA1) [11] with the time–activity curves for the initial 60 min and plasma input functions according to the previous quantitative analysis of [11C] TASP457–PET data [6]. The correlation analyses between MRI (ß value) and PET (VT) data were conducted with IBM SPSS Statistics, version 23 (IBM Corp., Armonk, NY). To compensate for the small sample size, we used a resampling procedure based on 5000 bootstrapped samples, using bias-correlated and accelerated (BCa) 95% confidence intervals (CIs) to estimate Pearson’s correlation coefficient for the neural activity and VT.
Results
Behavioural data
There were no significant differences in accuracy across the three N-back tasks (0-back, 98.0 ± 0.7%; 1-back, 98.1 ± 0.8; 2-back, 97.1 ± 1.1; Friedman test P = 0.66, χ2 = 0.84, df = 2). The performances of 1- and 2-backs showed the ceiling effects as they were not significantly different from the maximum accuracy of 100% (Wilcoxon signed-rank tests both Ps > 0.05, both Ws = − 15); thus, the behavioural data were not used for further analyses to avoid spurious estimates.
Imaging data
Three clusters, in which neural activities assessed by fMRI were associated with increased working memory load, were detected in the bilateral DLPFC (the left middle frontal gyrus, left superior frontal gyrus and right middle frontal gyrus; Table 1). Their VT values (mL/cm3), estimated from PET data, were 7.4 ± 0.6 for the left middle frontal gyrus, 7.2 ± 0.9 for the left superior frontal gyrus and 7.0 ± 0.6 for the right middle frontal gyrus. The ß value of the right middle frontal gyrus (Fig. 1a) was negatively correlated with the VT value of this region (Spearman r = − 0.65, P = 0.043, 95% BCa CI = (− 0.91, − 0.05), bias = 0.063, standard error = 0.21, Fig. 1b). No significant correlations were found in the other two clusters.
Table 1.
Neural activity as a function of increased working memory load
| Region | Side | BA | Cluster size | Z value | x | y | z |
|---|---|---|---|---|---|---|---|
| Middle frontal gyrus | Left | 9 | 83 | 3.91 | − 28 | 32 | 34 |
| Superior frontal gyrus | Left | 8 | 45 | 3.53 | − 26 | 8 | 60 |
| Middle frontal gyrus | Right | 8 | 21 | 3.33 | 28 | 10 | 60 |
(x, y, z) corresponds to Montreal Neurological Institute (MNI) coordinates
BA Brodmann area
Fig. 1.
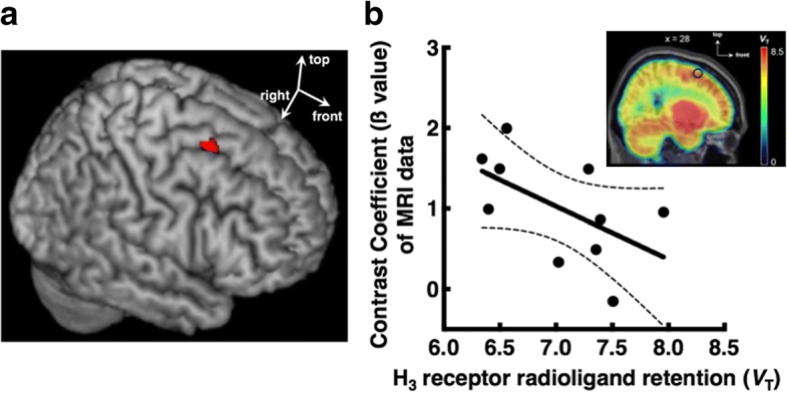
a Group map showing the activity in the right middle frontal gyrus (red, x, y, z = 28, 10, 60) in response to increased working memory load. b The activity in the right middle frontal gyrus was negatively correlated with the H3 receptor radioligand retention (VT), which reflects the H3 receptor density. The dashed lines indicate 95% confidence intervals. The PET image on the graph indicates the distribution of the H3 receptors in the brain. The sagittal image was obtained by averaging parametric images of the 10 subjects. The parametric images were calculated using Ichise’s multilinear analysis to estimate total distribution volume for each voxel and spatially normalised thereafter. The circle indicates the location of the activated region detected in fMRI during performing working memory task in the right middle frontal gyrus shown in a
Discussion
The present study revealed that higher activity in the right DLPFC due to increased working memory load, which was consistent with the findings of previous studies [1, 2], was associated with lower density of H3 receptors. Preclinical studies demonstrated that H3 receptor blockade with H3 antagonists increased the release of neurotransmitters such as acetylcholine and dopamine, resulting in enhanced cognition, whereas H3 agonists impaired cognition [3]. Enhanced cognition has also been reported in H3 receptor knockout rodents [3]. Thus, the individual variability of H3 receptor density may reflect the ability of neurotransmitter release. Taken together with our findings, these results indicate that inhibition of H3 receptors, which increases the release of histamine and other neurotransmitters, plays a role in working memory activation in the right DLPFC.
Although very few studies have investigated the role of H3 receptors in working memory in humans, one fMRI study revealed that an increase in histamine neurotransmission induced by betahistine (H3 antagonist/H1 agonist) moderately increased right DLPFC activity during N-back task performance [12]. This finding also supports the role of the H3 receptor inhibition in DLPFC activation, consistent with our study results.
One of the key limitations of this study is its small sample size, which decreased its statistical power. Moreover, we were unable to establish a causal relationship between H3 receptor density and working memory activity in the DLPFC or to examine the association between H3 receptors and working memory performance. Further studies with a larger sample size and the use of H3 agonists/antagonists are required to establish the causal relationship between H3 receptors and working memory activity and its performance.
Conclusion
In conclusion, the present study showed for the first time that H3 receptor density was associated with working memory activity in the right DLPFC in humans, revealing a histaminergic mechanism underlying working memory. This finding supports the potential of histaminergic modulators, especially those that affect H3 receptors, for treating cognitive impairments in neuropsychiatric disorders.
Acknowledgements
We thank the radiology technologists of the PET Department and members of the Brain Disorder Translational Research Team for their support with PET scans, Kazuko Suzuki and Shizuko Kawakami for their assistance as clinical coordinators, Hiromi Sano for her support with MRI scans, the staff of the Department of Radiopharmaceuticals Development for radioligand synthesis and metabolite analysis, and Atsuo Waki and his team for quality assurance of the radioligand. The [11C] TASP457 precursor and standard used in this study were provided by Taisho Pharmaceutical Co., Ltd.
Funding
This research was partly supported by JSPS KAKENHI grant numbers 26118518, 26119531, 24791251, 26860957, and 17H02173 and by AMED under grant numbers JP18dm0107094 and JP18dm0207007.
Availability of data and materials
Please contact the author for data requests.
Authors’ contributions
All authors contributed substantially to the scientific process leading to this manuscript. Authors TI, YK and MY contributed to the design of the study. Authors TI, YK, CS and KY acquired data. KK and MRZ prepared the PET radioligand. TI, YK, MI and MY analysed the data. TI, YK and MY drafted the manuscript. HT, MH and TS critically contributed to the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Radiation Drug Safety Committee and the Institutional Review Board of National Institute of Radiological Sciences of Japan. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all participants.
Competing interests
YK, MH, and TS are involved in a joint research and clinical trial sponsored by Taisho Pharmaceutical Co., Ltd. MH and TS hold a patent for [11C] TASP457 and related chemicals as H3 ligands (Japan patent JP2014-47209A).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Takehito Ito, Email: ito.takehito.0102@gmail.com.
Yasuyuki Kimura, Email: kimura.yasuyuki@qst.go.jp.
Chie Seki, Email: seki.chie@qst.go.jp.
Masanori Ichise, Email: ichise.masanori@qst.go.jp.
Keita Yokokawa, Email: yokokawa.keita@qst.go.jp.
Kazunori Kawamura, Email: kawamura.kazunori@qst.go.jp.
Hidehiko Takahashi, Email: hidehiko@kuhp.kyoto-u.ac.jp.
Makoto Higuchi, Email: higuchi.makoto@qst.go.jp.
Ming-Rong Zhang, Email: zhang.ming-rong@qst.go.jp.
Tetsuya Suhara, Email: suhara.tetsuya@qst.go.jp.
Makiko Yamada, Phone: +81-43-206-3251, Email: yamada.makiko@qst.go.jp.
References
- 1.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9:20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- 3.Esbenshade TA, Browman KE, Bitner RS, Strakhova M, Cowart MD, Brioni JD. The histamine H3 receptor: an attractive target for the treatment of cognitive disorders. Br J Pharmacol. 2008;154:1166–1181. doi: 10.1038/bjp.2008.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadek B, Saad A, Sadeq A, Jalal F, Stark H. Histamine H3 receptor as a potential target for cognitive symptoms in neuropsychiatric diseases. Behav Brain Res. 2016;312:415–430. doi: 10.1016/j.bbr.2016.06.051. [DOI] [PubMed] [Google Scholar]
- 5.Łażewska D, Kieć-Kononowicz K. Progress in the development of histamine H3 receptor antagonists/inverse agonists: a patent review (2013–2017) Expert Opin Ther Pat. 2018;28:175–196. doi: 10.1080/13543776.2018.1424135. [DOI] [PubMed] [Google Scholar]
- 6.Kimura Y, Seki C, Ikoma Y, Ichise M, Kawamura K, Takahata K, et al. [11C] TASP457, a novel PET ligand for histamine H3 receptors in human brain. Eur J Nucl Med Mol Imaging. 2016;43:1653–1663. doi: 10.1007/s00259-016-3332-6. [DOI] [PubMed] [Google Scholar]
- 7.Jin CY, Anichtchik O, Panula P. Altered histamine H3 receptor radioligand binding in post-mortem brain samples from subjects with psychiatric diseases. Br J Pharmacol. 2009;157:118–129. doi: 10.1111/j.1476-5381.2009.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koga K, Maeda J, Tokunaga M, Hanyu M, Kawamura K, Ohmichi M, et al. Development of TASP0410457 (TASP457), a novel dihydroquinolinone derivative as a PET radioligand for central histamine H 3 receptors. EJNMMI Res. 2016;6:11. doi: 10.1186/s13550-016-0170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diedrichsen J, Shadmehr R. Detecting and adjusting for artifacts in fMRI time series data. NeuroImage. 2005;27:624–634. doi: 10.1016/j.neuroimage.2005.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woo C-W, Krishnan A, Wager TD. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. NeuroImage. 2014;91:412–419. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ichise M, Toyama H, Innis RB, Carson RE. Strategies to improve neuroreceptor parameter estimation by linear regression analysis. J Cereb Blood Flow Metab. 2002;22:1271–1281. doi: 10.1097/01.WCB.0000038000.34930.4E. [DOI] [PubMed] [Google Scholar]
- 12.Van Ruitenbeek P, Mehta MA. Potential enhancing effects of histamine H1 agonism/H3 antagonism on working memory assessed by performance and bold response in healthy volunteers. Br J Pharmacol. 2013;170:144–155. doi: 10.1111/bph.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact the author for data requests.


