Abstract
背景与目的
就全世界范围内而言,肺癌是一种常见疾病。人8-羟基鸟嘌呤糖苷酶(human 8-hydroxyguanine glycosylase, hOGG1)是一种DNA修复酶,它能特异切除8-羟基鸟嘌呤(8-dihydro-8-oxoguanine, 8-OH-G),对损伤的DNA进行修复。hOGG1 Ser326Cys基因多态性与癌症易感性的关系一直是研究的热点,而该多态性与肺癌易感性的关系尚存在争议。本研究采用meta分析旨在更好地探讨hOGG1 Ser326Cys多态性与肺癌易感性之间的关系。
方法
使用MEDLINE数据库检索2010年11月以前的相关文献,按照纳入标准,全面搜索含有研究hOGG1 Ser326Cys多态性与肺癌易感性相关的信息。由至少两位评论员做独立文献筛选和资料提取,并交叉审核。使用STATA 10.1软件进行统计分析。
结果
根据检索条件,共有22篇文献(包括8, 575例肺癌患者和9, 484名正常对照个体)被纳入当前的meta分析。分析结果表明22项研究的结果存在明显异质性,当排除不符合Hard-Weinberg平衡定律的两篇文献后,其余的文献呈现出较好的同质性。与hOGG1 Ser326相比,Cys326基因型明显增加了肺癌发病风险(OR=1.24, 95%CI:1.10-1.39, P < 0.001)。这种正相关在亚洲人群和医院来源的样本中尤为明显(OR=1.28, 95%CI: 1.11-1.49; OR=1.26, 95%CI: 1.09-1.46)。
结论
hOGG1 Ser326Cys多态性与肺癌易感性之间存在明显相关性,Cys326基因型能明显增加肺癌发病风险。
Keywords: hOGG1, 肺肿瘤, 易感性, Meta分析
Abstract
Background and objective
Human 8-hydroxyguanine glycosylase (hOGG1) is one of the DNA repair genes, which can repair damaged DNA by specifically excising 8-dihydro-8-oxoguanine (8-OH-G). A considerable number of studies investigating hOGG1 Ser326Cys polymorphism were in relation to various cancers. However, the association of hOGG1 Ser326Cys polymorphism with risk of lung cancer is inconsistency. The aim of this study is to assess the association of hOGG1 Ser326Cys polymorphism with risk of lung cancer by conducting a meta-analysis.
Methods
To identify all studies that are qualified for meta-analysis, we conducted a computerized literature search of MEDLINE database (before November, 2010). Two investigators independently extracted the data and reached a consensus on all items.
Results
According to our search limits, twenty-two case-control studies, including 8, 575 lung cancer cases and 9, 484 controls, were selected for meta-analysis. Significant heterogeneity was found among those twenty-two case-control studies, when excluding two studies that were not accorded with Hard-Weinberg Equilibrium, the remains performed well homogeneity. Compared with Ser326 genotype, Cys326 genotype can significantly increase risk of lung cancer (OR=1.24, 95%CI: 1.10-1.39, P < 0.001), especially in Asian ethnic population and hospital population (OR=1.28, 95%CI: 1.11-1.49; OR=1.26, 95%CI: 1.09-1.46).
Conclusion
The present study indicates that the hOGG1 Ser326Cys polymorphism is associated with risk of lung cancer, Cys326 genotype can significantly increase risk of lung cancer.
Keywords: hOGG1, Lung neoplasms, Susceptibility, Meta-analysis
癌症的发生是一个复杂的逐步积累的过程,在这个过程中正常细胞遗传信息发生改变从而导致表型改变,进一步使其具有入侵和转移到其它部位的能力,这是导致癌症病人死亡的主要原因之一[1]。另外,癌症的发生与环境因素也有着直接的联系,吸烟便是一类众所周知的致癌因素。严重的致癌环境能导致多种DNA损伤,如DNA的氧化损伤[2]。人8-羟基鸟嘌呤糖苷酶(human 8-hydroxyguanine glycosylase, hOGG1)是一种DNA修复酶,它能特异切除8-羟基鸟嘌呤(8-dihydro-8-oxoguanine, 8-OH-G),对损伤的DNA进行修复[3]。从遗传的角度来说,倘若DNA修复基因的遗传变异能影响其对DNA的修复能力,这便意味着癌症发病风险的增加。
大量的分子流行病学研究对hOGG1基因多态性与各种癌症易感性进行了分析,这些研究大都集中在hOGG1基因的功能多态性位点与肺癌、肝癌和乳腺癌的相关性方面,尤以Ser326Cys最为常见[4]。该位点存在于hOGG1基因第7外显子的第1245位碱基处(1245C/G),由于C/G多态使第326位密码子可编码丝氨酸(ser,密码子TCC)或编码半胱氨酸(cys,密码子TGC),形成hOGG1-Ser326和hOGG1-Cys326两种蛋白,后者导致糖苷酶活性降低,致使hOGG1修复8-OH-G的能力低下,造成8-OH-G在细胞内的残留,从而增加了基因突变和癌症的风险。然而关于Ser326Cys多态性与肺癌易感性关系的研究结果并不一致[3, 4],为了更好地探讨hOGG1多态性与肺癌易感性之间的关系,我们收集、整理既往研究数据,并进行meta分析。
1. 材料与分析
1.1. 数据收集
将“human 8-hydroxyguanine glycosylase”、“hOGG1”、“polymorphisms”、“genetic variation”、“lung cancer”、“lung carcinoma”等作为关键词,使用MEDLINE数据库检索2010年11月以前的相关文献。在检索到的文章全文中仔细搜索是否含有hOGG1 Ser326Cys多态性与肺癌易感性相关的信息。
1.2. 纳入标准
包括:①运用相互独立的病例-对照试验设计;②基因多态性对应的基因型频率可以从文献中获取。
两位研究者(钱乾和刘仍允)通过独立阅读所获得文献题目和摘要,在排除明显不符合纳入标准的文献后,对可能符合纳入的文献阅读全文,以确定是否真正符合纳入标准,并交叉核对纳入文献的结果,且对有分歧的文献通过讨论或由第3个研究者(雷哲)决定其是否纳入。
1.3. 数据整理及统计分析
对于符合纳入标准的每一篇文献均按照作者、发表年份、试验人群的来源和人种,以及患者和对照组中各基因型携带者的数量进行整理(表 1)。采用Person拟合度卡方检验分析对照组基因型分布是否符合Hard-Weinberg平衡(HWE)。使用以Chi2检验为基础的Q检验衡量样本的同质性[5],并以I2的大小反应多组研究之间同质性的程度(I2值越小,同质性越强)[6]。当样本间不具有明显异质性的时候,采用基于Mantel-Haenszel方法的固定效应模型分析[7];反之,采用DerSimonian and Laird方法的随机效应模型分析。漏斗图(funnel plot)用来检测数据之间是否存在发表偏倚,其程度采用Egger检验进行计算。基因多态性与肺癌易感性的关系的强弱程度用OR值表示[8],而OR值是否明显采用Z检验。上述统计分析均采用STATA软件(Stata/SE version 10.1 for Windows; Stata Corp, College Station, Texas),P < 0.05为有统计学差异。
1.
纳入meta分析的22项独立研究的基本特征
Characteristics of twenty-two studies included in the meta -analysis
| Author | Year | Source of controls | Ethnicity | Cases | Controls | P for HWE | |||||
| CC | CG | GG | CC | CG | GG | ||||||
| *The studies significantly derived from HWE (P < 0.05). | |||||||||||
| Khono[12] | 1998 | Population | Asian | 16 | 19 | 10 | 15 | 20 | 7 | 0.939 | |
| Sugimura[13] | 1999 | Hospital | Mixed | 85 | 115 | 41 | 63 | 107 | 27 | 0.082 | |
| Wikman[14] | 2000 | Hospital | Caucasian | 68 | 32 | 5 | 60 | 43 | 2 | 0.067 | |
| Ito[15] | 2002 | Hospital | Asian | 40 | 71 | 27 | 68 | 118 | 54 | 0.837 | |
| Le[16] | 2002 | Population | Mixed | 123 | 110 | 65 | 177 | 175 | 53 | 0.350 | |
| Sunaga[17] | 2002 | Hospital | Asian | 54 | 106 | 38 | 50 | 66 | 36 | 0.126 | |
| Lan[18] | 2004 | Population | Asian | 37 | 61 | 20 | 51 | 43 | 15 | 0.232 | |
| Park[3] | 2004 | Hospital | Caucasian | 101 | 65 | 13 | 255 | 87 | 8 | 0.857 | |
| Vogel[4] | 2004 | Population | Caucasian | 149 | 93 | 14 | 159 | 91 | 19 | 0.237 | |
| Hung[19] | 2005 | Hospital | Caucasian | 1, 401 | 661 | 93 | 1, 368 | 716 | 79 | 0.215 | |
| Liang[20] | 2005 | Hospital | Asian | 154 | 69 | 4 | 158 | 66 | 3 | 0.178 | |
| Khono[21] | 2006 | Hospital | Asian | 285 | 544 | 268 | 123 | 190 | 81 | 0.628 | |
| Loft[22] | 2006 | Population | Caucasian | 144 | 93 | 14 | 154 | 88 | 19 | 0.200 | |
| Sorensen[23] | 2006 | Population | Caucasian | 254 | 155 | 22 | 479 | 284 | 33 | 0.258 | |
| Zienolddiny[10] | 2006 | Population | Caucasian | 182 | 100 | 44 | 194 | 117 | 75 | < 0.001* | |
| De Ruyck[24] | 2007 | Hospital | Caucasian | 74 | 33 | 3 | 60 | 46 | 4 | 0.176 | |
| Hatt[25] | 2007 | Population | Caucasian | 92 | 58 | 8 | 93 | 59 | 12 | 0.536 | |
| Karahalil[26] | 2008 | Population | Caucasian | 86 | 65 | 14 | 115 | 106 | 29 | 0.546 | |
| Chang[27] | 2009 | Hospital | Asian | 142 | 518 | 436 | 154 | 482 | 361 | 0.741 | |
| Miyaishi[28] | 2009 | Hospital | Asian | 27 | 55 | 26 | 39 | 54 | 28 | 0.271 | |
| Okasaka[29] | 2009 | Population | Asian | 117 | 257 | 141 | 250 | 544 | 236 | 0.070 | |
| Liu[9] | 2010 | Hospital | Asian | 68 | 158 | 132 | 110 | 294 | 312 | 0.004* | |
2. 结果
根据检索条件,共有22篇文献[3, 4, 9, 10, 12-29]符合纳入标准(表 1)。共有8, 575例肺癌患者和9, 484名正常对照个体被纳入meta分析,其中10项研究(包括3, 900例患者和4, 028名对照个体)来源于亚洲人群,10项研究(包括4, 136例患者和4, 854名对照者)来源于高加索人群,另外2项研究(包括539例患者和602名对照者)的样本均源于两种以上的人群。
2.1. hOGG1 Ser326Cys多态与肺癌易感性
将收集到的22篇文献[3, 4, 9, 10, 12-29]全部纳入meta分析,发现hOGG1 Ser326Cys多态性与肺癌易感性之间并无明显相关性,然而这些文献之间存在很强的异质性(表 2)。按照人种或者对照来源进行分层分析,结果表明22篇文献间的异质性主要由高加索人或医院来源的研究所引起。与hOGG1 Ser326相比,Cys326基因型可以明显增加亚洲人群肺癌的发病风险(OR=1.17, 95%CI: 1.02-1.35, P=0.023),但是这种明显相关性在高加索人群中并不存在。
2.
Ser326Cys基因多态性位点的基因型及其在肺癌中的OR值
Summary of genotyping for Ser326Cys polymorphism and their ORs in lung cancer
| Variables | n | Cases/Controls | CG versus CC | GG versus CC | CG/GG versus CC | ||||||||
| OR (95% CI) | PH* | I2(%) | OR (95% CI) | PH | I2(%) | OR (95% CI) | PH | I2(%) | |||||
| *The presence of heterogeneity (PH < 0.05). | |||||||||||||
| Total | 22 | 8, 575/9, 484 | 1.04 (0.94-1.14) | 0.028 | 40.0 | 1.11 (0.94-1.31) | 0.007 | 47.8 | 1.05 (0.94-1.16) | 0.003 | 51.3 | ||
| Ethnicities | |||||||||||||
| Asian | 10 | 3, 900/4, 028 | 1.14 (1.01-1.28) | 0.432 | 0.7 | 1.17 (1.02-1.35) | 0.135 | 34.1 | 1.15 (1.03-1.29) | 0.231 | 23.1 | ||
| Caucasian | 10 | 4, 136/4, 854 | 0.98 (0.84-1.15) | 0.025 | 52.7 | 0.97 (0.71-1.34) | 0.021 | 54.0 | 0.97 (0.82-1.14) | 0.004 | 62.6 | ||
| Mixed | 2 | 539/602 | 0.86 (0.66-1.12) | 0.641 | 0 | 1.51 (1.07-2.13) | 0.224 | 32.3 | 1.01 (0.79-1.28) | 0.334 | 0 | ||
| Source of controls | |||||||||||||
| Pop. | 10 | 2, 563/3, 712 | 1.02 (0.91-1.14) | 0.593 | 0 | 1.04 (0.79-1.36) | 0.023 | 53.2 | 1.02 (0.92-1.14) | 0.315 | 13.9 | ||
| Hosp. | 12 | 6, 012/5, 772 | 1.05 (0.89-1.24) | 0.004 | 60.1 | 1.16 (0.94-1.44) | 0.038 | 46.6 | 1.06 (0.90-1.26) | 0.001 | 66.3 | ||
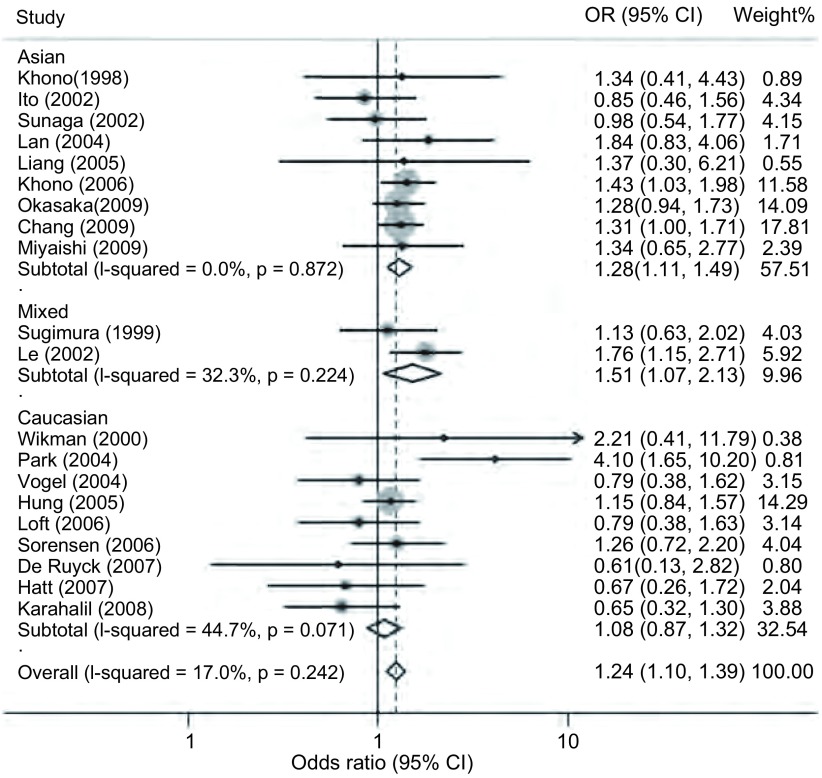
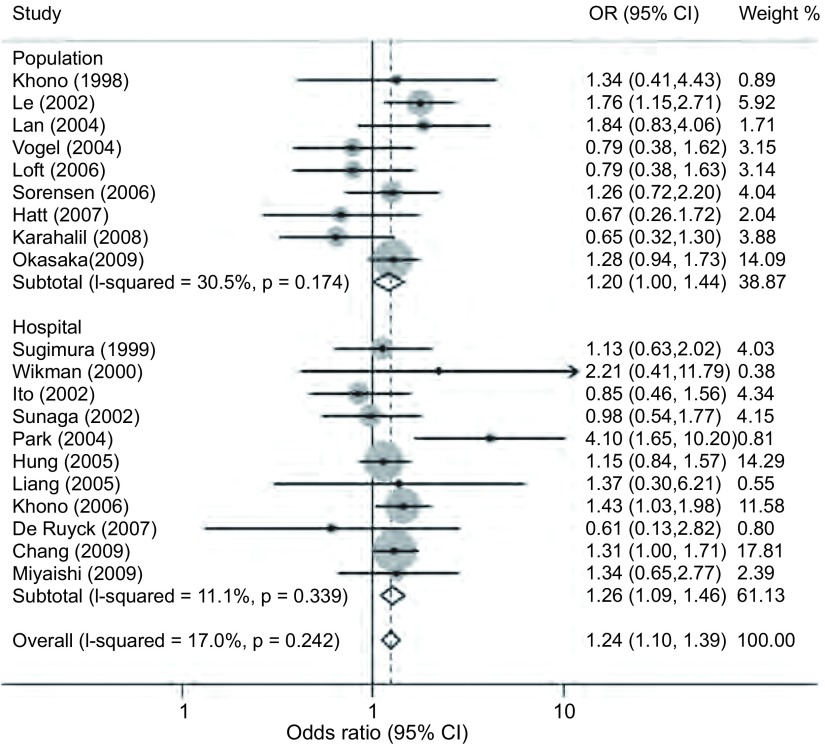
对纳入的22篇文献[3, 4, 9, 10, 12-29]的对照组基因型数据进行HWE检验,发现2篇文献[9, 10]的数据不符合HWE。其中一篇源于亚洲人群[9],另一篇源于高加索人群[10]。将这2篇文献排除后发现其它20篇文献之间显示出较好的同质性(P=0.242, I2=17.0%),且与hOGG1 Ser326相比Cys326基因型明显增加了24%的肺癌发病风险(OR=1.24, 95%CI: 1.10-1.39, P < 0.001)(图 1)。按照人种进行亚组分析后,在亚洲和混合型人群中仍然可见这种与肺癌易感性的正相关现象(P=0.001, P=0.020)(图 1)。按照样本来源进行亚组分析的结果表明,在人群来源的研究和医院来源的研究中hOGG1 Cys326基因型较Ser326基因型均明显增加了肺癌易感性(P=0.05, P=0.002)(图 2)。
1.
根据人种来源分组的Ser326Cys多态性位点在肺癌中的meta分析
Meta -analysis for Ser326Cys polymorphism in lung cancer by ethnic subgroup
2.
根据样本来源分组的Ser326Cys多态性位点在肺癌中的meta分析
Meta -analysis for Ser326Cys polymorphism in lung cancer by cohort subgroup
2.2. 发表偏倚
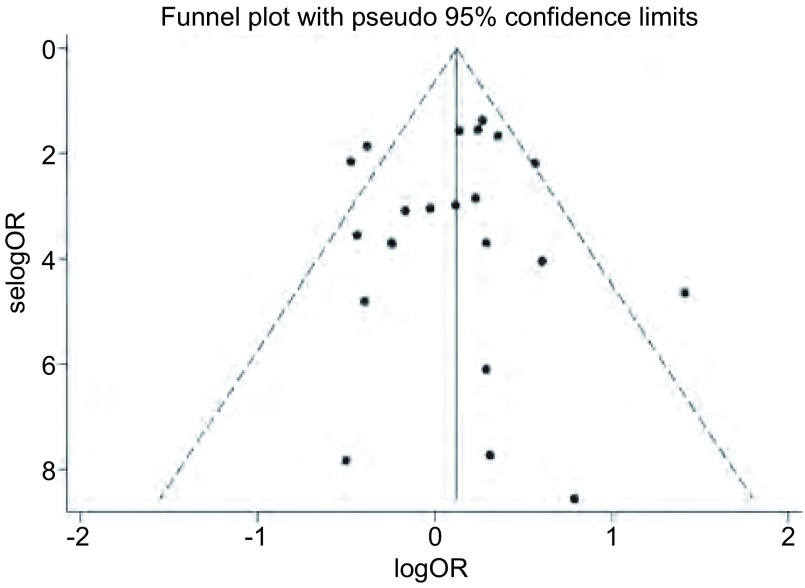
使用OR值的自然对数及其标准误创建漏斗图检测发表偏倚,漏斗图表现出良好的对称性,说明本meta分析并不存在发表偏倚(图 3)。此外,Egger检验结果证实了漏斗图的结果(t=-0.23, P=0.823)。亚组分析后发现在亚洲人群和高加索人群中均不存在发表偏倚(t=-0.06, P=0.955; t=0.20, P=0.847),人群来源的研究和医院来源的研究也不存在明显的发表偏倚(t=-0.70, P=0.506; t=0.34, P=0.739)。
3.
关于hOGG1 Ser326Cys多态性与肺癌发病风险研究的漏斗分析图
Funnel plot analysis on the association of hOGG1 Ser326Cys polymorphism with risk of lung cancer
3. 讨论
肺癌是世界上最为常见的一种癌症,尽管人们认为吸烟是导致肺癌的一个主要风险因素,但是在终生吸烟的人群中,最终患肺癌的人不超过20%[11],这说明遗传易感性对于癌症的发生起到重要的作用。
关于hOGG1基因,近年来分子流行病和遗传学研究主要集中在其第7外显子的Ser326Cys多态与癌症易感性的关系。然而关于Ser326Cys多态性与肺癌易感性是否相关尚存在争议,为了更好地探讨hOGG1多态性与肺癌易感性之间的关系,我们进行了meta分析。对纳入研究的22篇文献[3, 4, 9, 10, 12-29]进行综合分析,发现这些文献之间存在很强的异质性。对这些文献的对照组基因型数据进行HWE检验,有发现2篇文献[9, 10]的数据不符合HWE,将这两篇文献排除后,其余的20项研究[3, 4, 12-29]的结果则呈现出较好的同质性。这些结果提示我们,分析质量较高的研究结果有利于提高meta分析时数据的一致性。更为重要的是,我们发现在亚洲和混合型人群中,Ser326Cys多态与肺癌易感性存在明显正相关,Cys326基因型相比Ser326基因型能明显增加肺癌发病风险,这说明Ser326Cys多态与肺癌易感性之间的关系可能与种族差异有关。在亚洲或混合型人群中,Ser326Cys位点的改变更易导致肺癌发病风险的增加。同样,我们按照对照来源进行亚组分析,发现在人群来源和医院来源的研究中Cys326基因型相比Ser326基因型均明显增加了肺癌发病风险。
综上所述,为了更好地研究hOGG1 Ser326Cys多态与肺癌易感性之间的关系,需要对大样本量进行人种、肺癌病理分型、地域环境、吸烟史等更细致的划分,这样才能准确地评估遗传因素与环境因素分别对肺癌发病风险的贡献。
Funding Statement
本研究受国家自然基金(No.30973425)、教育部“新世纪优秀人才支持计划”(No.NCET-09-0165)、江苏省自然科学基金(No.BK2008162)、江苏省教育厅“青蓝工程”、江苏省人事厅“333工程”和天津市科技支撑计划中瑞合作重大项目(No.09ZCZDSF04100)资助
This work was partly supported by grants from National Natural Science Foundation of China (to Hong-Tao ZHANG) (No.30973425), Program for New Century Excellent Talents in University (to Hong-Tao ZHANG)(No.NCET-09-0165), Science and Technology Committee of Jiangsu Province (to Hong-Tao ZHANG)(No.BK2008162), Qing-Lan Project of Education Bureau of Jiangsu Province (to Hong-Tao ZHANG) and China-Sweden Cooperative Foundation (to Qinghua ZHOU) (No.09ZCZDSF04100)
Contributor Information
周 清华 (Qinghua ZHOU), Email: zhouqh1016@yahoo.com.cn.
张 洪涛 (Hong-Tao ZHANG), Email: htzhang@suda.edu.cn.
References
- 1.Fider IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 2.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411(6835):366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 3.Park J, Chen L, Tockman MS, et al. The human 8-oxoguanine DNA N-glycosylase 1 (hOGG1) DNA repair enzyme and its association with lung cancer risk. Pharmacogenetics. 2004;14(2):103–109. doi: 10.1097/00008571-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Vogel U, Nexo B, Wallin H, et al. No association between base excision repair gene polymorphisms and risk of lung cancer. https://link.springer.com/content/pdf/10.1023%2FB%3ABIGI.0000043957.03420.7e.pdf. Biochem Genet. 2004;42(11-12):453–460. doi: 10.1023/b:bigi.0000043957.03420.7e. [DOI] [PubMed] [Google Scholar]
- 5.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 6.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petitti D. Statistical methods in meta-analysis. In: Petitti DB ed. Metaanalysis, decision analysis, and cost-effectiveness analysis. New York: Oxford University Press, 1994. 90-114.
- 8.Woolf B. On estimating the relationship between blood group and disease. Ann Human Genet. 1955;19(4):251–253. doi: 10.1111/ahg.1955.19.issue-4. [DOI] [PubMed] [Google Scholar]
- 9.Liu CJ, Hsia TC, Tsai RY, et al. The joint effect of hOGG1 single nucleotide polymorphism and smoking habit on lung cancer in Taiwan. Anticancer Res. 2010;30(10):4141–4146. [PubMed] [Google Scholar]
- 10.Zienolddiny S, Campa D, Lind H, et al. Polymorphisms of DNA repair genes and risk of non-small cell lung cancer. Carcinogenesis. 2006;27(3):560–567. doi: 10.1093/carcin/bgi232. [DOI] [PubMed] [Google Scholar]
- 11.Shields PG. Molecular epidemiology of smoking and lung cancer. Oncogene. 2002;21(45):6870–6876. doi: 10.1038/sj.onc.1205832. [DOI] [PubMed] [Google Scholar]
- 12.Kohno T, Shinmura K, Tosaka M, et al. Genetic polymorphisms and alternative splicing of the hOGG1 gene, that is involved in the repair of 8-hydroxyguanine in damaged DNA. Oncogene. 1998;16(25):3219–3225. doi: 10.1038/sj.onc.1201872. [DOI] [PubMed] [Google Scholar]
- 13.Sugimura H, Kohno T, Wakai K, et al. hOGG1 Ser326Cys polymorphism and lung cancer susceptibility. Cancer Epidem Bioma. 1999;8(8):669–674. [PubMed] [Google Scholar]
- 14.Wikman H, Risch A, Klimek F, et al. hOGG1 polymorphism and loss of heterozygosity (LOH): significance for lung cancer susceptibility in a caucasian population. Int J Cancer. 2000;88(6):932–937. doi: 10.1002/(ISSN)1097-0215. [DOI] [PubMed] [Google Scholar]
- 15.Ito H, Hamajima N, Takezaki T, et al. A limited association of OGG1 Ser326Cys polymorphism for adenocarcinoma of the lung. J Epidemiol. 2002;12(3):258–265. doi: 10.2188/jea.12.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchand LL, Donlon T, Annette LJ, et al. Association of the hOGG1 Ser326Cys polymorphism with lung cancer risk. Cancer Epidem Bioma. 2002;11(4):409–412. [PubMed] [Google Scholar]
- 17.Sunaga N, Kohno T, Yanagitani N, et al. Contribution of the NQO1 and GSTT1 polymorphisms to lung adenocarcinoma susceptibility. Cancer Epidem Bioma. 2002;11(8):730–738. [PubMed] [Google Scholar]
- 18.Qing L, Mumford JL, Shen M, et al. Oxidative damage-related genes AKR1C3 and OGG1 modulate risks for lung cancer due to exposure to PAH-rich coal combustion emissions. Carcinogenesis. 2004;25(11):2177–2181. doi: 10.1093/carcin/bgh240. [DOI] [PubMed] [Google Scholar]
- 19.Hung J, Brennan P, Canzian F, et al. Large-scale investigation of base excision repair genetic polymorphisms and lung cancer risk in a multicenter study. J Natl Cancer. 2005;97(8):567–576. doi: 10.1093/jnci/dji101. [DOI] [PubMed] [Google Scholar]
- 20.Liang GY, Pu YP, Yin LH. Rapid detection of single nucleotide polymorphisms related with lung cancer susceptibility of chinese population. Cancer Lett. 2005;223(2):265–274. doi: 10.1016/j.canlet.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 21.Kohno T, Kunitoh H, Toyama K, et al. Association of the OGG1-326Cys polymorphism with lung adenocarcinoma risk. Cancer Sci. 2006;97(8):724–728. doi: 10.1111/cas.2006.97.issue-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loft S, Svoboda P, Kasai H, et al. Prospective study of 8-oxo-7, 8-dihydro- 2'-deoxyguanosine excretion and the risk of lung cancer. Carcinogenesis. 2006;27(6):1245–1250. doi: 10.1093/carcin/bgi313. [DOI] [PubMed] [Google Scholar]
- 23.Sorensen M, Ole RN, Hansen RD, et al. Interactions between the OGG1 Ser326Cys polymorphism and intake of fruit and vegetables in relation to lung cancer. Free Radic Res. 2006;40(8):885–891. doi: 10.1080/10715760600733129. [DOI] [PubMed] [Google Scholar]
- 24.De Ruyck K, Szaumkessel M, De Rudder I, et al. Polymorphisms in baseexcision repair and nucleotide-excision repair genes in relation to lung cancer risk. Mutat Res. 2007;631(2):101–110. doi: 10.1016/j.mrgentox.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Hatt L, Loft S, Risom L, et al. OGG1 expression and OGG1 Ser326Cys polymorphism and risk of lung cancer in a prospective study. https://www.sciencedirect.com/science/article/pii/S002751070700396X. Mutat Res. 2007;639(1-2):45–54. doi: 10.1016/j.mrfmmm.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Karahalil B, Emerce E, Kocer B, et al. The association of OGG1 Ser326Cys polymorphism and urinary 8-OHdG levels with lung cancer susceptibility: a hospital-based case-control study in turkey. https://hrcak.srce.hr/file/46460. Arh Hig Rada Toksikol. 2008;59(4):241–250. doi: 10.2478/10004-1254-59-2008-1924. [DOI] [PubMed] [Google Scholar]
- 27.Chang CH, Hsiao CF, Chang GC, et al. Interactive effect of cigarette smoking with human 8-Oxoguanine DNA N-Glycosylase 1 (hOGG1) polymorphisms on the risk of lung cancer: a case-control study in Taiwan. Am J Epidemiol. 2009;170(6):695–702. doi: 10.1093/aje/kwp019. [DOI] [PubMed] [Google Scholar]
- 28.Miyaishi A, Osawa K, Osawa Y, et al. MUTYH Gln324His gene polymorphism and genetic susceptibility for lung cancer in a Japanese population. J Exp Clin Cancer Res. 2009;28:10. doi: 10.1186/1756-9966-28-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okasaka T, Matsuo K, Suzuki T, et al. hOGG1 Ser326Cys polymorphism and risk of lung cancer by histological type. J Hum Genet. 2009;54(12):739–745. doi: 10.1038/jhg.2009.108. [DOI] [PubMed] [Google Scholar]