Abstract
背景与目的
已有研究表明Kelch样环氧氯丙烷相关蛋白1(Kelch-like ECH-associated protein1, Keap1)与铂类耐药相关。本研究旨在探讨Keap1在进展期非小细胞肺癌(non-small cell lung cancer, NSCLC)中的表达及其与一线含铂化疗方案疗效的相关性。
方法
应用免疫组化检测50例进展期NSCLC患者组织标本中Keap1的表达。
结果
进展期NSCLC患者中Keap1高表达率为26.0%;Keap1表达水平与化疗疗效、无进展生存期(progression free survival, PFS)有关(P < 0.05),而与性别、年龄、吸烟、病理类型、分化程度、转移和总生存期无关(P > 0.05)。Keap1高表达组的中位PFS明显高于低表达组(P=0.002)。多因素分析表明Keap1表达水平是一线化疗方案PFS的独立预测因素(P=0.007)。
结论
Keap1与进展期NSCLC一线化疗疗效和PFS有关,Keap1可能成为新的化疗疗效预测指标。
Keywords: 肺肿瘤, Keap1, 化疗, 无进展生存期
Abstract
Background and objective
It has been proven that Kelch-like ECH-associated protein 1 (Keap1) expression was correlated with the chemoresistance of platinum drugs. The aim of this study is to investigate the protein expression levels of Keap1 in non-small cell lung cancer (NSCLC) patients as well as to correlate its expression with the response rate (RR), progression-free survival (PFS), and overall survival (OS) of patients treated with platinum-based first-line chemotherapy.
Methods
The immunohistochemical analysis of Keap1 expression was performed using tumor samples from 50 patients with stage Ⅲ or Ⅳ NSCLC.
Results
The high expression ratio of Keap1 was 26.0%. The Keap1 expression was significantly correlated with the RR and PFS after platinum-based chemotherapy (P < 0.05) but not with the gender, age, smoking, pathology type, differentiation, metastasis, and OS (P > 0.05). The PFS was significantly longer in patients with high Keap1 expression than in those with low/negative expression (P=0.002). Furthermore, the level of Keap1 expression was an independent predictive factor of PFS after platinum-based first-line chemotherapy (P=0.007).
Conclusion
The expression of Keap1 was significantly correlated with the RR and PFS of platinum-based first-line chemotherapy. Therefore, Keap1 may be a useful biomarker for predicting the chemosensitivity of patients with advanced-stage NSCLC.
Keywords: Lung neoplasms, Kelch-like ECH-associated protein 1, Chemotherapy, Progression free survival
目前肺癌死亡率已位居男、女性恶性肿瘤的第一位[1],全球每年至少有160万的新发病例和130万的死亡病例。非小细胞肺癌(non-small cell lung cancer, NSCLC)占肺癌的80%左右[2],发现时多处于进展期,化疗仍是进展期NSCLC治疗的主要手段。含铂两药方案是一线化疗首选方案,耐药是化疗失败的主要原因。目前关于肿瘤耐药机制尚不完全清楚,因此探索与肿瘤耐药相关的生物指标,对提高化疗疗效和患者生活质量显得尤为重要。
Kelch样环氧氯丙烷相关蛋白-1(Kelch-like ECH-associated protein1, Keap1)是细胞应对氧化应激和亲电性应激损伤的重要防御基因之一[3]。基础研究[3, 4]表明Keap1的表达水平与NSCLC细胞对卡铂、顺铂、依托泊苷等药物的敏感性相关。因此,Keap1表达水平或许与NSCLC患者含铂化疗方案疗效相关。为此,本研究通过免疫组化方法检测了50例进展期NSCLC患者组织标本中Keap1的表达水平,并分析了Keap1表达水平与患者临床特征和一线含铂化疗方案疗效之间的关系。
1. 对象与方法
1.1. 研究对象
选取2008年1月-2011年12月在北京大学第三医院接受化疗的共50例NSCLC患者。入组前需病理确诊为NSCLC;不能手术切除的Ⅲ期和Ⅳ期患者(依据国际肺癌研究协会颁布的第7版分期标准[5]);有足够的病理组织标本供免疫组化检测;一线方案是含铂两药方案;治疗前、治疗2个周期和4个周期后均有影像学检查(胸腹部CT、头颅MRI)。共纳入符合条件的病例50例,包括男性29例,女性21例;年龄范围48岁-77岁,中位年龄64岁;鳞癌患者23例,腺癌患者27例;Ⅲ期患者20例,Ⅳ期患者30例;接受紫杉类药物联合铂类方案化疗患者10例,接受吉西他滨联合铂类方案化疗患者28例,接受长春瑞宾联合铂类化疗患者5例,接受培美曲塞联合铂类化疗患者4例,其它药物联合铂类化疗患者3例。
1.2. 随访及后续治疗
所有患者每3个月通过定期来院或电话随访,随访开始时间为2008年1月,末次随访时间为2012年3月1日,最短随访时间为3个月,最长为50个月。50例患者中仅接受1种治疗方案的患者14例,接受≥2种治疗方案的患者36例;后续治疗中接受放疗患者5例,根治性手术切除2例,接受表皮生长因子受体酪氨酸激酶抑制剂(epidermal growth factor receptor tyrosine kinase inhibitor, EGFR-TKI)靶向治疗患者13例。
1.3. 临床资料收集及疗效评价标准
记录患者临床特征、化疗方案和影像学指标。疗效指标包括近期和远期疗效。近期疗效按照实体瘤疗效评价标准1.1版[6]分为完全缓解(complete response, CR)、部分缓解(partial response, PR)、疾病稳定(stable disease, SD)和疾病进展(progressive disease, PD),获得CR或PR的患者4周或以后确认。远期疗效为无疾病进展生存期(progression free survival, PFS)和总生存期(overall survival, OS)。PFS定义为从治疗开始至疾病进展或任何原因导致死亡的时间,OS定义为从初次治疗开始至死亡或随访终点时间。
1.4. 免疫组化检测Keap1表达
1.4.1. 实验方法
活检组织标本经10%甲醛固定后,常规石蜡包埋,切片,4 μm厚度。采用免疫组化SP法检测(兔抗人Keap1抗体购于proteintech®公司,免疫组化二抗SP检测试剂盒购于北京中杉金桥生物技术有限公司,Keap1抗体按1:100稀释),应用PBS代替一抗作为阴性对照,按照试剂说明书进行操作。
1.4.2. 结果判定
采用双盲法,由两位独立的病理医师进行阅片,Keap1抗原阳性反应位于细胞浆内。随机选取5个高倍镜视野(200倍),每个视野记数200个肿瘤细胞。共计数1, 000个细胞。阳性结果参照Solis等研究[7]的评判标准,按染色强度分为:0分,无染色;1分,染色呈淡黄色;2分,染色呈棕黄色;3分,染色呈棕褐色;按阳性细胞比例分为0%-100%;按照染色强度和阳性细胞比例乘积判定免疫组织化学表达水平。Keap1低或不高表达是指二者乘积 < 150%,反之为高表达。
1.5. 统计学方法
应用SPSS 17.0统计学软件分析。率的比较采用卡方检验或Fisher精确检验,相关性检验采用Pearson检验,多因素分析采用Logistic回归模型(逐步后退法)。Kaplan-Meier方法进行生存分析,Log-rank检验差异性,多因素分析采用Cox多因素分析模型。P < 0.05为差异有统计学意义。
2. 结果
2.1. 随访及疗效
随访率为100%。中位随访时间16个月,随访范围3个月-50个月。近期疗效中无患者为CR,17例(34.0%)患者为PR,23例(46.0%)患者为SD,10例(20.0%)患者为PD。因3例获得PR患者中2例接受了根治性手术切除,1例接受了根治性放射治疗,后期检查方式和间隔差别较大,故在评价远期疗效时将其剔除。远期疗效:PFS为1个月-12个月,中位PFS为5个月;OS为3个月-50个月,中位OS为16个月。
2.2. Keap1蛋白检测结果及与临床特征间的关系
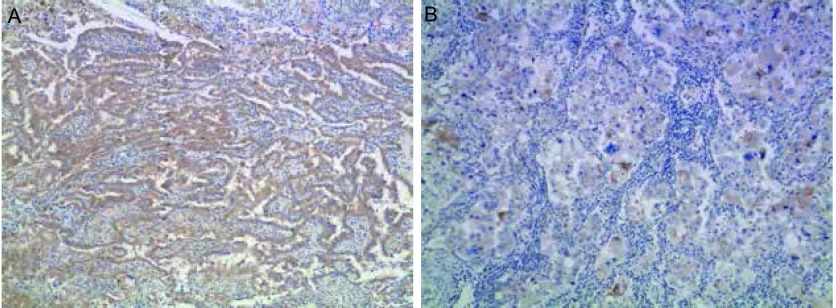
Keap1主要表达在细胞浆中(图 1)。Keap1高表达率为26.0%(13/50)。Keap1在PR患者中的高表达率为47.1%(8/17),明显高于PD患者的10.0%(1/10)(P=0.047)。但Keap1表达水平在性别、年龄、病理类型、分化程度、远处转移、吸烟和化疗方案组间无差异(P>0.05),见表 1。
1.
免疫组化检测Keap1在肺癌中的表达情况(×100)。A:Keap1高表达;B:Keap1低表达
Expression of Keap1 in lung cancer (×100). A: Keap1 high expression; B: Keap1 low expression
1.
Keap1表达水平同接受化疗的NSCLC患者临床特征关系(n=50)
The relationship between the protein expression level of Keap1 and clinical features of NSCLC patients with chemotherapy (n=50)
| Clinical characterstic | n | High expression of Keap1 | χ2 | P |
| SQC: squamous cell carcinoma; ADC: adenocarcinoma; NSCLC: non-small cell lung cancer; PR: partial response; SD: stable disease; PD: progressive disease. | ||||
| Gender | 2.582 | 0.108 | ||
| Male | 29 | 10 (34.5%) | ||
| Female | 21 | 3 (14.3%) | ||
| Age (yr) | 0.008 | 0.928 | ||
| < 70 | 38 | 10 (26.3%) | ||
| ≥70 | 12 | 3 (25.0%) | ||
| Pathological type | < 0.001 | 0.990 | ||
| SQC | 23 | 6 (26.1%) | ||
| ADC | 27 | 7 (25.9%) | ||
| Differentiation | 3.175 | 0.204 | ||
| Low | 2 | 1 (50.0%) | ||
| Moderate | 33 | 6 (18.2%) | ||
| High | 15 | 6 (40.0%) | ||
| Smoking history | 1.661 | 0.198 | ||
| Yes | 31 | 10 (32.3%) | ||
| No | 19 | 3 (15.8%) | ||
| Metastasis | 0.017 | 0.895 | ||
| M0 | 20 | 5 (25.0%) | ||
| M1 | 30 | 8 (26.7%) | ||
| Chemotherapeutic regimen | 2.022 | 0.732 | ||
| Taxanes-based | 10 | 3 (30.0%) | ||
| Gemcitabine-based | 28 | 8 (28.6%) | ||
| Novibine-based | 5 | 0 | ||
| Pemtrexed-based | 4 | 1 (25.0%) | ||
| Others | 3 | 1 (33.3%) | ||
| Response | 6.135 | 0.047 | ||
| PR | 17 | 8 (47.1%) | ||
| SD | 23 | 4 (17.4%) | ||
| PD | 10 | 1 (10.0%) | ||
2.3. Keap1表达与化疗疗效、PFS、OS相关性分析
Pearson相关分析表明,Keap1表达与化疗疗效(r=-0.327, P=0.020)和PFS(r=0.439, P=0.002)相关,但与OS(r=0.018, P=0.904)无关,Keap1高表达NSCLC患者的疗效及PFS明显优于低或不表达者。
2.4. 生存分析
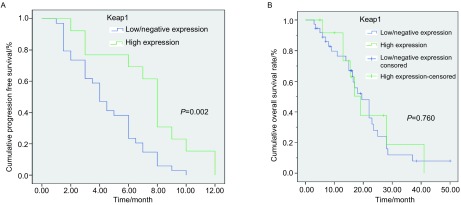
Kaplan-Meier生存分析显示Keap1高表达NSCLC患者组的中位PFS和OS分别为8.0个月和19.0个月;低表达组分别为4.0个月和19.5个月,Keap1高表达组中位PFS明显高于低表达组(P=0.002,图 2A),但两组在OS方面无统计学差异(P=0.760,图 2B)。
2.
Kaplan-Meier生存曲线分析。A:Keap1高表达和低/不表达NSCLC患者的PFS生存曲线;B:Keap1高表达和低/不表达NSCLC患者的OS生存曲线
Kaplan-Meier cumulative survival time curves analysis. A: The PFS curves of Keap1 high expression and low/negative expression group of NSCLC patients; B: The OS curves of Keap1 high expression and low/negative expression group of NSCLC patients. PFS: progression free survival
2.5. 多因素分析
校正性别、年龄、病理类型、转移等因素后,Logistic回归分析表明Keap1表达水平有成为一线化疗疗效的独立预测指标的趋势(P=0.065),见表 2。校正性别、年龄、病理类型、分化程度、化疗方案、转移、接受化疗方案种类、接受EGFR-TKI治疗与否等因素后,多因素分析表明Keap1表达水平是一线化疗PFS的独立预测因素(P=0.007),但不是OS的独立预测因素(P=0.700)。病理类型(P=0.026)、后续是否接受EGFR-TKI治疗(P=0.007)及接受化疗方案种类(P=0.046)是OS的预测因素,见表 3。
2.
回归模型比较一线化疗疗效相关的各组变量(n=50)
Comparsion of variable for chemotherapy induced response (n=50)
| Characteristic | Regression coefficient β | Standard error | Wald | P | Exp(B) | 95%CI |
| Gender | 0.481 | 0.910 | 0.279 | 0.597 | 1.617 | 0.272-9.615 |
| Age | -0.569 | 1.285 | 0.196 | 0.658 | 0.566 | 0.046-7.030 |
| Pathological type | 0.617 | 0.959 | 0.414 | 0.520 | 1.853 | 0.283-12.147 |
| Metastasis | 0.442 | 0.973 | 0.206 | 0.650 | 1.555 | 0.231-10.471 |
| Keap1 | -2.204 | 1.194 | 3.407 | 0.065 | 0.110 | 0.011-1.146 |
3.
多因素分析NSCLC特异性生存率的预后因素(n=47)
Cox regression analysis of the disease-specific survival of NSCLC patients (n=47)
| Characteristic | Regression coefficient β | Standard error | Wald | P | Exp(B) | 95%CI |
| Progression free survival | ||||||
| Gender | 0.339 | 0.478 | 0.503 | 0.478 | 1.403 | 0.550-3.577 |
| Age | 0.418 | 0.433 | 0.929 | 0.335 | 1.519 | 0.649-3.551 |
| Pathological type | 0.186 | 0.380 | 0.240 | 0.624 | 1.205 | 0.572-2.538 |
| Differentiation | -0.131 | 0.316 | 0.173 | 0.677 | 0.877 | 0.472-1.628 |
| Metastasis | -0.023 | 0.427 | 0.003 | 0.956 | 0.977 | 0.423-2.254 |
| Chemotherapeutic regimen | 0.237 | 0.221 | 1.151 | 0.283 | 1.268 | 0.822-1.956 |
| Keap1 | -1.079 | 0.398 | 7.358 | 0.007 | 0.340 | 0.156-0.741 |
| Overall survival | ||||||
| Gender | 0.288 | 0.657 | 0.193 | 0.661 | 1.334 | 0.368-4.831 |
| Age | -0.421 | 0.683 | 0.380 | 0.538 | 0.657 | 0.172-2.504 |
| Pathological type | -2.212 | 0.992 | 4.976 | 0.026 | 0.109 | 0.016-0.765 |
| Differentiation | 0.863 | 0.587 | 2.164 | 0.141 | 2.370 | 0.751-7.486 |
| Number of chemo therapeutic regimen | -0.904 | 0.454 | 3.966 | 0.046 | 0.405 | 0.166-0.986 |
| EGFR-TKI treatment | -2.375 | 0.880 | 7.290 | 0.007 | 0.093 | 0.017-0.522 |
| Keap1 | -0.276 | 0.716 | 0.149 | 0.700 | 0.759 | 0.186-3.088 |
| EGFR-TKI: epidermal growth factor receptor tyrosine kinase inhibitor. | ||||||
3. 讨论
Keap1是细胞防御氧化和亲电性应激损伤的重要蛋白,其与核因子E2相关因子2(nuclear factor erythroid-2-related factor 2, Nrf2)组成重要的细胞防御体系。生理状况下,Keap1结合Nrf2,并与E3泛素化连接酶结合,通过泛素化介导Nrf2蛋白降解,维持细胞浆内Nrf2处于较低水平。一旦细胞受到外源性或内源性的氧化应激或亲电性应激刺激,Keap1便成为敏感的传感器,其通过对自身半胱氨酸残基的修饰,阻止Nrf2降解,并促进Nrf2释放,累积的Nrf2进入细胞核内,激活抗氧化反应元件(antioxidant response element, ARE),进而激活ARE下游的细胞保护基因,包括:①细胞内氧化还原基因,如谷氨酸半胱氨酸连接酶、血红素氧合酶-1等;②Ⅱ相解毒基因,如谷胱甘肽-S转移酶、NAD(P)H苯醌氧化还原酶-1等;③编码转运蛋白的基因,如多药耐药蛋白(multidrug resistant protein, MRP)等,从而保护细胞免受氧化应激或亲电性应激所致损伤[3, 8]。
Solis等[7]应用免疫组化的方法发现Keap1高表达组NSCLC患者的PFS和OS明显高于低表达组;Takahashi等[9]和Li等[10]应用基因检测方法证实NSCLC Keap1的失活与预后差密切相关。近期研究[3, 11-13]表明Keap表达下降后Nrf2表达升高,导致肺癌细胞对阿霉素、足叶乙甙、顺铂和紫杉类药物耐药。因此Keap1表达水平除与NSCLC患者预后相关,还应与化疗疗效相关。但目前缺乏Keap1表达水平与进展期NSCLC患者一线化疗疗效的相关研究。因进展期患者组织标本通常是通过支气管镜或CT穿刺活检获得,组织标本较小,而免疫组化方法所需组织标本量明显少于基因检测方法。因此,本研究以临床肺穿刺或支气管活检标本为研究对象,应用免疫组织化学方法检测Keap1蛋白,拟明确进展期NSCLC患者中Keap1表达情况及与一线化疗疗效的相关性。
本研究结果显示进展期NSCLC患者中Keap1表达存在个体差异,Keap1高表达率为26.0%。Keap1表达产生个体差异机制包括:①遗传背景本身所致;②Keap1自身突变不同所致,近期多项研究发现NSCLC标本中检测出Keap1突变[10, 14, 15],突变导致Keap1表达下降或缺失;③Keap1甲基化所致,Wang等[11]和Muscarella等[16]发现NSCLC组织标本和细胞株中存在Keap1基因启动子CpG岛甲基化,甲基化导致Keap1功能减低;④Nrf2突变所致,Nrf2突变的NSCLC细胞株中Nrf2过表达,间接抵消Keap1功能[17];⑤外源性刺激Nrf2活性增强,间接减弱Keap1功能所致,张凯茹等[18]发现耐顺铂的A549细胞株中Nrf2水平较亲本的A549明显升高。上述因素可以单一或同时存在。
本研究中Keap1高表达的NSCLC患者组获得PR率高于低表达或不表达患者组。其原因可能为在Keap1不表达或低表达的患者中,Keap1对Nrf2调控受限,从而促使Nrf2进入细胞核内激活ARE的下游基因,如Ⅱ相解毒基因、编码转运蛋白的基因等,从而促进药物解毒、外排,产生耐药[3];而Keap1高表达者,可维持Nrf2处于较低水平,进而提高药物敏感性[4]。结合本研究结果,Keap1表达水平或许是预测化疗疗效的理想指标。
本研究中Keap1高表达组患者的PFS明显延长,可能与Keap1高表达患者组客观缓解率高或Keap1高表达的肿瘤细胞增殖、转移能力下降[4, 19]相关。但在OS方面,本研究发现Keap1表达水平与OS无关,与Solis[7]和Merikallio[20]研究结果不同。其差异产生的原因可能在于:①Keap1表达情况随着外界环境刺激发生改变;②后续治疗不平衡,如患者接受化疗方案的种类以及是否接受EGFR-TKI治疗等不同;③二者研究对象不同,本研究涉及均是进展期患者,而Solis[7]和Merikallio[20]研究中多为术后患者。
本研究的主要限制在于样本量偏小,可能存在选择偏移。但本研究发现Keap1表达水平与一线化疗疗效、PFS相关,Keap1是PFS的独立预测因素。因此Keap1或许可成为预测NSCLC化疗疗效的生物指标,需要进一步扩大样本量,进行前瞻性研究验证Keap1对化疗疗效预测的临床价值。
Funding Statement
本研究受北京大学第三医院中青年骨干基金(No.76476-01)资助
This study was supported by the grant from Peking University Third Hospital (to Baoshan CAO)(No.76476-01)
References
- 1.Jemal A, Bray F, Center MM, et al. Global Cancer Statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.v61:2. [DOI] [PubMed] [Google Scholar]
- 2.Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997;111(6):1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 3.Singh A, Misra V, Thimmulappa RK, et al. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3(10):e420. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qu LY, Gao P, Wang HY, et al. Nrf2 down-regulated cell line H460-N5 with Keap1 over-expression increased sensitivity to anti-cancer drugs. http://med.wanfangdata.com.cn/Paper/Detail/PeriodicalPaper_zjdxxb-yxb201001002. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2010;39(1):5–9. doi: 10.3785/j.issn.1008-9292.2010.01.002. [DOI] [PubMed] [Google Scholar]; 曲 丽艳, 高 鹏, 王 洪燕, et al. 稳定表达Keap1的H460-N5细胞株的建立及增敏抗肿瘤药物作用的研究. http://med.wanfangdata.com.cn/Paper/Detail/PeriodicalPaper_zjdxxb-yxb201001002 浙江大学学报(医学版) 2010;39(1):5–9. [Google Scholar]
- 5.Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136(1):260–271. doi: 10.1378/chest.08-0978. [DOI] [PubMed] [Google Scholar]
- 6.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Solis LM, Behrens C, Dong W, et al. Nrf2 and Keap1 abnormalities in non-small cell lung carcinoma and association with clinicopathologic features. Clin Cancer Res. 2010;16(14):3743–3753. doi: 10.1158/1078-0432.CCR-09-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Que LL, Wang HX, Cao BS, et al. The regulation and functions of transcription factor Nrf2 in cancer chemoprevention and chemoresistance. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4677237/table/tab2/ J Chin Pharm Sci. 2011;20(1):5–19. [Google Scholar]
- 9.Takahashi T, Sonobe M, Menju T, et al. Mutations in Keap1 are a potential prognostic factor in resected non-small cell lung cancer. http://europepmc.org/abstract/MED/24137397. J Surg Oncol. 2010;101(6):500–506. doi: 10.1002/jso.21520. [DOI] [PubMed] [Google Scholar]
- 10.Li QK, Singh A, Biswal S, et al. KEAP1 gene mutations and NRF2 activation are common in pulmonary papillary adenocarcinoma. J Hum Genet. 2011;56(3):230–234. doi: 10.1038/jhg.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang R, An J, Ji F, et al. Hypermethylation of the Keap1 gene in human lung cancer cell lines and lung cancer tissues. Biochem Biophys Res Commun. 2008;373(1):151–154. doi: 10.1016/j.bbrc.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Ramos-Gomez M, Kwak MK, Dolan PM, et al. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci USA. 2001;98(6):3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shim GS, Manandhar S, Shin DH, et al. Acquisition of doxorubicin resistance in ovarian carcinoma cells accompanies activation of the NRF2 pathway. Free Radic Biol Med. 2009;47(11):1619–1631. doi: 10.1016/j.freeradbiomed.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Ohta T, Iijima K, Miyamoto M, et al. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res. 2008;68(5):1303–1309. doi: 10.1158/0008-5472.CAN-07-5003. [DOI] [PubMed] [Google Scholar]
- 15.Yoo NJ, Kim HR, Kim YR, et al. Somatic mutations of the KEAP1 gene in common solid cancers. Histopathology. 2012;60(6):943–952. doi: 10.1111/his.2012.60.issue-6. [DOI] [PubMed] [Google Scholar]
- 16.Muscarella LA, Parrella P, D'Alessandro V, et al. Frequent epigenetics inactivation of KEAP1 gene in non-small cell lung cancer. Epigenetics. 2011;6(6):710–719. doi: 10.4161/epi.6.6.15773. [DOI] [PubMed] [Google Scholar]
- 17.Shibata T, Saito S, Kokubu A, et al. Global downstream pathway analysis reveals a dependence of oncogenic NF-E2-related factor 2 mutation on the mTOR growth signaling pathway. Cancer Res. 2010;70(22):9095–9105. doi: 10.1158/0008-5472.CAN-10-0384. [DOI] [PubMed] [Google Scholar]
- 18.Zhang KR, Yan HQ, Wang Y, et al. Expression and significance of nrf2 and its target genes in pulmonary adenocarcinoma A549 cells resistant to Cisplatin. Zhongguo Fei Ai Za Zhi. 2009;12(11):1150–1154. doi: 10.3779/j.issn.1009-3419.2009.11.04. [DOI] [PubMed] [Google Scholar]; 张 凯茹, 闫 惠琴, 王 燕, et al. Nrf2及其靶基因在人肺腺癌A549顺铂耐药细胞株中的表达和意义. 中国肺癌杂志. 2009;12(11):1150–1154. doi: 10.3779/j.issn.1009-3419.2009.11.04. [DOI] [Google Scholar]
- 19.Singh A, Boldin-Adamsky S, Thimmulappa RK, et al. RNAi-mediated silencing of nuclear factor erythroid-2-related factor 2 gene expression in non-small cell lung cancer inhibits tumor growth and increases efficacy of chemotherapy. Cancer Res. 2008;68(19):7975–7984. doi: 10.1158/0008-5472.CAN-08-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merikallio H, Paakko P, Kinnula VL, et al. Nuclear factor erythroid-derived 2-like 2 (Nrf2) and DJ1 are prognostic factors in lung cancer. Hum Pathol. 2012;43(4):577–584. doi: 10.1016/j.humpath.2011.05.024. [DOI] [PubMed] [Google Scholar]