Abstract
May Thurner syndrome (MTS) is an anatomic variant that can present as acute or chronic deep vein thrombosis. Although it is classically reported in young and middle-aged women, it is also seen in both young and older men. Multiple cases of anatomic variations of MTS have been described. We present an uncommon case of variant MTS, including diagnostic imaging and approach to treatment.
Keywords: May-Thurner syndrome, Deep vein thrombosis, Thrombolysis
Case presentation
A 57-year-old man presented with a 2-week history of progressive left lower extremity swelling and redness. The swelling started in the calf and gradually progressed along the entire leg with significant redness. He noted discomfort that was worse with activity or while the leg was in a dependent position. Physical examination was notable for 4+ pitting left lower extremity edema, diffuse erythema, and mild tenderness in the popliteal fossa. Dorsalis pedis and posterior tibialis pulses were 1+ on the right and nonpalpable on the left. There were no overlying skin ulcers or pigmentation.
Left lower extremity venous duplex ultrasound demonstrated extensive occlusive deep vein thrombosis (DVT) in the lower extremity from the peroneal veins to the external iliac vein (Fig. 1A). The common iliac vein was patent. Initial workup was otherwise unremarkable. The patient was started on intravenous heparin, and interventional radiology was consulted to perform catheter-directed thrombolysis.
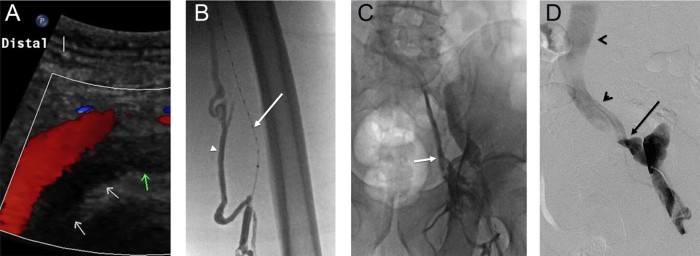
Fig. 1.
(A) Left lower extremity venous duplex ultrasound demonstrating occlusive deep vein thrombus (DVT) in the external iliac vein (arrows). (B) Extensive left lower extremity occlusive DVT was noted on venography. Note the lysis catheter in the occluded superficial femoral vein (arrow) and filling of collateral muscular veins (arrowhead). (C) Venography demonstrates filling of collaterals draining into the internal iliac vein (arrow). Thrombolysis was initiated in the affected segments. (D) Pelvic venography demonstrated an abrupt change in contrast opacity at the confluence of the external iliac vein with the internal iliac vein (arrow), with the common iliac vein and inferior vena cava remaining patent (arrowheads).
Left leg venogram demonstrated large expansile filling defects extending from the popliteal vein to the external iliac vein with filling of numerous collaterals (Fig. 1B and C). Thrombolysis was initiated along the length of the thrombosed venous segments using recombinant tissue plasminogen activator, with resolution of significant thrombus burden after 24 hours. Venography demonstrated an irregular, slightly angulated course of the left external iliac vein with a high-grade stenosis at the confluence of the internal iliac vein and external iliac vein with the common iliac vein (Fig. 1D, arrow). The common iliac vein and confluence with the inferior vena cava (IVC) were widely patent (Fig. 1D, arrowheads). The stenosis was crossed with a wire, and serial venoplasty and balloon maceration were performed. The stenosis was severely painful during venoplasty and was highly elastic. The decision was therefore made to stent the lesion using a self-expanding stent (Wallstent, Boston Scientific, Natick, MA). Of note, a significant waist remained within the stent at the level of the stenosis, even after dilation with high-pressure angioplasty balloons (Fig. 2A). Despite this residual stenosis, flow had significantly improved after stenting (Fig. 2B). Therapeutic low-molecular-weight heparin was prescribed for outpatient anticoagulation along with compression stocking therapy.
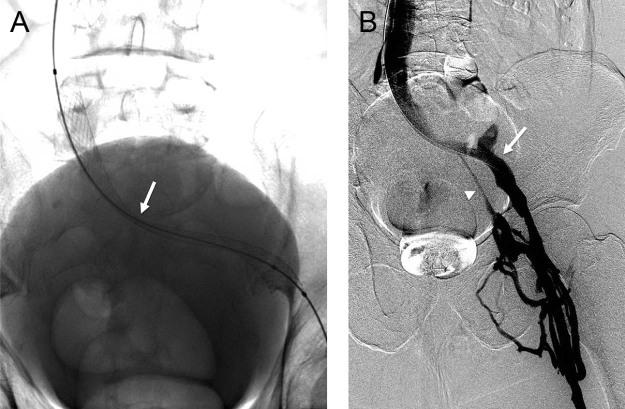
Fig. 2.
(A) After thrombolysis, balloon angioplasty and stenting was performed, with a significant residual waist at the region of stenosis (arrow). (B) Flow had significantly improved, however, after stenting. Note increased opacification of the external iliac vein (arrow) and less collateral filling of the internal iliac vein (arrowhead).
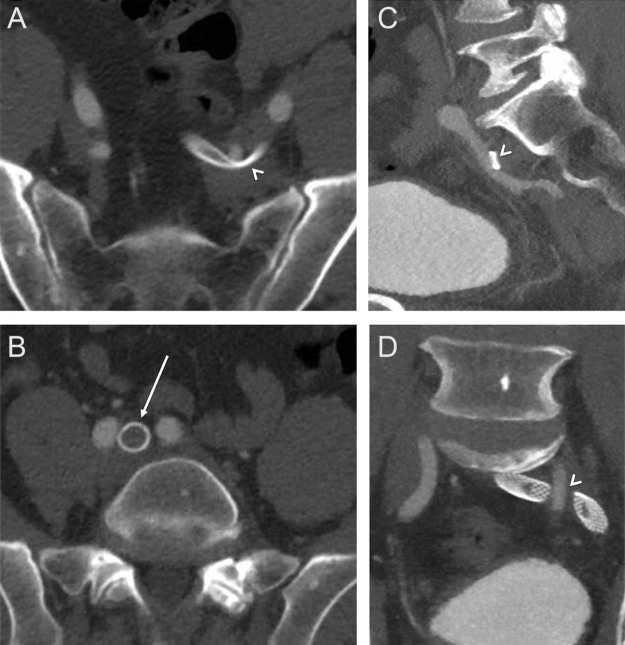
Because of the unusual location of venous stenosis, a computed tomography (CT) with intravenous contrast was performed to look for extrinsic compression, such as a mass or aneurysm. CT revealed compression of the left common iliac vein near the confluence of the external iliac vein by the overlying left internal iliac artery as it crossed over the vein (Fig. 3, arrowheads). The more cephalad portion of the left common iliac vein, a more typical location for May-Thurner compression, was widely patent as it coursed under the right common iliac artery (Fig. 3B, arrow). No aneurysm or solid lesion was noted. The patient was thus diagnosed with a variant of May-Thurner syndrome (MTS). A follow-up pelvic venogram demonstrated a patent venous system with brisk flow and interval spontaneous dilation of the compressed portion of the stent (Fig. 4A and B). On follow up 2 months after stenting, his symptoms were markedly improved, and there was significant resolution of pitting edema and erythema. Ultrasound demonstrated a widely patent stent (Fig. 4C).
Fig. 3.
(A, C, D) Computed tomography (CT) with contrast demonstrates compression of the stented iliac veins by the overlying left internal iliac artery (arrowheads). No compression was seen at the confluence of the left common iliac vein with the inferior vena cava (IVC), at the location of typical May-Thurner compression.
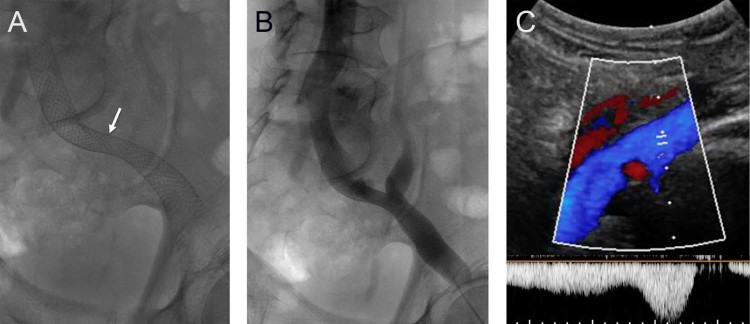
Fig. 4.
(A) Follow-up radiograph demonstrates interval spontaneous dilation of the waist within the stented left iliac veins (arrow). (B) Venogram demonstrates brisk flow through the stented iliac system. (C) Ultrasound evaluation at follow-up demonstrated a widely patent iliac venous system with normal augmentation of flow.
Discussion
MTS is a condition where the left common iliac vein is compressed between the overlying right common iliac artery and spine, resulting in venous webs, synechiae, and, ultimately DVT. MTS can present as acute or chronic DVT and may occur more commonly in patients with reduced left common iliac vein diameter [1]. It is classically reported as a finding in young females, presenting as acute or chronic isolated left leg DVT with symptoms of unilateral leg pain, edema, or new superficial venous varicosities.
There have been reports of various causes of iliac vein compression, including by a distended bladder, endometriosis, common iliac artery aneurysm, and internal iliac artery aneurysm [2]. The left common iliac vein can also be compressed by the right iliac artery and a tortuous left common iliac artery, which represent some variants of MTS [2]. Previous variants of MTS that have been described include obstruction of the left common iliac vein by the left internal iliac artery, compression of IVC by right common iliac artery or a tortuous left common iliac artery, and compression of the right common iliac vein by the right internal iliac artery or by the right common iliac artery [3], [4], [5]. This case demonstrates compression of the left common iliac vein by the overlying left internal iliac artery. This is an uncommon area of compression, and only a few similar cases have been described previously [5].
MTS is believed to be caused by both mechanical and physiological factors. The chronic pulsatile compression of the left common iliac vein by the right common iliac artery stimulates the formation of fibrotic intravascular lesions that can cause partial or complete vein obstruction over time [6]. MTS is frequently recurrent and is poorly responsive to anticoagulation or thrombolysis alone because of the underlying anatomic provocation [7], [8]. Patients remain at risk of developing post-thrombotic syndrome (PTS). Other provoking factors usually associated with DVT such as recent surgeries, prolonged periods of immobilization, hypercoagulable states, trauma, and malignancies should be ruled out. The clinical presentation and history of the patient are critical components in formulating a diagnosis of MTS. An acute presentation of MTS, which includes the sudden onset of left leg edema, is usually easier to diagnose, whereas the chronic phase of MTS can be more difficult to identify and requires a comprehensive investigation of patient history, physical examination, and diagnostic imaging studies. Diagnostic imaging tests help confirm the presence of venous compression and determine the best modality of treatment.
Imaging findings on ultrasound, CT venography, or magnetic resonance venography (MRV) demonstrate extrinsic compression of the vessel with stenosis and venous collaterals. Although venous ultrasonography is useful to identify the extent of DVT, a diagnosis of MTS is usually made by cross-sectional CT or MRV, which demonstrate the venous anatomy and location of the stenosis in relation to the artery [9]. Color Doppler ultrasonography (CDUS) is often the initial diagnostic modality because it is noninvasive and accurate in determining the location and extent of venous thrombosis [10]. CT with intravenous contrast is a useful modality in confirming the diagnosis of MTS. The relationship of the vein relative to the artery and spine is clearly seen, and occasionally a chronic occlusion with calcifications can be seen. In addition, CT can help rule out other reasons for extrinsic compression such as masses and aneurysms. Advantages of CT venography over CDUS or traditional venography include lack of operator dependence, better imaging of the pelvic vasculature, and a shorter examination time. MRV can also be used to confirm the diagnosis of MTS. The primary advantages of MRV in the diagnosis of MTS include its noninvasiveness, ability to analyze all pelvic structures, and lack of ionizing radiation [11]. MRV can also be performed without contrast, which is beneficial for patients with contraindications.
Intravascular ultrasound (IVUS) can accurately determine left common iliac vein vessel size and morphology, and verify the presence of MTS anatomy by demonstrating focal stenosis and the presence of vascular webs or fibrotic bands [12]. IVUS can be used to provide alternative imaging that complements traditional venography, and can aid endovascular interventions. IVUS of the major axial veins may be useful in assessing luminal diameter, providing a 360-degree 2-dimensional gray scale ultrasound image of lumen and vessel wall structures [13]. In catheter-based procedures, IVUS can give an accurate location and size of veins such as the common femoral vein and IVC, and it can determine important landmarks and venous branches. It can also visualize other important abnormalities such as fibrosis, mural wall thickening, spurs, and trabeculations seen commonly in patients with MTS.
Traditional treatment of DVT, including anticoagulation and leg compression, do not address the underlying mechanical compression seen in MTS. Consequently, patients managed by these therapies alone may be predisposed to developing recurrent DVT, PTS, or both [1], [14]. As such, early thrombolysis and stenting can potentially decrease symptoms more rapidly, and may also reduce the risk of PTS [9], [15]. Patients with iliofemoral DVT successfully treated with catheter-directed thrombolysis correlated with increased clinical benefit for up to 5 years; however, allocation to this therapy has not shown to directly impact quality of life during that time frame, according to the results of the CaVenT study [16], [17]. For MTS, isolated balloon angioplasty or catheter directed thrombolysis alone has a high re-occlusion rate and is typically supplemented with stent placement to improve patency [9]. Recently, both retrospective and prospective studies have suggested that endovascular management with thrombolysis and stenting can result in better long-term outcomes, as this treats both thrombus burden and mechanical compression [18], [19]. With the improvement in percutaneous endovascular techniques, balloon angioplasty and stenting have become the standard of care for MTS [7], [8]. The technical success of the endovascular approach is upward of 96%, with a 1-year primary and secondary patency rate of 58%-100% and 76%-98% [20], [21]. Several studies have demonstrated efficacy in treatment of MTS with thrombectomy and endovascular stenting [18], [22], [23], [24]. A recent study of 225 patients treated with percutaneous transluminal angioplasty and stent placement showed primary patency rate of 93.2% in 1 year, 84.3% in 3 years, and 74.5% in 5 years [25]. A 982-patient study demonstrated that patients with nonthrombotic iliac vein lesions tend to experience significantly better long-term stent patency than patients with DVT [18]. Primary and secondary patency rates at 72-month follow-up were 79% and 100%, respectively, for nonthrombotic vascular lesions. In another study of stents for nonthrombotic iliac vein lesions, a 98% primary stent patency was observed after 4 years [26]. Considering these favorable results, early intervention and stent placement in symptomatic patients may be beneficial; however, the optimal endovascular thrombolytic approach still requires further investigation [17].
The true incidence rate of MTS is not currently known. Original autopsy studies estimated a range of 22%-32%, and the prevalence of symptomatic MTS has been reported in 18%-49% of patients with left-sided lower extremity DVT [1]. MTS-related DVTs account for only about 2%-3% of all cases. MTS is thought to occur predominantly in patients who present with moderate to severe symptoms, but many patients with iliofemoral compression remain asymptomatic [15]. Existing studies have confirmed the presence of left common iliac vein compression in an asymptomatic patient population [2], [27]. One study noted that the mean age of subjects without symptoms was 40 years with 60% females and 40% males, 24% of which had greater than 50% compression, but without symptoms [2]. Another study of an asymptomatic population found a mean compression of 37.8% [28]. Given the relatively high incidence of this anatomic finding in the general population, it is important to consider MTS as a cause of acute DVT symptoms not only in young and middle-aged women but also in men.
Conclusion
The classic presentation of MTS is described as compression of the left common iliac vein by the overlying right common iliac artery. Anatomic variants of MTS involving other vessels have been described in the literature. We present a case of variant MTS involving compression of the left common iliac vein by the overlying left internal iliac artery, successfully treated with catheter-directed thrombolysis and stenting. MTS is found at a relatively high incidence in the general population in symptomatic and asymptomatic patients of both sexes. As awareness of this entity increases, it will be important to consider variants of MTS in order to direct appropriate care.
References
- 1.Galang L. May Thurner Syndrome: an important differential diagnosis for DVT. J Vasc Med Surg. 2016;4(2):261. [Google Scholar]
- 2.Kibbe M.R., Ujiki M., Goodwin A.L., Eskandari M., Yao J., Matsumura J. Iliac vein compression in an asymptomatic patient population. J Vasc Surg. 2004;39(5):937–943. doi: 10.1016/j.jvs.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 3.Fretz V., Binkert C.A. Compression of the inferior vena cava by the right iliac artery: a rare variant of May-Thurner syndrome. Cardiovasc Intervent Radiol. 2010;33(5):1060–1063. doi: 10.1007/s00270-009-9671-y. [DOI] [PubMed] [Google Scholar]
- 4.Molloy S., Jacob S., Buckenham T., Khaw K.T., Taylor R.S. Arterial compression of the right common iliac vein; an unusual anatomical variant. Cardiovasc Surg. 2002;10(3):291–292. doi: 10.1016/s0967-2109(01)00139-9. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg J.B., Jacocks M.A. May-Thurner syndrome: a previously unreported variant. Ann Vasc Surg. 1993;7(6):577–581. doi: 10.1007/BF02000154. [DOI] [PubMed] [Google Scholar]
- 6.Brinegar K.N., Sheth R.A., Khademhosseini A., Bautista J., Oklu R. Iliac vein compression syndrome: clinical, imaging and pathologic findings. World J Radiol. 2015;7(11):375–381. doi: 10.4329/wjr.v7.i11.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brazeau N.F., Harvey H.B., Pinto E.G., Deipolyi A., Hesketh R.L., Oklu R. May-Thurner syndrome: diagnosis and management. Vasa. 2013;42(2):96–105. doi: 10.1024/0301-1526/a000252. [DOI] [PubMed] [Google Scholar]
- 8.Mussa F.F., Peden E.K., Zhou W., Lin P.H., Lumsden A.B., Bush R.L. Iliac vein stenting for chronic venous insufficiency. Tex Heart Inst J. 2007;34(1):60–66. [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta S., Bhuiyan T., Shah A.R., Lyon R.T., Rivera A., Sokol S.I. May-Thurner Syndrome: a case based review. J Heart Circ. 2014;1(1):17000101. [Google Scholar]
- 10.Lamba R., Tanner D.T., Sekhon S., McGahan J.P., Corwin M.T., Lall C.G. Multidetector CT of vascular compression syndromes in the abdomen and pelvis—erratum. Radiographics. 2015;35(3):973. doi: 10.1148/rg.2015154007. [DOI] [PubMed] [Google Scholar]
- 11.Wolpert L.M., Rahmani O., Stein B., Gallagher J.J., Drezner A.D. Magnetic resonance venography in the diagnosis and management of May-Thurner syndrome. Vasc Endovascular Surg. 2002;36(1):51–57. doi: 10.1177/153857440203600109. [DOI] [PubMed] [Google Scholar]
- 12.Forauer A.R., Gemmete J.J., Dasika N.L., Cho K.J., Williams D.M. Intravascular ultrasound in the diagnosis and treatment of iliac vein compression (May-Thurner) syndrome. J Vasc Interv Radiol. 2002;13(5):523–527. doi: 10.1016/s1051-0443(07)61535-8. [DOI] [PubMed] [Google Scholar]
- 13.McLafferty R.B. The role of intravascular ultrasound in venous thromboembolism. Semin Intervent Radiol. 2012;29(1):10–15. doi: 10.1055/s-0032-1302446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibrahim W., Al Safran Z., Hasan H., Abu Zeid W. Endovascular management of May-Thurner syndrome. Ann Vasc Dis. 2012;5(2):217–221. doi: 10.3400/avd.cr.12.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holper P., Kotelis D., Attigah N., Hyhlik-Dürr A., Böckler D. Longterm results after surgical thrombectomy and simultaneous stenting for symptomatic iliofemoral venous thrombosis. Eur J Vasc Endovasc Surg. 2010;39(3):349–355. doi: 10.1016/j.ejvs.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 16.Comerota A.J., Paolini D. Treatment of acute iliofemoral deep venous thrombosis: a strategy of thrombus removal. Eur J Vasc Endovasc Surg. 2007;33(3):351–360. doi: 10.1016/j.ejvs.2006.11.013. discussion 361-2. [DOI] [PubMed] [Google Scholar]
- 17.Haig Y., Enden T., Grøtta O., Kløw N.E., Slagsvold C.E., Ghanima W. Post-thrombotic syndrome after catheter-directed thrombolysis for deep vein thrombosis (CaVenT): 5-year follow-up results of an open-label, randomised controlled trial. Lancet Haematol. 2016;3(2):e64–e71. doi: 10.1016/S2352-3026(15)00248-3. [DOI] [PubMed] [Google Scholar]
- 18.Neglen P., Hollis K.C., Olivier J., Raju S. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg. 2007;46(5):979–990. doi: 10.1016/j.jvs.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 19.Kwak H.S., Han Y.M., Lee Y.S., Jin G.Y., Chung G.H. Stents in common iliac vein obstruction with acute ipsilateral deep venous thrombosis: early and late results. J Vasc Interv Radiol. 2005;16(6):815–822. doi: 10.1097/01.RVI.0000157690.91690.38. [DOI] [PubMed] [Google Scholar]
- 20.Binkert C.A., Schoch E., Stuckmann G., Largiader J., Wigger P., Schoepke W. Treatment of pelvic venous spur (May-Thurner syndrome) with self-expanding metallic endoprostheses. Cardiovasc Intervent Radiol. 1998;21(1):22–26. doi: 10.1007/s002709900205. [DOI] [PubMed] [Google Scholar]
- 21.Mousa A.Y., AbuRahma A.F. May-Thurner syndrome: update and review. Ann Vasc Surg. 2013;27(7):984–995. doi: 10.1016/j.avsg.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Kim J.-Y., Choi D., Ko Y.G., Park S., Jang Y., Lee D.Y. Treatment of May-Thurner Syndrome with catheter-guided local thrombolysis and stent insertion. Korean Circ J. 2004;34(7):655–659. [Google Scholar]
- 23.Mickley V., Schwagierek R., Rilinger N., Görich J., Sunder-Plassmann L. Left iliac venous thrombosis caused by venous spur: treatment with thrombectomy and stent implantation. J Vasc Surg. 1998;28(3):492–497. doi: 10.1016/s0741-5214(98)70135-1. [DOI] [PubMed] [Google Scholar]
- 24.Berger A., Jaffe J.W., York T.N. Iliac compression syndrome treated with stent placement. J Vasc Surg. 1995;21(3):510–514. doi: 10.1016/s0741-5214(95)70295-4. [DOI] [PubMed] [Google Scholar]
- 25.Shi W.Y., Gu J.P., Liu C.J., He X., Lou W.S. Endovascular treatment for iliac vein compression syndrome with or without lower extremity deep vein thrombosis: a retrospective study on mid-term in-stent patency from a single center. Eur J Radiol. 2016;85(1):7–14. doi: 10.1016/j.ejrad.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 26.Ye K., Lu X., Li W., Huang Y., Huang X., Lu M. Long-term outcomes of stent placement for symptomatic nonthrombotic iliac vein compression lesions in chronic venous disease. J Vasc Interv Radiol. 2012;23(4):497–502. doi: 10.1016/j.jvir.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 27.Oguzkurt L., Ozkan U., Ulusan S., Koc Z., Tercan F. Compression of the left common iliac vein in asymptomatic subjects and patients with left iliofemoral deep vein thrombosis. J Vasc Interv Radiol. 2008;19(3):366–370. doi: 10.1016/j.jvir.2007.09.007. quiz 371. [DOI] [PubMed] [Google Scholar]
- 28.Moreland N.C., Ujiki M., Matsumura J.S., Morasch M.D., Eskandari M.K., Pearce W.H. Decreased incidence of left common iliac vein compression in patients with abdominal aortic aneurysms. J Vasc Surg. 2006;44(3):595–600. doi: 10.1016/j.jvs.2006.05.046. [DOI] [PubMed] [Google Scholar]