Abstract
Medulloblastoma is the most common posterior fossa tumor of childhood typically within the fourth ventricle. However, extra-axial medulloblastoma in posterior fossa is an uncommon diagnosis. We report a case in a 33-month-old male who presented with repeated complaints of abdominal pain, intermittent emesis, and diarrhea, and diagnosed with right cerebellar extra-axial medulloblastoma, which was surgically resected. Majority of the reported extra-axial medulloblastoma in posterior fossa in the United States are located in the cerebellopontine angle. However, to the best of our knowledge, our case is the first to document medulloblastoma occurring exclusively in the cerebellar hemispheric extra-axial space rather than the cerebellopontine angle. Although the diagnosis can present as a radiological dilemma, a systematic multimodality imaging approach can aid in narrowing the differential diagnosis and timely management. In this case report, we will discuss the imaging characteristics, differential diagnosis, and management strategies, alongside a brief review of the world literature of extra-axial medulloblastoma.
Keywords: Medulloblastoma, Pediatric neoplasm, Posterior fossa tumors, Extra-axial
Introduction
Medulloblastoma is the most common malignant, invasive, childhood embryonal tumor that occurs typically in midline posterior fossa. Approximately 500 children are diagnosed with medulloblastoma each year in the United States. It accounts for 20% of all pediatric tumors, with a peak age distribution of 5-9 years old and a preponderance for males [1]. Medulloblastoma typically presents midline or paramedian in the cerebellum, often compressing and invading the fourth ventricle. Diagnosis requires histopathologic confirmation at time of resection [2]. Children diagnosed at younger than 5 years of age, and especially those younger than 3, have been shown to have poorer prognosis with an estimated 5-year survival of 32% [3]. That said, histopathologic and molecular subtype have been used to stratify patients into risk categories and determine appropriate therapy [4]. Herein, we present the atypical case of a 33-month-old patient diagnosed with medulloblastoma presenting in a rare location overlying the cerebellar hemisphere posterolateral to the cerebellopontine angle.
Case report
A 33-month-old white male presented with 1-week history of increased sleepiness, abdominal pain, emesis, and diarrhea previously diagnosed as constipation and dehydration. Initial contrast-enhanced (CE) head computed tomography (CT) scan demonstrated an enhancing mass overlying the right cerebellar hemisphere with secondary mass effect on adjacent structures including effacement of the fourth ventricle, resulting in hydrocephalus (Fig. 1). Subsequent contrast-enhanced magnetic resonance imaging (CEMRI) brain depicted a well-circumscribed, extra-axial T2 hyperintense mass in the right posterior fossa measuring 4.3 × 4.7 × 3.1 cm, which exerted mass effect on the right cerebellar hemisphere, causing slight inferior herniation of the right cerebellar tonsil as well as effacement of the fourth ventricle and resulting in obstructive hydrocephalus. The mass demonstrated restricted diffusion on diffusion-weighted images (Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8). Given the infratentorial location in the posterior fossa, mild heterogeneous enhancement, and age of the patient, extra-axial medulloblastoma was considered with hemangioblastoma, astrocytoma, meningioma, or an atypical teratoid-rhabdoid tumor in the differential. Concurrently performed whole-spine CEMRI was unremarkable, except for a small linear enhancement along the posterior margin of the thoracic spinal cord at T9-T10, which was not definitive of metastasis.
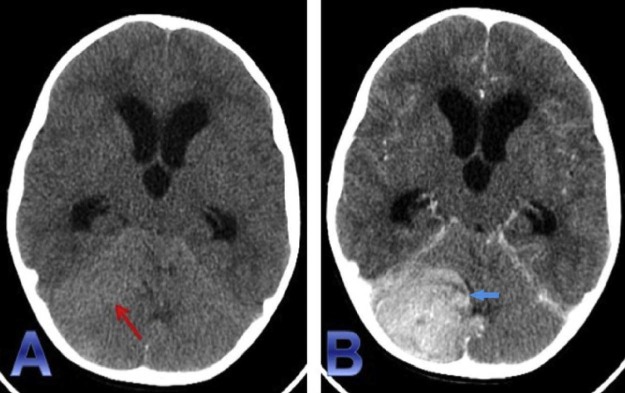
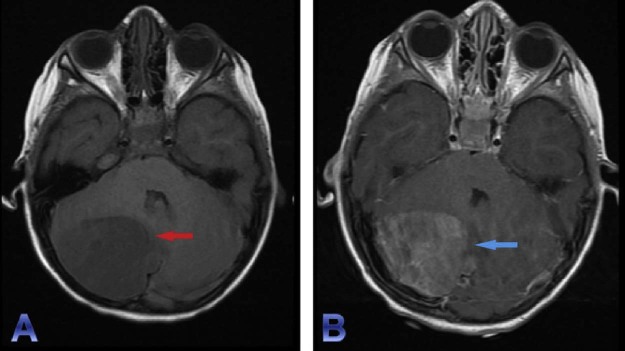
Fig. 1.
Noncontrast CT head (A) demonstrates a mildly hyperattenuating focus (red arrow) in the region of right cerebellar hemisphere. The lesion demonstrates homogenous enhancement (blue arrow) seen on the contrast-enhanced CT (B). In addition, mass effect of the right cerebellar hemisphere and brainstem, along with near complete effacement of the fourth ventricle results in obstructive hydrocephalus. CT, computed tomography.
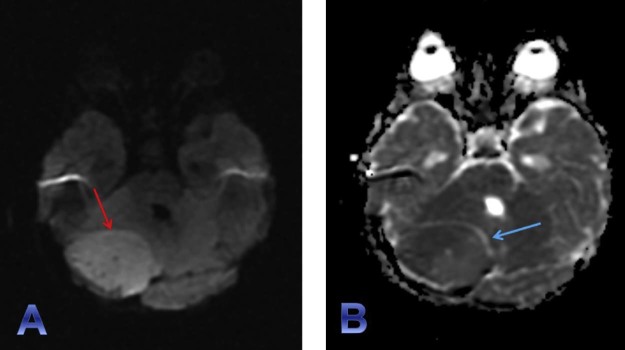
Fig. 2.
Diffusion-weighted imaging (DWI) (A) and apparent diffusion coefficient (ADC) sequences (B) clearly depict a large well-circumscribed focus of diffusion restriction (red arrow) within the right cerebellar hemisphere with corresponding signal loss on ADC maps. The hypercellularity of the lesion can be attributed to its low ADC value. A small rim of CSF (T2 signal intensity) well outlines the mass anteromedially (blue arrow). This finding along with the nature of the mass effect on the adjacent cerebellum and fourth ventricle guides the diagnosis toward an extra-axial tumor. CSF, cerebrospinal fluid.
Fig. 3.
Axial T2WI (A) and axial T2 FLAIR (B) sequences demonstrate T2 prolongation (hyperintensity) within and surrounding the posterior fossa mass. On the FLAIR sequence, the normal CSF signal is nullified and the remaining rim (red arrow) of adjacent T2 hyperintensity corresponds to vasogenic edema of the adjacent cerebellar tissue compressed by the mass. Mass effect on the right cerebellar hemisphere, effacement of the right perimesencephalic cistern, and partial effacement of the fourth ventricle (blue arrow) is well appreciated on these fluid-sensitive sequences. CSF, cerebrospinal fluid; FLAIR, fluid-attenuated inversion recovery; T2WI, T2-weighted imaging.
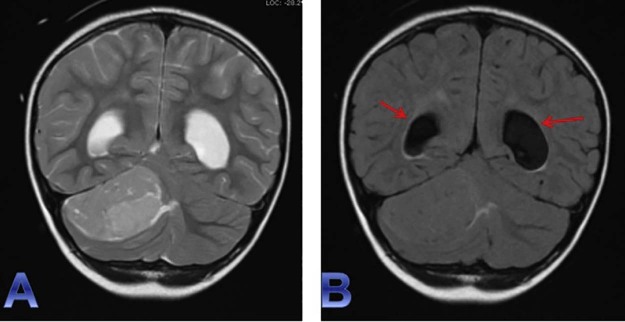
Fig. 4.
Coronal T2WI (A) and coronal T2 FLAIR (B) sequences provide additional views of the previously described (see Fig. 3) mass effect, vasogenic edema, and significant effacement of the fourth ventricle with consequential ventricular enlargement—CSF obstructive hydrocephalus (red arrows) caused by the posterior fossa extra-axial mass. CSF, cerebrospinal fluid; FLAIR, fluid-attenuated inversion recovery; T2WI, T2-weighted imaging.
Fig. 5.
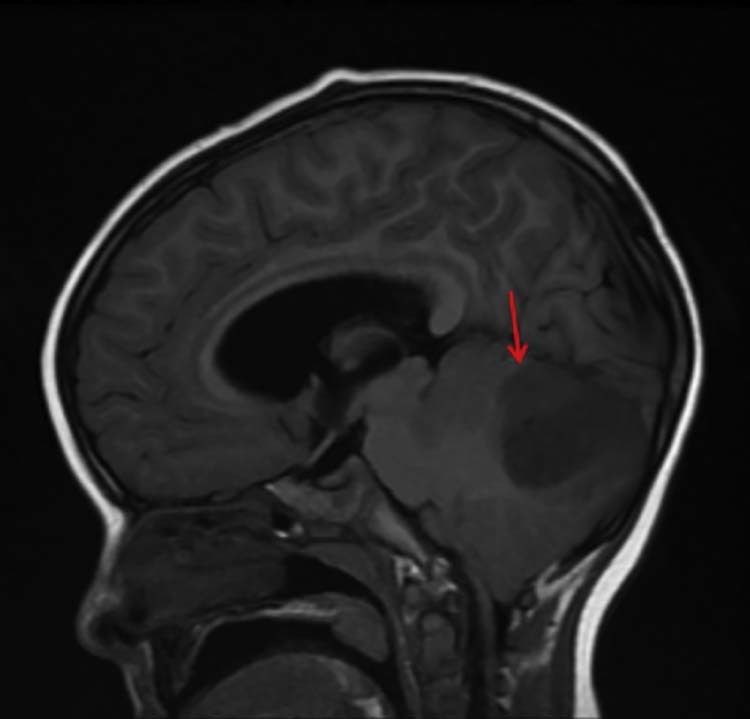
On this sagittal noncontrast T1 FLAIR sequence, a T1 hypointensity in the posterior fossa (red arrow) corresponds to the previously described mass. The degree of mass effect and effacement of the fourth ventricle is difficult to assess here; however, mild tonsillar herniation inferiorly is seen, which is also in turn due to mass effect from the lesion. FLAIR, fluid-attenuated inversion recovery.
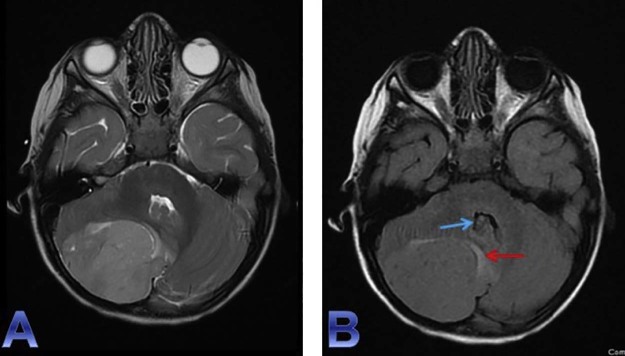
Fig. 6.
Precontrast axial T1WI (A) reiterates an extra-axial T1 hypointense lesion (red arrow) in the right posterior fossa that shows corresponding mild heterogeneous enhancement (blue arrow) on the postgadolinium axial T1WI (B). T1WI, T1-weighted imaging.
Fig. 7.
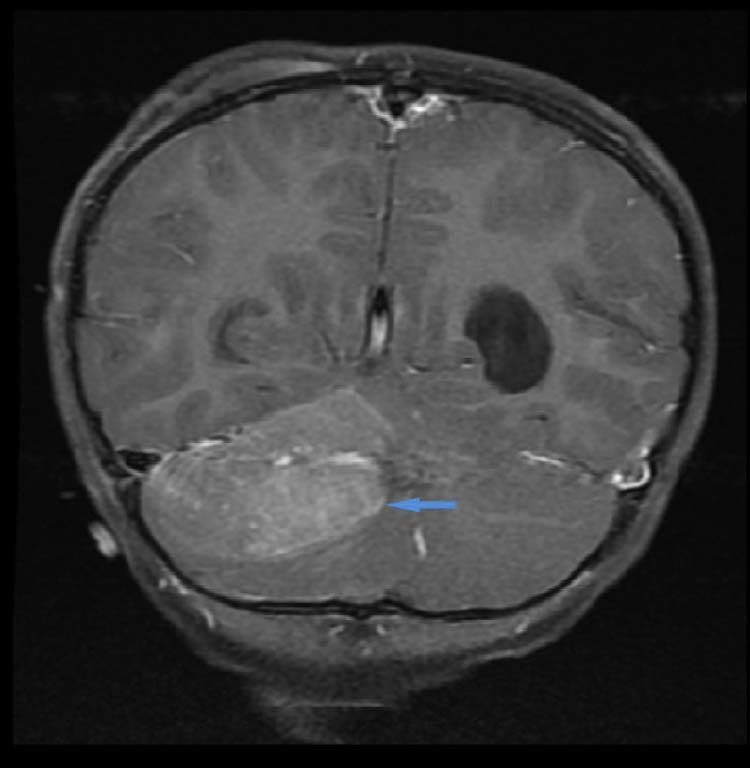
An additional view of the heterogenous enhancement (blue arrow) of the right infratentorial extra-axial lesion on this coronal post gadolinium T1WI. T1WI, T1-weighted imaging.
Fig. 8.
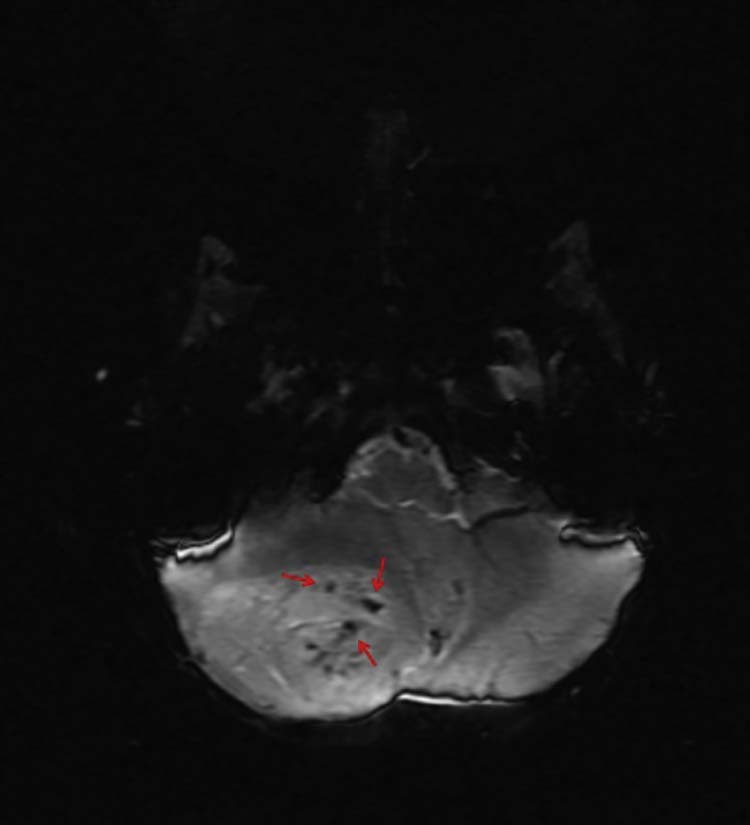
Axial SWI can provide useful information regarding micro-hemorrhages not easily seen on CT or conventional MR sequences, such as hemosiderin deposition, normal vascular flow voids, and, as depicted in this case, hypervascularity (red arrows) within a neoplasm. CT, computed tomography; MR, magnetic resonance; SWI, susceptibility-weighted imagining.
An emergent craniectomy with resection of the mass was performed. Macroscopically, a significantly bloody, slightly discolored mass was observed extra-axially in the right cerebellar hemisphere. A gross total surgical excision of the tumor was performed. Microscopically, frozen section showed a small, round, blue cell tumor. Hemotoxylin and eosin stain revealed marked hypercellular tissue with areas of primitive histology and areas of mild differentiation with a small foci of cells in a desmoplastic pattern with anaplasia (Fig. 9, Fig. 10). Ki-67 was over 5% positive nuclei and GFAP was found to be focally positive. These findings were consistent with medulloblastoma (World Health Organization grade IV).
Fig. 9.
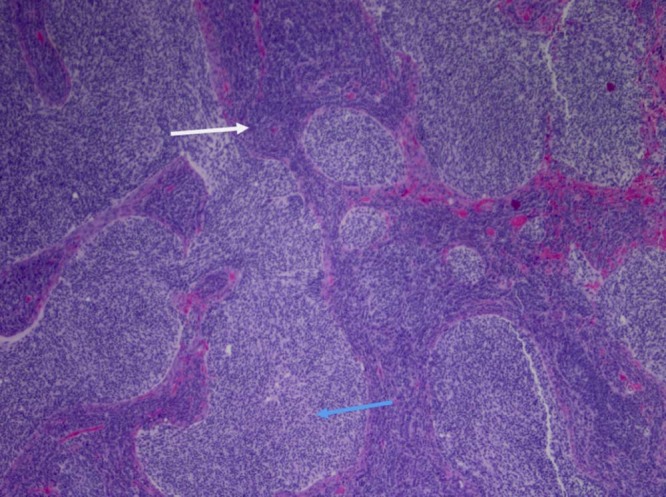
(4×) Medulloblastoma with classic histology. Markedly hypercellular areas with primitive small blue cells with hyperchromatic, pleomorphic nuclei are present (white arrow). Mitotic figures and apoptotic cells are present. A second population of less primitive but still overtly neoplastic cells is also present (blue arrow).
Fig. 10.
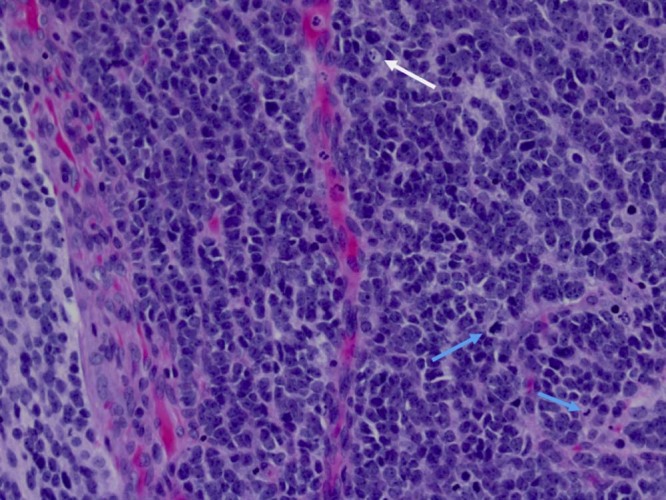
(20×) Higher power images highlight the mitotic activity (blue arrows), apoptosis (white arrow), microvascular proliferation, nuclear hyperchromasia, and atypia.
Postoperative CEMRI brain revealed no evidence of remnant enhancing lesion in the right cerebellar hemisphere and showed improvement of hydrocephalus and mass effect on the fourth ventricle when compared to the previous study. The postoperative course was uneventful, with rapid resolution of symptoms. Although medulloblastomas are radiosensitive, for children less than 3 years, radiotherapy is avoided. Adjuvant chemotherapy beginning 21 days postoperative to the resection is instead suggested. The patient was enrolled in the “Head Start 4” study [5].
Discussion
Medulloblastomas are the most common malignant brain tumor of childhood, accounting for 16%-20% of all childhood tumors [6], [7]. They are invasive embryonal tumors occurring almost exclusively in the posterior fossa involving cerebellum. Medulloblastomas were previously thought to represent a subset of primitive neuroectodermal tumors (PNETs) of the posterior fossa [8], but have recently been shown to be distinct via DNA microarray gene expression data [9]. The cell of origin is controversial, but is thought to originate from germinal cells or their remnant situated at the end of the posterior medullary velum or from the proliferating remnants of the external granular layer of the cerebellar hemispheres, including the flocculus [10], [11], [12].
The tumor typically presents as a contrast-enhancing mass midline in the cerebellar vermis, often compressing the fourth ventricle [13]. Zee et al. reported a study of 129 histologically verified tumors of the posterior fossa and determined that medulloblastoma frequently exhibits increased density compared with adjacent cerebellum and can be diagnosed with high likelihood when there is a central mass exhibiting well-demarcated borders [14]. Only 35 cases of medulloblastoma have been reported to be extra-axial in nature, most of which occur in the cerebellopontine angle [15], [16], [17]. The extra-axial finding alongside a purely hemispheric presentation of the tumor makes our patient's case quite rare in the pediatric population. It has been suggested that 50% of adult medulloblastomas arise from the surface of the cerebellum or pons in a lateral location. Histopathologically, medulloblastoma is a heterogenous, small, blue cell tumor. They are highly cellular, with abundant mitoses and little cytoplasmic differentiation. Neuroblastic Homer Wright rosettes are present in up to 40% of cases [18].
No clinical manifestations of medulloblastoma have been related specifically to location of the tumor. However, some characteristics of posterior fossa tumors such as progressive cerebellar signs and gait ataxia may be seen, although usually favoring an intra-axial location. Headache, nausea, and vomiting are less distinguishable symptoms commonly reported in medulloblastoma cases [17]. Additional differential diagnoses, such as posterior fossa epidermoid cyst, hemangioblastoma, pilocytic astrocytoma, and atypical tertoid-rhabdoid tumor, may also be included. Epidermoid cysts are uncommon benign tumors that can present in the posterior fossa in both adult and pediatric patients. They are often referred to as “pearly tumors,” considering their high lipid and cholesterol components that make them characteristically well-demarcated and hypodense on CT. They are further differentiated by cerebrospinal fluid intensity on MRI, including minimal peripheral enhancement and characteristic restricted diffusion on diffusion-weighted images [19]. Juvenile pilocytic astrocytomas (JPAs) often demonstrate pathognomonic mural nodule [20] enhancement within an infratentorial cystic lesion, and they can also be seen within the hypothalamus or along the optic pathways. In contrast to medulloblastoma, these tumors are hypoattenuated on unenhanced CT in view of ample cytoplasm and relatively less compact matrix [21]. These tumors are rarely seen in adulthood. In contrast, hemangioblastomas present in young and middle-aged adults and have nearly identical imaging characteristics to that of JPAs. Other distinctions from JPAs include their relatively high intrinsic vascularity as evidenced by high rCBV values on perfusion MRI, and if seen in multiples, their high association with Von Hippel-Lindau (VHL) syndrome [20], [21], [22]. Atypial teratoid-rhabdoid tumors constitute approximately 1%-2% of all pediatric central nervous system tumors and present as a heterogenous solid-cystic mass, occurring off-midline in children <3 years of age. They are commonly misdiagnosed as medulloblastoma owing to difficulty in distinction on radiology alone [23].
Treatment and prognosis of extra-axial medulloblastoma is undefined because of the small number of cases reported. Histologic variants, “classic” vs “desmoplastic,” were initially thought to provide some insight, but have subsequently been refuted [24]. Genetically defined molecular subgroups, via the World Health Organization, may play a better role in prognosis. Four distinct subgroups have been defined: sonic hedgehog (SHH), Wingless (WNT), group 3, and group 4. WNT has been shown to have the best prognosis, whereas group 3 tends to have the worst [25]. However, treatment is well-defined in all cases of medulloblastoma and consists of combined surgery with radiation therapy or chemotherapy in most patients [26]. Metastasis presents at diagnosis in one-third of children [27]. In contrast to nonmetastatic medulloblastoma, gold-standard treatment for metastatic medulloblastoma has not been well defined. A recent study by von Bueren et al. suggests that combined intensive non-high dose chemotherapy or autologous stem-cell transplantation with hyperfractionated, nonaccelerated radiotherapy is encouraging for improved 5-year survival and overall survival trends [28].
Conclusion
Medulloblastoma is an exceedingly common childhood tumor that nearly always presents intra-axially involving the fourth ventricle. Nonetheless, our case illustrates that an extra-axial enhancing mass found in the posterior fossa of a pediatric patient requires consideration of infrequent extra-axial medulloblastoma in differential diagnosis.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Smoll N.R., Drummond K.J. The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. J Clin Neurosci. 2012;19(11):1541–1544. doi: 10.1016/j.jocn.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Wolpert S.M. Mosby Year Book; St. Louis, MO: 1992. MRI in pediatric neuroradiology. [Google Scholar]
- 3.Zeltzer P.M., Boyett J.M., Finlay J.L., Albright A.L., Rorke L.B., Milstein J.M. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children's Cancer Group 921 randomized phase III study. J Clin Oncol. 1999;17(3):832–845. doi: 10.1200/JCO.1999.17.3.832. [DOI] [PubMed] [Google Scholar]
- 4.Rutkowski S., von Hoff K., Emser A., Zwiener I., Pietsch T., Figarella-Branger D. Survival and prognostic factors of early childhood medulloblastoma: an international meta-analysis. J Clin Oncol. 2010;28(33):4961–4968. doi: 10.1200/JCO.2010.30.2299. [DOI] [PubMed] [Google Scholar]
- 5.Head Start 4 Randomized Clinical Trial Nationwide Children's Hospital. 2015. http://www.nationwidechildrens.org/head-start-4 [accessed 1 September 2017]
- 6.Beygi S., Saadat S., Jazayeri S.B., Rahimi-Movaghar V. Epidemiology of pediatric primary malignant central nervous system tumors in Iran: a 10 year report of National Cancer Registry. Cancer Epidemiol. 2013;37(4):396–401. doi: 10.1016/j.canep.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Davis F.G., Kupelian V., Freels S., McCarthy B., Surawicz T. Prevalence estimates for primary brain tumors in the United States by behavior and major histology groups. Neuro Oncol. 2001;3(3):152–158. doi: 10.1093/neuonc/3.3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bigner D.D., McLendon R.E., Brunder J.M. Arnold; London: 1998. Russell and Rubenstein's pathology of tumors of the nervous system. [Google Scholar]
- 9.Pomeroy S.L., Tamayo P., Gaasenbeek M., Sturla L.M., Angelo M., McLaughlin M.E. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature. 2002;415(6870):436–442. doi: 10.1038/415436a. [DOI] [PubMed] [Google Scholar]
- 10.Jaiswal A.K., Mahapatra A.K., Sharma M.C. Cerebellopontine angle medulloblastoma. J Clin Neurosci. 2004;11(1):42–45. doi: 10.1016/j.jocn.2003.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Cugati G., Singh M., Symss N.P., Pande A., Chakravarthy V.M., Ramamurthi R. Extra-axial cerebello pontine angle medulloblastoma: a rare site of tumor. Indian J Med Paediatr Oncol. 2011;32(2):123–124. doi: 10.4103/0971-5851.89801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar R., Bhowmick U., Kalra S.K., Mahapatra A.K. Pediatric cerebellopontine angle medulloblastomas. J Pediatr Neurosci. 2008;3:127–130. [Google Scholar]
- 13.Poretti A., Meoded A., Huisman T.A. Neuroimaging of pediatric posterior fossa tumors including review of the literature. J Magn Reson Imaging. 2012;35(1):32–47. doi: 10.1002/jmri.22722. [DOI] [PubMed] [Google Scholar]
- 14.Zee C.S., Segall H.D., Miller C., Ahmadi J., McComb J.G., Han J.S. Less common CT features of medulloblastoma. Radiology. 1982;144(1):97–102. doi: 10.1148/radiology.144.1.6979760. [DOI] [PubMed] [Google Scholar]
- 15.Chung E.J., Jeun S.S. Extra-axial medulloblastoma in the cerebellar hemisphere. J Korean Neurosurg Soc. 2014;55(6):362–364. doi: 10.3340/jkns.2014.55.6.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fallah A., Banglawala S.M., Provias J., Jha N.K. Extra-axial medulloblastoma in the cerebellopontine angle. Can J Surg. 2009;52(4):E101–E102. [PMC free article] [PubMed] [Google Scholar]
- 17.Pant I., Chaturvedi S., Gautam V.K., Pandey P., Kumari R. Extra-axial medulloblastoma in the cerebellopontine angle: report of a rare entity with review of literature. J Pediatr Neurosci. 2016;11(4):331–334. doi: 10.4103/1817-1745.199477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleihues P., Burger P.C., Scheithauer B.W. Springer-Verlag; Berlin: 1993. Histological typing of tumors of the central nervous system. [Google Scholar]
- 19.Bohara M., Yonezawa H., Hanaya R., Takeshita S., Sumida M., Arita K. Posterior fossa epidermoid cysts presenting with unusual radiological appearances—two case reports. Neurol Med Chir (Tokyo) 2011;51(1):85–88. doi: 10.2176/nmc.51.85. [DOI] [PubMed] [Google Scholar]
- 20.Koeller K.K., Rushing E.J. From the archives of the AFIP: medulloblastoma: a comprehensive review with radiologic-pathologic correlation. Radiographics. 2003;23(6):1613–1637. doi: 10.1148/rg.236035168. [DOI] [PubMed] [Google Scholar]
- 21.Barkovich A.J. 3rd ed. Lippincott Williams & Wilkins; Philadelphia, Pa: 2000. Pediatric neuroimaging. [Google Scholar]
- 22.Borja M.J., Plaza M.J., Altman N., Saigal G. Conventional and advanced MRI features of pediatric intracranial tumors: supratentorial tumors. AJR Am J Roentgenol. 2013;200(5):W483–W503. doi: 10.2214/AJR.12.9724. [DOI] [PubMed] [Google Scholar]
- 23.Biswas A., Kashyap L., Kakkar A., Sarkar C., Julka P.K. Atypical teratoid/rhabdoid tumors: challenges and search for solutions. Cancer Manag Res. 2016;8:115–125. doi: 10.2147/CMAR.S83472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pramanik P., Sharma M.C., Mukhopadhyay P., Singh V.P., Sarkar C. A comparative study of classical vs. desmoplastic medulloblastomas. Neurol India. 2003;51(1):27–34. [PubMed] [Google Scholar]
- 25.Sure U., Berghorn W.J., Bertalanffy H., Wakabayashi T., Yoshida J., Sugita K. Staging, scoring and grading of medulloblastoma. A postoperative prognosis predicting system based on the cases of a single institute. Acta Neurochir (Wien) 1995;132(1–3):59–65. doi: 10.1007/BF01404849. [DOI] [PubMed] [Google Scholar]
- 26.Park T.S., Hoffman H.J., Hendrick E.B., Humphreys R.P., Becker L.E. Medulloblastoma: clinical presentation and management. Experience at the hospital for sick children, Toronto, 1950-1980. J Neurosurg. 1983;58(4):543–552. doi: 10.3171/jns.1983.58.4.0543. [DOI] [PubMed] [Google Scholar]
- 27.Packer R.J., Rood B.R., MacDonald T.J. Medulloblastoma: present concepts of stratification into risk groups. Pediatr Neurosurg. 2003;39(2):60–67. doi: 10.1159/000071316. [DOI] [PubMed] [Google Scholar]
- 28.von Bueren A.O., Kortmann R.D., von Hoff K., Friedrich C., Mynarek M., Müller K. Treatment of children and adolescents with metastatic medulloblastoma and prognostic relevance of clinical and biologic parameters. J Clin Oncol. 2016;34(34):4151–4160. doi: 10.1200/JCO.2016.67.2428. [DOI] [PubMed] [Google Scholar]