Abstract
Background
Studies suggest that the related proteins nucleoplasmin and nucleophosmin (also called B23, NO38 or numatrin) are nuclear chaperones that mediate the assembly of nucleosomes and ribosomes, respectively, and that these activities are accomplished through the binding of basic proteins via their acidic domains. Recently discovered and less well characterized members of this family of acidic phosphoproteins include mouse nucleophosmin/nucleoplasmin 3 (Npm3) and Xenopus NO29. Here we report the cloning and initial characterization of the human ortholog of Npm3.
Results
Human genomic and cDNA clones of NPM3 were isolated and sequenced. NPM3 lies 5.5 kb upstream of FGF8 and thus maps to chromosome 10q24-26. In addition to amino acid similarities, NPM3 shares many physical characteristics with the nucleophosmin/nucleoplasmin family, including an acidic domain, multiple potential phosphorylation sites and a putative nuclear localization signal. Comparative analyses of 14 members of this family from various metazoans suggest that Xenopus NO29 is a candidate ortholog of human and mouse NPM3, and they further group both proteins closer with the nucleoplasmins than with the nucleophosmins. Northern blot analysis revealed that NPM3 was strongly expressed in all 16 human tissues examined, with especially robust expression in pancreas and testis; lung displayed the lowest level of expression. An analysis of subcellular fractions of NIH3T3 cells expressing epitope-tagged NPM3 revealed that NPM3 protein was localized solely in the nucleus.
Conclusions
Human NPM3 is an abundant and widely expressed protein with primarily nuclear localization. These biological activities, together with its physical relationship to the chaparones nucleoplasmin and nucleophosmin, are consistent with the proposed function of NPM3 as a molecular chaperone functioning in the nucleus.
Background
The proper assembly of basic proteins with nucleic acids, such as occurs in the packaging of histones with DNA to produce chromatin and in the packaging of ribosomal proteins with rRNA to form ribosomes, is a reaction that must be facilitated so as to prevent the aggregation of these oppositely charged groups of molecules. Proteins that mediate these reactions, generally termed molecular (or nuclear) chaperones, have been identified biochemically. Two well studied proteins that participate in the processes of chromatin and ribosome assembly are nucleoplasmin and nucleophosmin, respectively, two related proteins whose characteristic acidic domains have been shown to bind the basic proteins involved in these processes and present them to the nucleic acid.
Nucleoplasmin is the most abundant protein in the Xenopus oocyte nucleus and is the protein for which the term molecular chaperone was coined due to its multiple roles in the assembly of nucleosomes during early frog development [1]. Nucleoplasmin forms a pentamer and its stretches of acidic residues bind to histone H2A and H2B. In concert with the unrelated acidic protein N1/N2, which binds histones H3 and H4, they act together and with other factors to assemble nucleosomes [2,3]. In addition to assembly, nucleoplasmin and other proteins are involved in the chromatin remodeling and nucleosome disassembly that occurs, for example, during transcription to allow the access of transcription factors to nucleosomal DNA [4]. Another major function of nucleoplasmin is the decondensation of sperm chromatin at fertilization. In this case, nucleoplasmin acts to exchange the sperm specific basic proteins, which allow the dense packing of DNA in sperm, with the histones H2A and H2B, thus effecting chromatin decondensation [5,6]. Phosphorylation of nucleoplasmin appears to be important in regulating this activity, as heavily phosphorylated nucleoplasmin is significantly more active [7].
Nucleophosmin (also called B23 [8], NO38 [9] or numatrin [10]), a protein related to nucleoplasmin, is implicated in ribosome assembly due to its abundance, localization in the nucleolus and its multiple activities that are consistent with such a function. Some of these activities include nucleic acid binding [11], ribonuclease activity (for processing preribosomal RNA) [12] and association with maturing preribosomal ribonucleoprotein particles [13,14]. It may also be involved in the transport of ribosomal or other nucleosomal proteins across the nuclear membrane, as it is known to shuttle between the cytoplasm and nucleus and to stimulate the nuclear importation of proteins [15,16]. Nucleophosmin also appears to be intimately involved in centrosome duplication. It associates specifically with unduplicated centrosomes, and its phosphorylation by CDK-2/cyclin E, the trigger for centrosome duplication, is required for duplication to occur [17]. The nucleophosmin gene is also known for its fusion with the anaplastic lymphoma kinase (ALK) receptor tyrosine kinase in cases of this disease with (2;5)(p23;q35) translocations [18]. The nucleophosmin portion contributes to transformation by providing a dimerization domain, which allows activation of the fused kinase and signal transduction [19].
We previously discovered and initially characterized a novel member of this family in the mouse, namely nucleophosmin/nucleoplasmin 3 (Npm3). To identify the human ortholog of Npm3 and begin its characterization, we have cloned a human NPM3 cDNA, determined its genomic structure and relationship to other family members and show its expression in multiple tissues and subcellular localization in the nucleus.
Results and Discussion
NPM3 cDNA cloning and genomic structure
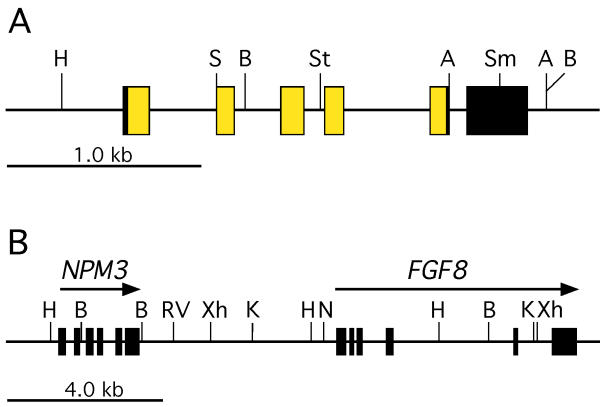
Mouse Npm3 is located approximately 5 kb upstream of the Fgf8 gene in the same transcriptional orientation [20,21]. To determine if human NPM3 is similarly located and to identify genomic clones of NPM3, we analyzed FGF8-containing human genomic lambda clones [22] by Southern blotting using a mouse Npm3 cDNA probe. A 4.3-kb Hind III-Xho I fragment that hybridized to Npm3 was identified, subcloned and used to screen a human liver cDNA library. Several partial cDNAs with strong sequence homology to mouse Npm3 were isolated. The 5' coding region was subsequently isolated using a reverse transcriptase-PCR approach and was fused to one of the partial cDNAs at a common restriction site to produce a cDNA with full coding potential. Sequencing of subcloned genomic fragments and comparison with the cDNA sequence allowed us to construct an exon map of the NPM3 gene (Figure 1A). NPM3 has the same exon structure as the mouse ortholog and is located approximately 5.5 kb upstream of FGF8, in the same transcriptional orientation (Figure 1B). The NPM3 exon/intron boundaries and organization information is presented in Table 1. Based on its close linkage to FGF8, NPM3 maps to chromosome 10q24-26 [23,24].
Figure 1.
NPM3 genomic structure and linkage to FGF8. (A) Genomic structure of NPM3. Boxed areas represent NPM3 exons. The yellow and black shadings represent translated and untranslated regions, respectively. (B) Linkage of NPM3 to FGF8. The location of genes and restriction map were determined by a combination of sequencing and restriction mapping of lambda clones and subclones in this study and a previous study [[22]]. An NPM3 cDNA probe will hybridize to restriction fragments of the following sizes in human genomic DNA: Avr II, 0.4 and 3.9 kb; Bam H I, 1.6 and 6.0 kb; Eco R V, 7.2 kb; Hind III, 6.8 kb; Kpn I, 6.6 kb; Sac I, 1.6 and 4.0 kb; Sma I, 1.0 and 5.1 kb; Stu I, 2.6 and 14.1 kb;Xho I, 8.4 kb. Restriction enzyme abbreviations: A, Avr II; B, Bam H I; H, Hind III; K, Kpn I; RV, Eco R V; N, Not I; S, Sac I; Sm, Sma I; St, Stu I; Xh, Xho I.
Table 1.
Exon/intron organization of the NPM3 gene
| Exon no. | Exon size (bp) | 5' Splice site* | Intron size (bp) | 3' Splice site* | Exon no. | Amino acid at splice** | ||
| 1 | 133 | GTTTTTTCTTCG | gtattagtgaag | 348 | tatttctaccag | GCTGTGAGCTCT | 2 | G40 |
| 2 | 87 | GCACTAACCATG | gtgaggggcagg | 241 | taccgttcccag | CTCTGCCTCACC | 3 | M68 |
| 3 | 120 | TGCCAACCCATG | gtgagttcccca | 126 | tcttcctcccag | CTCAGTCTGGAT | 4 | M108 |
| 4 | 94 | GGCACCAGATTG | gtgagaagaggg | 403 | tgtgtccaccag | TTACGATGAGCA | 5 | V140 |
| 5 | 128 | TAGCCCTCCTAG | gtgagttgcggg | 94 | tcctcttttcag | GTCAGCTCCATG | 6 | None |
| 6 | 308 | |||||||
*Exon bases are capitalized; intron bases are in lower case. **The amino acid codon that is split by, or located immediately before, the splice site is denoted.
NPM3 sequence analysis
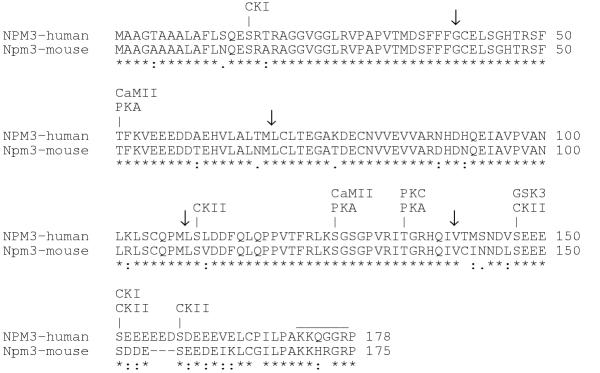
The amino acid sequence deduced from the human NPM3 cDNA sequence was 87% identical and 95% similar to mouse Npm3 (Figure 2). The human sequence is three amino acids longer than that of the mouse; these additional residues lie in the acidic domain in the C-terminal portion of the protein. At least eight potential serine and threonine phosphorylation sites are present in the human protein involving such kinases as casein kinase I and II, protein kinase A and C, glycogen synthase kinase 3 and calmodulin-dependent protein kinase II (Figure 2). All of these consensus sites are also found in mouse Npm3 with the exception of the casein kinase I site at residue 16. A cluster of amino acids at the C-terminus that is rich in basic residues and glycines forms a potential nuclear localization signal [25,26] in the human as well as the mouse protein (Figure 2). Multiple phosphorylation sites and nuclear localization signals, or the ability to bind proteins with such signals, are also characteristics of nucleophosmin and nucleoplasmin [16,27-29]. When the functions of NPM3 are elucidated, it will be of interest to determine the contribution of phosphorylation to these activities, since the functions of both nucleoplasmin and nucleophosmin are regulated by phosphorylation, which is extensive in both proteins [7,17]).
Figure 2.
Comparison and features of human NPM3 and mouse Npm3 amino acid sequences. Identical amino acids are denoted by an asterisk, highly similar residues by a colon and less similar residues by a period, as determined by CLUSTAL W software. The putative nuclear localization signal is overlined. Potential phosphorylation sites in NPM3 for the indicated kinases are shown, as predicted by NetPhos 2.0. Arrows denote splice points in the mRNA. Dashes represent gaps in the alignment. Kinase abbreviations: CKI, casein kinase I; CKII, casein kinase II; CaMII, calmodulin-dependent protein kinase II; GSK3, glycogen synthase kinase 3; PKA, protein kinase A; PKC, protein kinase C.
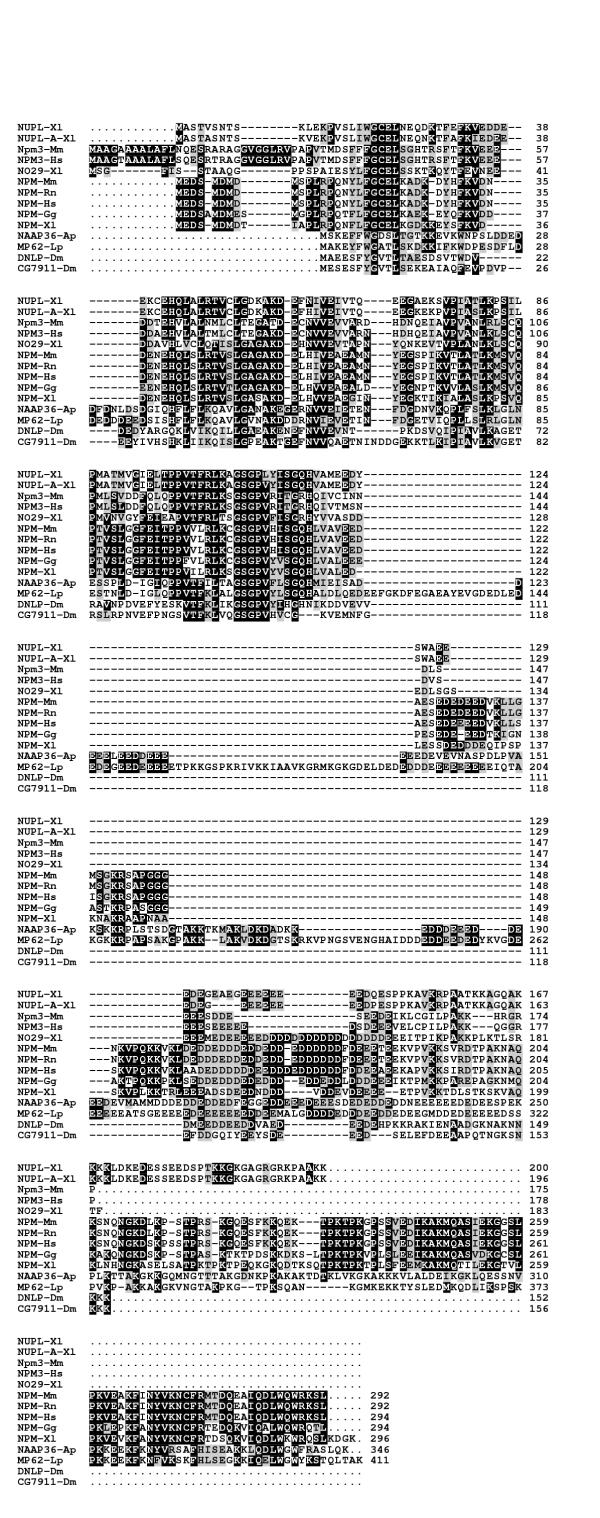
To enable an amino acid comparison between NPM3 with other members of the nucleophosmin/nucleoplasmin family, we searched the nonredundant GenBank database using human NPM3, Xenopus nucleoplasmin and human nucleophosmin as BLASTP queries, and 11 additional full-length proteins were retrieved (Table 2). An amino acid comparison of NPM3 with these other members of the nucleophosmin/nucleoplasmin family from various metazoans reveals extensive sequence identities and similarities throughout all of NPM3 except the C-terminal 16 residues (Figure 3). All members of this family have a core region of relatively close similarity in the N-terminal half of the proteins. C-terminal to this region, all have one or more acidic domains consisting of a total of 17 to more than 100 aspartic acid and glutamic acid residues per molecule. These two residues can comprise more than 25% of the total amino acids in some proteins of this family; in NPM3 they make up approximately 18% of the residues. The N-terminal core region of high similarity between family members correlates to a region in nucleophosmin/B23 that has been shown to be involved in oligomerization as well as chaperone activity [44]. A central portion between nucleophosmin's two acidic domains is required for ribonuclease activity [44]; this region does not have a corresponding domain in either NPM3 or nucleoplasmin, suggesting that these proteins would lack such activity. A nucleic acid binding domain in the C-terminus of nucleophosmin is also lacking in NPM3 and nucleoplasmin, but the known functions of nucleoplasmin in nucleosome assembly and sperm decondensation suggest that this protein, and possibly NPM3, can accomplish intermolecular reactions involving nucleic acids in other ways, perhaps by associating with other proteins that have this binding activity. Indeed, NO29, a Xenopus protein with significant si milarity to NPM3, is found to associate with NO38, the Xenopus ortholog of mammalian nucleophosmin [43].
Table 2.
Accession numbers of amino acid sequences
| Symbol* | Name(s) | Species | Accession No. | Ref. No. |
| NUPL-X1 | Nucleoplasmin | Xenopus laevis | CAA28460 | [34] |
| NUPL-A-X1 | Nucleoplasmin A | Xenopus laevis | CAA68363 | [31] |
| Npm3-mm | Nucleophosmin/nucleoplasmin-3 | Mus musculus | NP_032749 | [21] |
| NPM3-Hs | Nucleophosmin/nucleoplasmin-3 | Homo sapiens | AY049737 | this study |
| NO29-X1 | NO29; NOVA | Xenopus laevis | CAB06652 | [43] |
| NPM-Mm | Nucleophosmin; NO38; B23; Numatrin | Mus musculus | NP_032748 | [40] |
| NPM-Rn | Nucleophosmin; NO38; B23; Numatrin | Rattus norvegicus | P13084 | [33] |
| NPM-Hs | Nucleophosmin; NO38; B23; Numatrin | Homo sapiens | NP_002511 | [32] |
| NPM-Gg | Nucleophosmin; NO38; B23; Numatrin | Gallus gallus | P16039 | [37] |
| NPM-X1 | Nucleophosmin; NO38; B23; Numatrin | Xenopus laevis | A41730 | [39] |
| NAAP36-Ap | Nucleic acid-associated protein 36; ANO39 | Asterina pectinifera | BAA90827 | [38] |
| MP62-Lp | Mitotic apparatus protein p62 | Lytechinus pictus | P91753 | [42] |
| DNLP-Dm | Nucleoplasmin-like protein | Drosophila melanogaster | Q27415 | [35] |
| CG7911-Dm | CG7911 gene product | Drosophila melanogaster | AAF56987 | [30] |
| N1/N2-X1 | Nuclear histone-binding protein N1/N2 | Xenopus laevis | A25680 | [36] |
| NASP-Hs | Nuclear autoantigenic sperm protein | Homo sapiens | NP_002473 | [41] |
*Symbol as used in this study
Figure 3.
A comparison of nucleophosmin/nucleoplasmin family members from metazoans. The alignment was made with CLUSTAL W. Aligned residues that were identical or similar in at least 50% of the sequences, excluding gaps, were shaded with a black or gray background, respectively; additionally, any residues that were similar to a block of identical residues were shaded in gray. Similar amino acids were grouped as follows: I, L, M, V; F, W, Y; H, K, R; D, E; N, Q; A, G; S, T; P; C. The names, species, accession numbers and references for these sequences are presented in Table 2.
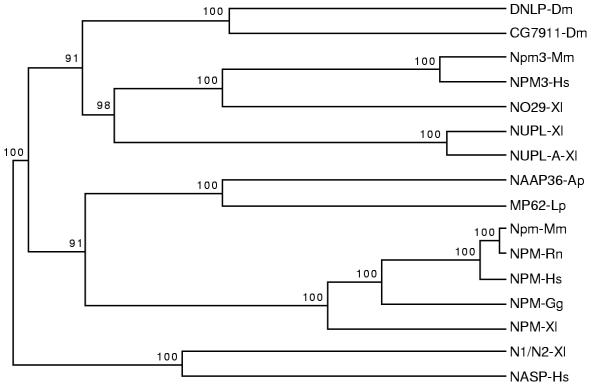
To gain further insight into the relationship of NPM3 with the other known members of this family, we used CLUSTAL W and TreeTop [45] to produce a dendrogram of these relationships (Figure 4). In this analysis, we included the 14 proteins from Figure 3 together with two other histone-binding proteins that are unrelated to this family. These two proteins, Xenopus N1/N2 [36] and human NASP (nuclear autoantigenic sperm protein) [41], are closely related to each other and may be orthologs [46]. This analysis shows that NPM3 is more closely related to NO29 and the nucleoplasmins from Xenopus than to the nucleophosmins from multiple species. No nucleoplasmin ortholog in humans has been reported to date, and our screening of GenBank has not detected such a sequence. The relationship between N1/N2 and NASP can be seen in this dendrogram and appears similar in closeness to that of NPM3 and NO29. The kinship of NPM3 and NO29 is supported by recent GenBank screens with the NPM3 amino acid sequence as a TBLASTN query against the entire nonredundant GenBank database, which resulted in NO29 sequences as the best non-NPM3 match, and vice versa. Although other genomic and functional studies would be required to prove an orthologous relationship between NPM3 and NO29, they appear by several analyses to share a relatively close evolutionary history and as such could be considered candidate orthologs.
Figure 4.
Dendrogram of nucleophosmin/nucleoplasmin family members. Amino acid sequences from the 16 proteins shown were aligned with CLUSTAL W, and a dendrogram was produced using the TreeTop algorithm as described in Materials and Methods. The 16 sequences included 14 from the nucleophosmin/nucleoplasmin family (see Table 2 and Figure 3) and two other histone-binding proteins (NASP and N1/N2), which are closely related to each other but unrelated to the nucleophosmin/nucleoplasmin family. Bootstrap significance values are shown at the branchpoints for 100 resamplings. The analysis suggests that NPM3 is more related to nucleoplasmins than to nucleophosmins and that NPM3 is closely related to Xenopus NO29.
NPM3 expression in human tissues
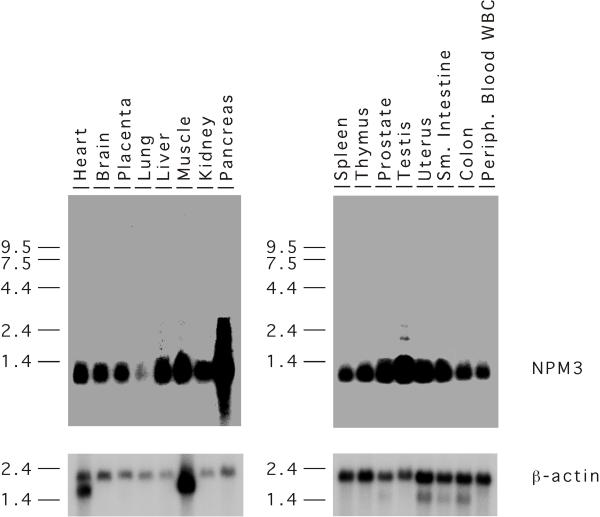
We examined the expression of NPM3 in 16 human tissues by Northern blot analysis (Figure 5). Abundant and relatively equal expression of NPM3 was found in all tissues with the highest levels, relative to actin, in pancreas and testis and the lowest in lung. Previous analysis of Npm3 expression in mouse tissues also showed generally equal and strong expression in the tissues tested with especially strong expression in testis [21]. However, in the mouse, the brain, rather than the lung, displayed the lowest Npm3 RNA levels. Nevertheless, the widespread and relatively strong expression of the gene in both species suggests that the protein has a fundamental function(s) in cells of many, if not all, tissues. The apparently ubiquitous expression and abundant levels of NPM3 RNA are consistent with other members of this gene family and with a proposed role as a molecular chaperone.
Figure 5.
Northern blot expression analysis of NPM3 in human tissues. Upper panels, hybridization with the human NPM3 cDNA probe. Lower panels, hybridization with the mouse beta-actin cDNA probe. The location of the molecular weight standards in kb is indicated to the left of the blots.
NPM3 protein localizes to the nucleus
All of the nucleophosmin and nucleoplasmin homologs that have been studied to date are localized in the nucleus. To allow a determination of the subcellular localization of the NPM3 protein, we tagged the protein at its N-terminus with a hemagglutinin (HA) epitope. We then expressed this protein in NIH3T3 cells, fractionated the cells into nuclear, cytoplasmic and extracellular (culture medium) fractions and analyzed these by immunoblotting using an anti-HA antibody. This analysis revealed that NPM3 was localized only in the nuclear fraction (Figure 6). The NPM3 protein migrated in SDS-PAGE gels as a doublet with apparent molecular masses of 25 and 27 kDa, which are larger than the 20.5 kDa mass predicted by the deduced amino acid sequence including the HA tag. Similarly, other members of the nucleophosmin/nucleoplasmin family also migrate more slowly than expected: Xenopus NO29 migrates at 29 kDa versus the expected 20 kDa [44], Xenopus NO38 migrates at 38 kDa versus the expected 33.5 kDa [9], and Xenopus nucleoplasmin migrates at 38 kDa rather than 33.5 kDa [9]. The slower migration is likely primarily due to an electrophoretic anomaly reflecting the amino acid composition, although phosphorylation or other modifications could conceivably contribute. Supporting this notion, the in vitro transcription/translation of a plasmid encoding the closely related NO29 protein produces a product, presumably unmodified, with slow mobility that is similar to the mobility of NO29 extracted from cells [43]. The smaller-sized polypeptide of the NPM3 doublet is probably a degradation product, as is observed in analyses of the NO29 protein [43]. This subcellular localization of NPM3, together with the other results here, suggests that this protein is a molecu lar chaperone with functions in the nucleus.
Figure 6.
Immunoblot analysis of the cellular localization of HA-tagged NPM3. Protein samples (50 mg) were subjected to SDS-PAGE, electrophoretic transfer to nitrocellulose and immunoblotting with an anti-HA antibody. Media, cytoplasm and nucleus are the subcellular fractions analyzed. The locations of the molecular weight standards in kDa are indicated to the left of the blot. Arrows indicate bands associated with HA-tagged NPM3. (A) cells mock-transfected; (B) cells transfected with antisense HA-tagged NPM3 cDNA construct; (C) cells transfected with sense HA-tagged NPM3 cDNA construct.
Conclusions
The striking similarities in amino acid sequence and domain structure between NPM3 and its chaperone relatives, nucleophosmin and nucleoplasmin, together with abundant expression levels and nuclear localization, strongly suggests that NPM3 shares fundamental nuclear chaperone functions with these proteins. It will be important in future work to refine these results and test, for example, whether NPM3 may have chromatin or ribosome assembly functions that are similar or complementary to nucleoplasmin or nucleophosmin, or whether it may have completely independent functions.
Materials and methods
Isolation of NPM3 genomic probes
We digested previously isolated lambda genomic clones of the human FGF8 region [22] with restriction endonucleases (Promega, Madison, WI) and analyzed them by Southern blotting using a mouse Npm3 cDNA [21] probe, as previously described [20]. An 8.4-kb Xho I fragment from these clones that hybridized to the probe was subcloned into pBluescript (Stratagene) for analysis. Deletion of non-Npm3 sequences from this clone resulted in a 4.3-kb Hind III-Xho I fragment, which was then isolated and used as a probe for the isolation of a human NPM3 cDNA.
Isolation of NPM3 cDNA
The 4.3-kb Hind III-Xho I human genomic fragment described above was used as a probe to screen a λgt11 human liver cDNA library for the NPM3 cDNA. Three positive clones were obtained from 250,000 plaques. Following isolation of pure plaques, the insert DNAs were prepared by PCR methods with the following conditions: 100 μl reactions containing 1X Pfu buffer (20 mM Tris-Cl, pH 8.75, 10 mM KCl, 2 mM MgSO4, 10 mM (NH4)2SO4, 0.1% v/v Triton X-100, 100 μg/ml bovine serum albumin), 1 μM λgt11 forward and reverse primers (Promega, Madison, WI), 0.2 mM deoxyribonucleotides, and 5 Units of recombinant Pfu DNA Polymerase (Stratagene, La Jolla, CA). The thermocycling conditions were as follows: 95°C for 3 minutes, then 30 cycles of 95°C for 45 seconds, 50°C for 30 seconds and 75°C for 60 seconds, then 75°C for 10 minutes, using a PTC-100 Thermocycler (M.J. Research, Watertown, MA). The resulting cDNA inserts were purified by agarose gel electrophoresis, digested with EcoR I and cloned into pBluescript KS- (Stratagene, La Jolla, CA). The cDNA inserts were thermocycle-sequenced using the fmol kit (Promega, Madison, WI) and found to be identical and to lack a portion of the 5' coding region.
To obtain the 5' end of the NPM3 cDNA, we performed 5' Rapid Amplification of cDNA Ends (RACE), using a Marathon-Ready™ human testis cDNA library (Clontech, Palo Alto, CA) and the Marathon™ cDNA amplification kit (Clontech, Palo Alto, CA). The RACE conditions were as follows: 50 μl reactions containing 1X KlenTaq buffer and Advantage KlenTaq Polymerase Mix (Clontech, Palo Alto, CA), 0.2 mM deoxyribonucleotides, 0.2 μM NPM3 GSP1 (5'-CGG TGA GGC AGA GCA TGG TTA GTG C-3'), and 0.2 μM API primer (Clontech, Palo Alto, CA). Touchdown PCR conditions were employed as follows: 95°C for 5 minutes, then 5 cycles of 95°C for 10 seconds, 72°C for 2 minutes, then 5 cycles of 95°C for 10 seconds, 70°C for 2 minutes, then 25 cycles of 95°C for 10 seconds, 68°C for 2 minutes. The resulting band was purified by agarose gel electrophoresis and subcloned into pCR II (Invitrogen, Carlsbad, CA). Multiple clones were thermocycle sequenced to confirm the 5' sequence of human NPM3 cDNA. Finally, to produce a full-length human NPM3 cDNA, we spliced together the 5' RACE NPM3 cDNA with the original partial cDNA obtained from the lambda library at a unique Sac I site present in both fragments. The final cDNA sequence was submitted to GenBank (accession number AY049737).
Determination of exon structure
Fragments of the 4.3-kb Hind III-Xho I NPM3 genomic DNA isolated above that hybridized to a mouse Npm3 cDNA probe were further subcloned into pBS and sequenced using an automated ABI 377 sequencer. Comparison of these compiled sequences with the human NPM3 cDNA sequence allowed the localization of exon sequences within the genomic DNA. The genomic sequence and exon placement was later confirmed with human genome sequences that subsequently appeared in GenBank (accession number AC010789).
Sequence analysis
Amino acid sequences were aligned using CLUSTAL W (v. 1.81) with default parameters on a European Molecular Biology Laboratory web server http://www.ebi.ac.uk. An alignment output from this source was used to create a dendrogram using TreeTop [45] with PHYLIP output (default parameters) at a node of the European Molecular Biology Network http://www.genebee.msu.su[47]. Potential phosphorylation sites were identified using NetPhos 2.0 http://www.cbs.dtu.dk/services/NetPhos[48]. After alignment with CLUSTAL W, identical and similar amino acids in Figure 3 were identified using MacBoxshade 2.15 http://www.isrec.isb-sib.ch/ftp-server/boxshade/MacBoxshade.
Northern blot analysis
Human tissue northern blots of poly(A)+ RNA were obtained from Clontech (Palo Alto, CA) and sequentially hybridized with radiolabeled NPM3 cDNA and beta-actin probes as previously described [20].
Hemagglutinin-tagged NPM3
A cDNA that encodes a hemagglutinin-tagged NPM3 protein was produced by PCR under the following conditions: 100 μl reactions with 1X Pfu buffer, 0.2 mM deoxyribonucleotides, 1 μM HA-F primer (5'-AAA GAA TTC AGC ATG TAC CCA TAC GAC GTC CCA GAC TAC GCC GCC GCC GGT ACT GCA GCT GCC-3'), 1 μM NPM3-R2 primer (5'-AAA GAA TTC CTA GGG CCT GCC CCC CTG CTT TTT GGC AGG AAG GAT GGG-3'), 1 ng of human NPM3 cDNA insert, and 2.5 Units of recombinant Pfu DNA Polymerase. The thermocycling conditions were as follows: 95°C for 3 minutes, then 30 cycles of 95°C for 45 seconds, 75°C for 60 seconds, then 75°C for 10 minutes, using a PTC-100 Thermocycler (M.J. Research, Watertown, MA). The resulting DNA fragment was purified by agarose gel electrophoresis, cleaved with EcoR I, and subcloned into pBluescript KS-. The resulting plasmids were thermocycle sequenced, and an insert with the correct sequence was identified and subcloned into pMIRB [49]. The orientations of the resulting inserts were determined by restriction digests with Sac I.
Cellular localization of HA-NPM3 by cell fractionation and immunoblotting
The resulting pMIRB-HA-NPM3 plasmids (both sense and antisense orientations) were transiently transfected into NIH 3T3 cells, using transfection conditions with Lipofectamine and OptiMEM serum-free medium (Gibco-BRL, Bethesda, MD) as described [49]. Following a six-hour incubation of the DNA-Lipofectamine complexes in OptiMEM, the cells were washed and incubated in 10-cm dishes with their usual growth media (DMEM with 10% v/v fetal calf serum, 2 mM L-glutamine, 100 Units/ml of Penicillin G and 100 μg/ml Streptomycin) for 48–72 hours at 37°C in humidified 5% CO2 incubators.
Following the 48–72 hour incubation, the media was collected and placed on ice with the following protease inhibitors added: 1 mM DTT (Sigma, St. Louis, MO), 0.5 mM PMSF (Sigma, St. Louis, MO), 5 μg/ml Pepstatin (Sigma, St. Louis, MO), 3 μg/ml Leupeptin (Sigma, St. Louis, MO) and 5 μg/ml Aprotinin (Sigma, St. Louis, MO). The cells were washed 3X in cold PBS and collected by cell scrapers (Nunc) in 1 ml of cold PBS. The cells were transferred to microfuge tubes, pelleted (5 seconds at 14,000 rpm, Eppendorf 5415C, 4°C) and resuspended in hypotonic Buffer A (10 mM K-HEPES pH 7.9, 1.5 mM MgCl2, and 10 mM KCl, with inhibitors added as for the media above) for 15 minutes on ice. The suspension was vortexed vigorously for 10 seconds to lyse the cells, and then microcentrifuged (1 minute at 14,000 rpm, Eppendorf 5415C, 4°C). The resulting supernatant was called "cytoplasm" but actually contained cytoplasmic membranes as well. The resulting nuclear pellet was resuspended in 20 μl of Buffer C (20 mM K-HEPES pH 7.9, 1.5 mM MgCl2, 0.2 mM EDTA, 20% v/v glycerol, and 0.42 M NaCl, with protease inhibitors as above), incubated on ice for 20 minutes, then microcentrifuged (5 minutes at 14,000 rpm, Eppendorf 5415C, 4°C). This final supernatant was the nuclear extract.
The protein solutions were quantified by Bradford Assay (Biorad, Hercules, CA), and 50 mg of each sample were subjected to SDS-PAGE (15% acrylamide). Following electrophoresis, the gel contents were electrophoretically-transferred to nitrocellulose (500 mA, 60–90 V, 1 hour, 4°C, Transfer Buffer II from Harlow and Lane [50]). The membrane was blocked with 5% w/v nonfat dried milk in PBS-0.1% v/v Tween 20 (PBS-T) overnight at 4°C, then subjected to immunoblotting and ECL (Amersham, Arlington, IL) detection. The primary antibody was monoclonal mouse anti-hemagglutinin (dilution 1:1000 in PBS-T, gift of Guojon Bu, Washington University, St. Louis), and the secondary antisera was horseradish peroxidase-conjugated sheep anti-mouse IgG (dilution 1:1000 in PBS-T, Amersham, Arlington, IL).
Acknowledgments
Acknowledgements
This work was supported by grants to G.M.S. from the Department of Defense Breast Cancer Research Program (DAMD 17-96-1-6039) and the T.J. Martell Foundation for Leukemia, Cancer and AIDS, and by grants to C.A.M. from the National Institutes of Health (CA70106), Edward G. Mallinckrodt, Jr., Foundation, and the Elsa U. Pardee Foundation.
Contributor Information
Gregory M Shackleford, Email: shacklef@hsc.usc.edu.
Amit Ganguly, Email: aganguly@ucla.edu.
Craig A MacArthur, Email: cmacar@bellsouth.net.
References
- Laskey RA, Honda BM, Mills AD, Finch JT. Nucleosomes are assembled by an acidic protein which binds histones and transfers them to DNA. Nature. 1978;275:416–420. doi: 10.1038/275416a0. [DOI] [PubMed] [Google Scholar]
- Dingwall C, Laskey RA. Nucleoplasmin: the archetypal molecular chaperone. Semin Cell Biol. 1990;1:11–17. [PubMed] [Google Scholar]
- Philpott A, Krude T, Laskey RA. Nuclear chaperones. Semin Cell Dev Biol. 2000;11:7–14. doi: 10.1006/scdb.1999.0346. [DOI] [PubMed] [Google Scholar]
- Chen H, Li B, Workman JL. A histone-binding protein, nucleoplasmin, stimulates transcription factor binding to nucleosomes and factor-induced nucleosome disassembly. EMBO J. 1994;13:380–390. doi: 10.1002/j.1460-2075.1994.tb06272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsumi K, Katagiri C. Characterization of the ooplasmic factor inducing decondensation of and protamine removal from toad sperm nuclei: involvement of nucleoplasmin. Dev Biol. 1991;148:295–305. doi: 10.1016/0012-1606(91)90338-4. [DOI] [PubMed] [Google Scholar]
- Philpott A, Leno GH, Laskey RA. Sperm decondensation in Xenopus egg cytoplasm is mediated by nucleoplasmin. Cell. 1991;65:569–578. doi: 10.1016/0092-8674(91)90089-H. [DOI] [PubMed] [Google Scholar]
- Leno GH, Mills AD, Philpott A, Laskey RA. Hyperphosphorylation of nucleoplasmin facilitates Xenopus sperm decondensation at fertilization. J Biol Chem. 1996;271:7253–7256. doi: 10.1074/jbc.271.13.7253. [DOI] [PubMed] [Google Scholar]
- Chan PK, Aldrich M, Cook RG, Busch H. Amino acid sequence of protein B23 phosphorylation site. J Biol Chem. 1986;261:1868–1872. [PubMed] [Google Scholar]
- Schmidt-Zachmann MS, Hugle-Dorr B, Franke WW. A constitutive nucleolar protein identified as a member of the nucleoplasmin family. EMBO J. 1987;6:1881–1890. doi: 10.1002/j.1460-2075.1987.tb02447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein N, Mond JJ. "Numatrin," a nuclear matrix protein associated with induction of proliferation in B lymphocytes. J Biol Chem. 1987;262:11389–11397. [PubMed] [Google Scholar]
- Dumbar TS, Gentry GA, Olson MO. Interaction of nucleolar phosphoprotein B23 with nucleic acids. Biochemistry. 1989;28:9495–9501. doi: 10.1021/bi00450a037. [DOI] [PubMed] [Google Scholar]
- Herrera JE, Savkur R, Olson MO. The ribonuclease activity of nucleolar protein B23. Nucleic Acids Res. 1995;23:3974–3979. doi: 10.1093/nar/23.19.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson MO, Wallace MO, Herrera AH, Marshall-Carlson L, Hunt RC. Preribosomal ribonucleoprotein particles are a major component of a nucleolar matrix fraction. Biochemistry. 1986;25:484–491. doi: 10.1021/bi00350a031. [DOI] [PubMed] [Google Scholar]
- Prestayko AW, Klomp GR, Schmoll DJ, Busch H. Comparison of proteins of ribosomal subunits and nucleolar preribosomal particles from Novikoff hepatoma ascites cells by two-dimensional polyacrylamide gel electrophoresis. Biochemistry. 1974;13:1945–1951. doi: 10.1021/bi00706a026. [DOI] [PubMed] [Google Scholar]
- Borer RA, Lehner CF, Eppenberger HM, Nigg EA. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell. 1989;56:379–390. doi: 10.1016/0092-8674(89)90241-9. [DOI] [PubMed] [Google Scholar]
- Szebeni A, Herrera JE, Olson MO. Interaction of nucleolar protein B23 with peptides related to nuclear localization signals. Biochemistry. 1995;34:8037–8042. doi: 10.1021/bi00025a009. [DOI] [PubMed] [Google Scholar]
- Okuda M, Horn HF, Tarapore P, Tokuyama Y, Smulian AG, Chan PK, Knudsen ES, Hofmann IA, Snyder JD, Bove KE, Fukasawa K. Nucleophosmin/B23 is a target of CDK2/cyclin E in centrosome duplication. Cell. 2000;103:127–140. doi: 10.1016/S0092-8674(00)00093-3. [DOI] [PubMed] [Google Scholar]
- Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, Look AT. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science. 1994;263:1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- Bischof D, Pulford K, Mason DY, Morris SW. Role of the nucleophosmin (NPM) portion of the non-Hodgkin's lymphoma- associated NPM-anaplastic lymphoma kinase fusion protein in oncogenesis. Mol Cell Biol. 1997;17:2312–2325. doi: 10.1128/mcb.17.4.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur CA, Shankar DB, Shackleford GM. Fgf-8, activated by proviral insertion, cooperates with the Wnt-1 transgene in murine mammary tumorigenesis. J Virol. 1995;69:2501–2507. doi: 10.1128/jvi.69.4.2501-2507.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur CA, Shackleford GM. Npm3: a novel, widely expressed gene encoding a protein related to the molecular chaperones nucleoplasmin and nucleophosmin. Genomics. 1997;42:137–140. doi: 10.1006/geno.1997.4353. [DOI] [PubMed] [Google Scholar]
- Gemel J, Gorry M, Ehrlich GD, MacArthur CA. Structure and sequence of human FGF8. Genomics. 1996;35:253–257. doi: 10.1006/geno.1996.0349. [DOI] [PubMed] [Google Scholar]
- White RA, Dowler LL, Angeloni SV, Pasztor LM, MacArthur CA. Assignment of FGF8 to human chromosome 10q25-q26: mutations in FGF8 may be responsible for some types of acrocephalosyndactyly linked to this region. Genomics. 1995;30:109–111. doi: 10.1006/geno.1995.0020. [DOI] [PubMed] [Google Scholar]
- Yoshiura K, Leysens NJ, Chang J, Ward D, Murray JC, Muenke M. Genomic structure, sequence, and mapping of human FGF8 with no evidence for its role in craniosynostosis/limb defect syndromes. Am J Med Genet. 1997;72:354–362. doi: 10.1002/(SICI)1096-8628(19971031)72:3<354::AID-AJMG21>3.3.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Bonifaci N, Moroianu J, Radu A, Blobel G. Karyopherin beta2 mediates nuclear import of a mRNA binding protein. Proc Natl Acad Sci USA. 1997;94:5055–5060. doi: 10.1073/pnas.94.10.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulikas T. Nuclear localization signals. Crit Rev Eukaryot Gene Expr. 1993;3:193–227. [PubMed] [Google Scholar]
- Cotten M, Sealy L, Chalkley R. Massive phosphorylation distinguishes Xenopus laevis nucleoplasmin isolated from oocytes or unfertilized eggs. Biochemistry. 1986;25:5063–5069. doi: 10.1021/bi00366a014. [DOI] [PubMed] [Google Scholar]
- Dingwall C, Robbins J, Dilworth SM. Characterisation of the nuclear location sequence of Xenopus nucleoplasmin. J Cell Sci Suppl. 1989;11:243–248. doi: 10.1242/jcs.1989.supplement_11.18. [DOI] [PubMed] [Google Scholar]
- Mamrack MD, Olson MO, Busch H. Negatively charged phosphopeptides of nucleolar nonhistone proteins from Novikoff hepatoma ascites cells. Biochem Biophys Res Commun. 1977;76:150–157. doi: 10.1016/0006-291X(77)91680-1. [DOI] [PubMed] [Google Scholar]
- Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, George RA, Lewis SE, Richards S, Ashburner M, Henderson SN, Sutton GG, Wortman JR, Yandell MD, Zhang Q, Chen LX, Brandon RC, Rogers YH, Blazej RG, Champe M, Pfeiffer BD, Wan KH, Doyle C, Baxter EG, Helt G, Nelson CR, Gabor GL, Abril JF, Agbayani A, An HJ, Andrews-Pfannkoch C, Baldwin D, Ballew RM, Basu A, Baxendale J, Bayraktaroglu L, Beasley EM, Beeson KY, Benos PV, Berman BP, Bhandari D, Bolshakov S, Borkova D, Botchan MR, Bouck J, Brokstein P, Brottier P, Burtis KC, Busam DA, Butler H, Cadieu E, Center A, Chandra I, Cherry JM, Cawley S, Dahlke C, Davenport LB, Davies P, de Pablos B, Delcher A, Deng Z, Mays AD, Dew I, Dietz SM, Dodson K, Doup LE, Downes M, Dugan-Rocha S, Dunkov BC, Dunn P, Durbin KJ, Evangelista CC, Ferraz C, Ferriera S, Fleischmann W, Fosler C, Gabrielian AE, Garg NS, Gelbart WM, Glasser K, Glodek A, Gong F, Gorrell JH, Gu Z, Guan P, Harris M, Harris NL, Harvey D, Heiman TJ, Hernandez JR, Houck J, Hostin D, Houston KA, Howland TJ, Wei MH, Ibegwam C, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- Burglin TR, Mattaj IW, Newmeyer DD, Zeller R, De Robertis EM. Cloning of nucleoplasmin from Xenopus laevis oocytes and analysis of its developmental expression. Genes Dev. 1987;1:97–107. doi: 10.1101/gad.1.1.97. [DOI] [PubMed] [Google Scholar]
- Chan WY, Liu QR, Borjigin J, Busch H, Rennert OM, Tease LA, Chan PK. Characterization of the cDNA encoding human nucleophosmin and studies of its role in normal and abnormal growth. Biochemistry. 1989;28:1033–1039. doi: 10.1021/bi00429a017. [DOI] [PubMed] [Google Scholar]
- Chang JH, Dumbar TS, Olson MO. cDNA and deduced primary structure of rat protein B23, a nucleolar protein containing highly conserved sequences. J Biol Chem. 1988;263:12824–12827. [PubMed] [Google Scholar]
- Dingwall C, Dilworth SM, Black SJ, Kearsey SE, Cox LS, Laskey RA. Nucleoplasmin cDNA sequence reveals polyglutamic acid tracts and a cluster of sequences homologous to putative nuclear localization signals. EMBO J. 1987;6:69–74. doi: 10.1002/j.1460-2075.1987.tb04720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Tyler JK, Bulger M, Kobayashi R, Kadonaga JT. ATP-facilitated chromatin assembly with a nucleoplasmin-like protein from Drosophila melanogaster. J Biol Chem. 1996;271:25041–25048. doi: 10.1074/jbc.271.40.25041. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt JA, Dingwall C, Maier G, Franke WW. Molecular characterization of a karyophilic, histone-binding protein: cDNA cloning, amino acid sequence and expression of nuclear protein N1/N2 of Xenopus laevis. EMBO J. 1986;5:3547–3552. doi: 10.1002/j.1460-2075.1986.tb04681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maridor G, Nigg EA. cDNA sequences of chicken nucleolin/C23 and NO38/B23, two major nucleolar proteins. Nucleic Acids Res. 1990;18:1286. doi: 10.1093/nar/18.5.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima H, Matoba K, Matsumoto Y, Hongo T, Kiritaka K, Sugino H, Nagamatsu Y, Hamaguchi Y, Ikegami S. Molecular characterization of a novel nucleolar protein in starfish oocytes which is phosphorylated before and during oocyte maturation. Eur J Biochem. 2000;267:295–304. doi: 10.1046/j.1432-1327.2000.00931.x. [DOI] [PubMed] [Google Scholar]
- Peculis BA, Gall JG. Localization of the nucleolar protein NO38 in amphibian oocytes. J Cell Biol. 1992;116:1–14. doi: 10.1083/jcb.116.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Zachmann MS, Franke WW. DNA cloning and amino acid sequence determination of a major constituent protein of mammalian nucleoli. Correspondence of the nucleoplasmin-related protein NO38 to mammalian protein B23. Chromosoma. 1988;96:417–426. doi: 10.1007/BF00303035. [DOI] [PubMed] [Google Scholar]
- Welch JE, Zimmerman LJ, Joseph DR, O'Rand MG. Characterization of a sperm-specific nuclear autoantigenic protein. I. Complete sequence and homology with the Xenopus protein, Biol Reprod. 1990;43:559–568. doi: 10.1095/biolreprod43.4.559. [DOI] [PubMed] [Google Scholar]
- Ye X, Sloboda RD. Molecular characterization of p62, a mitotic apparatus protein required for mitotic progression. J Biol Chem. 1997;272:3606–3614. doi: 10.1074/jbc.272.6.3606. [DOI] [PubMed] [Google Scholar]
- Zirwes RF, Schmidt-Zachmann MS, Franke WW. Identification of a small, very acidic constitutive nucleolar protein (NO29) as a member of the nucleoplasmin family. Proc Natl Acad Sci USA. 1997;94:11387–11392. doi: 10.1073/pnas.94.21.11387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani K, Szebeni A, Olson MO. Mapping the functional domains of nucleolar protein B23. J Biol Chem. 2000;275:24451–24457. doi: 10.1074/jbc.M003278200. [DOI] [PubMed] [Google Scholar]
- Chumakov KM, Yushmanov SV. The maximum topological similarity principle in molecular systematics. Mol Genet Microbiol Virusol. 1988;3:3–9. [PubMed] [Google Scholar]
- O'Rand MG, Richardson RT, Zimmerman LJ, Widgren EE. Sequence and localization of human NASP: conservation of a Xenopus histone-binding protein. Dev Biol. 1992;154:37–44. doi: 10.1016/0012-1606(92)90045-I. [DOI] [PubMed] [Google Scholar]
- Brodsky LI, Ivanov VV, Kalaidzidis YL, Leontovich AM, Nikolaev VK, Feranchuk SI, Drachev VA. GeneBee-NET: Internet-based server for analyzing biopolymers structure. Biochemistry. 1995;60:923–928. [PubMed] [Google Scholar]
- Blom N, Gammeltoft S, Brunak S. Sequence- and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294:1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- MacArthur CA, Lawshe A, Shankar DB, Heikinheimo M, Shackleford GM. FGF-8 isoforms differ in NIH3T3 cell transforming potential. Cell Growth Differ. 1995;6:817–825. [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: a laboratory manual Cold Spring Harbor, NY; 1988.