Abstract
Although the lung is a common site for metastatic disease from extrathoracic malignancies, a pattern of lepidic growth of these metastases is considered rare. A 67-year-old man with a history of partial hepatectomy for hepatocellular carcinoma (HCC) presented to our hospital with dyspnea and a nonproductive cough. Chest radiographs and computed tomography imaging demonstrated consolidation in the right upper lobe and an ipsilateral pleural effusion. Findings were initially suspected to be secondary to infection, given the radiographic appearance and the rapid development from a normal computed tomography 3 months previously. However, the patient did not have convincing clinical evidence of pneumonia, and after little change after antibiotic therapy, a thoracentesis and pleural biopsy were performed that were positive for malignancy. Although immunostaining and morphology closely resembled the patient's primary HCC, new pathologic features of cholangiocarcinoma were found. We herein report the first case of rapidly progressing lepidic pulmonary metastases from an HCC that dedifferentiated into a hepatocholangiocarcinoma.
Keywords: Lepidic metastases, Dedifferentiated HCC
Introduction
The lungs are the second most common site for metastatic disease. Pulmonary metastases are seen in 20%-54% of extrathoracic malignancies with isolated pulmonary metastasis observed in 20% of cases [1]. Early diagnosis of pulmonary metastatic disease is crucial for disease staging and treatment planning with high-resolution computed tomography (HRCT), a sensitive and widely available imaging modality. Typical computed tomography (CT) imaging patterns of pulmonary metastatic disease have been widely described, including multiple solid round nodules of varying sizes and a random distribution (hematogenous spread), smooth interlobular septal thickening with perilymphatic micronodules (lymphangitic carcinomatosis), and solid masses within the bronchial lumen (endobronchial spread) [2], [3], [4], [5]. Rarer, atypical patterns of metastatic disease, including lepidic spread of metastatic malignancy along intact alveolar walls, have been reported and may mimic benign entities such as pneumonia or other malignancies such as primary pulmonary adenocarcinoma (formerly bronchioalveolar carcinoma) [6], [7]. Overlap of the imaging characteristics of these entities may complicate or delay diagnosis. Therefore, recognition of this atypical pattern of metastases and avoidance of this potential pitfall are crucial. We herein report, to the best of our knowledge, the first case of lepidic spread of pulmonary metastases from a poorly differentiated hepatocellular carcinoma (HCC) with the pulmonary metastases demonstrating acquisition of new pathologic features of cholangiocarcinoma.
Case report
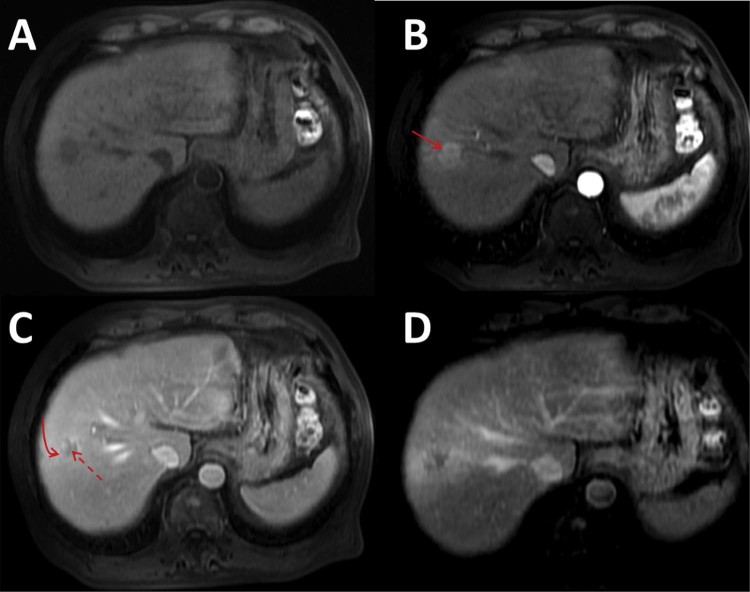
A 67-year-old Caucasian man was admitted to our hospital with abnormal thoracic findings on routine outpatient surveillance CT imaging for HCC, which he had been diagnosed with 7 months previously. At that time, magnetic resonance imaging had demonstrated a 2.3 × 2.0 cm (anteroposterior × transverse) segment VIII liver lesion with arterial hyperenhancement, washout kinetics, and a peripheral enhancing capsule with imaging features in keeping with an HCC confirmed on pathology obtained after partial hepatectomy (Fig. 1). No portal venous invasion, inferior vena cava invasion, or findings of distant metastases were observed at that time. Before this hospital admission, the patient reported 2 weeks of intermittent shortness of breath and a nonproductive cough. Chest auscultation revealed diminished breath sounds in the right mid-lung zone with tactile fremitus. Vital signs were within normal limits without fever or tachypnea. Laboratory data on admission demonstrated a mildly increased white count of 12,000/µL but otherwise were within normal limits. Chest radiographs demonstrated consolidation within the right middle and lower lung zones with a unilateral right pleural effusion, findings that were new from radiographs dated 7 months previously and the chest CT dated 3 months previously (Fig. 2). Noncontrast CT imaging of the thorax demonstrated consolidation within the anterior segment of the right upper lobe with surrounding patchy areas of ground-glass opacification, smooth interlobular septal thickening, centrilobular micronodules, and bronchial wall thickening. Multistation mediastinal lymphadenopathy had markedly progressed from the prior examination. Additionally, a small loculated right pleural effusion of simple fluid attenuation was present. This constellation of intrathoracic findings was new from the CT examination dated 3 months previously (Fig. 3, Fig. 4). Given the appearance and rapid progression, the patient was diagnosed as having community-acquired pneumonia with endobronchial spread of infection, treated as an inpatient with azithromycin and ceftriaxone, and was subsequently discharged.
Fig. 1.
Axial magnetic resonance images of the liver in the precontrast (A), arterial (B), portal venous (C), and delayed phases (D) demonstrate a hepatic segment VIII T1 hypointense lesion demonstrating hyperenhancement on arterial phase imaging (solid arrow), washout kinetics on portal venous-phase imaging (dashed arrow), and an enhancing capsule (curved arrow) with imaging features in keeping with a hepatocellular carcinoma.
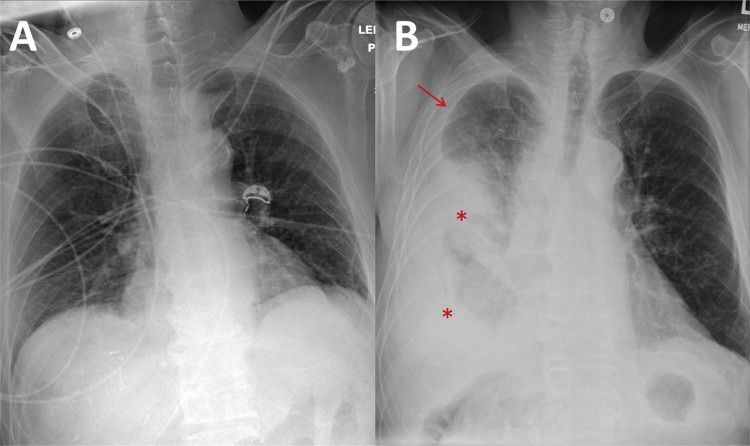
Fig. 2.
A frontal supine radiograph performed 7 months before the patient's admission (A) demonstrates clear lungs. A frontal supine radiograph performed at the time of admission (B) demonstrates interval development of consolidative changes in the right mid and lower lung zones (asterisks) and a new right pleural effusion (arrow).
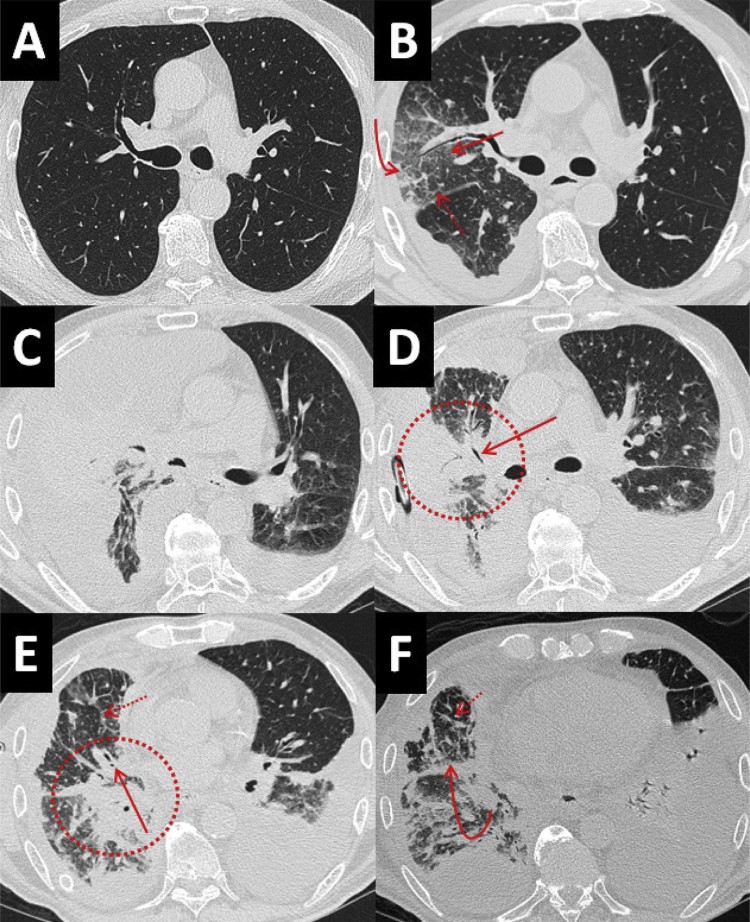
Fig. 3.
(A) Axial noncontrast CT image in the lung window at the level of the mainstem bronchi 3 months before admission demonstrates clear lungs. (B) Axial noncontrast CT image in the lung window at the level of the mainstem bronchi on initial admission demonstrates interval development of consolidation in the anterior segment of the right upper lobe (curved arrow), surrounding ground-glass opacification (straight arrow), and smooth interlobular septal thickening (dashed arrow). (C) Axial noncontrast CT image in the lung window at the level of the mainstem bronchi 1 week later demonstrates progression of consolidation. (D-F) Serial axial noncontrast CT images from the same examination in the lung window at the level of the mainstem bronchi (D), right middle lobar bronchus (E), and right lower lobe segmental bronchi (F) taken status post thoracentesis 1 week later demonstrate progressive consolidation (circles) with interval development of air bronchograms (solid arrows), worsening interlobular septal thickening (dashed arrows), and peribronchovascular nodularity (curved arrow), suggesting lymphangitic carcinomatosis. CT, computed tomography.
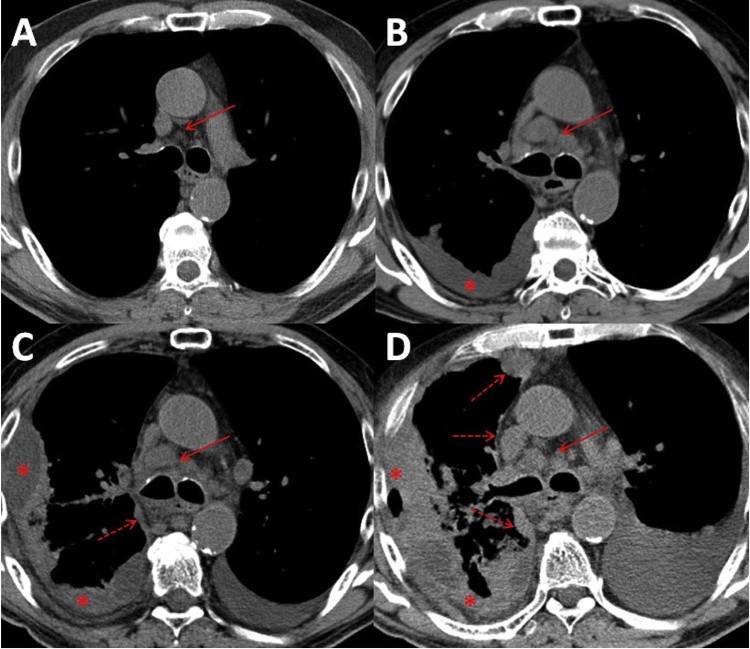
Fig. 4.
(A) Axial noncontrast computed tomography in the soft tissue window demonstrating small subcentimeter normal-appearing inferior paratracheal lymph nodes (arrow). (B) Three months later, there has been an increase in the size of pretracheal lymph nodes (arrow) and interval development of a unilateral loculated right pleural effusion (asterisk). (C) One week later, there has been an increase in the size of the loculated right pleural effusion (asterisk) with interval development of mediastinal pleural thickening (dashed arrow), a finding more commonly seen in malignant pleural disease. Multistation mediastinal lymphadenopathy persists (solid arrow). There has also been interval development of a left pleural effusion. (D) Status post thoracentesis 1 week later, there has been progressive multistation mediastinal lymphadenopathy (solid arrow), persistence of the loculated right pleural effusion with pleural thickening (asterisks), and worsening mediastinal pleural thickening (dashed arrows). There has also been interval worsening of a left pleural effusion. Note: a small amount of air in the lateral right pleural space is iatrogenic from chest tube placement.
The patient returned 8 days later with worsening shortness of breath and nonproductive cough with new-onset subjective fevers despite compliance with outpatient antibiotic therapy. Chest auscultation demonstrated diminished breath sounds in the right mid-lung zone with tactile fremitus and rhonchi. Laboratory data on admission demonstrated a mildly increased white count of 14,300/µL. Serum alpha-fetoprotein was 4.3 ng/mL and, although within normal limits, increased from 3 to 6 months previously when it measured 2.8 and 1.7 ng/mL, respectively. CT imaging demonstrated an interval increase in the size of a now moderately sized right-sided loculated pleural effusion with new chest wall and mediastinal nodular pleural thickening, as well as persistent consolidation, ground-glass opacification, and bronchial wall thickening in the anterior segment of the right upper lobe (Fig. 3, Fig. 4). New similar opacities were seen in the left upper lobe. The patient was admitted and diagnosed as having a hospital-acquired pneumonia with an associated parapneumonic effusion and was treated with levofloxacin and vancomycin.
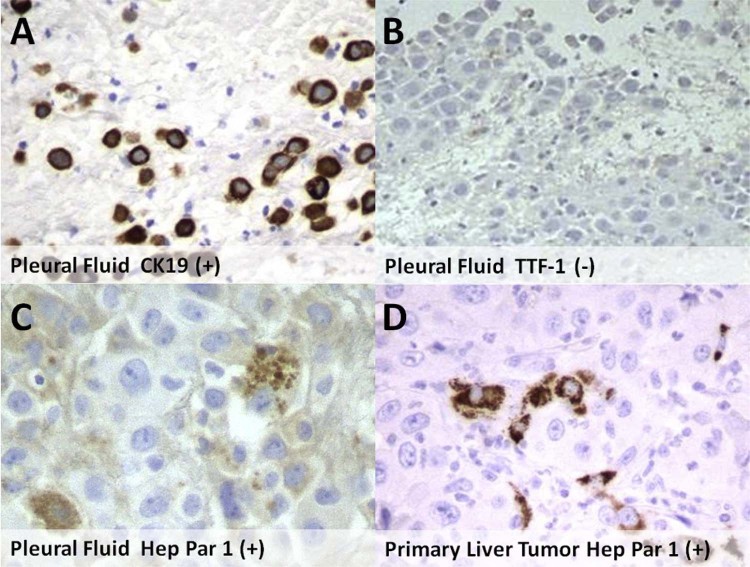
The patient underwent diagnostic thoracentesis of the right pleural effusion with cytology demonstrating atypical cells compatible with malignancy. Immunohistochemical staining was positive for CK7, MOC31, and BerEP4, with the morphology and staining pattern indicative of a poorly differentiated carcinoma. The sample was negative for thyroid transcription factor 1 (TTF-1) and napsin A, making a primary pulmonary adenocarcinoma an unlikely etiology. Hepatocyte paraffin 1 monoclonal antibody (Hep Par 1) immunostain, which has been expressed by the patient's known poorly differentiated HCC on the hepatectomy specimen 7 months previously was positive on the pleural effusion specimen. Subsequently, a right pleural biopsy was performed and glandular formation was identified. A cytokeratin 19 (CK19) immunostain, a marker of biliary differentiation, was positive on both the pleural effusion and the pleural biopsy specimens (Fig. 5). With the overall morphology of the pleural biopsy specimen demonstrating new glandular formation and the immunohistochemical staining pattern of the thoracentesis specimen resembling that of the previously diagnosed poorly differentiated HCC albeit with acquisition of new glandular morphology and markers of cholangiocarcinoma, the final diagnosis was rapidly developed lepidic pulmonary, pleural, and nodal metastases from a poorly differentiated HCC with new features of cholangiocarcinoma. Within 3 weeks, the patient degraded rapidly, becoming more hypoxic and delirious, and ultimately died of respiratory failure.
Fig. 5.
Immunohistochemical analysis of pleural fluid demonstrates positive staining with cytokeratin 19 (A) and negative staining with thyroid transcription factor 1 (B). Positivity with hepatocyte paraffin 1 monoclonal antibody immunostaining is demonstrated on both the pleural fluid (C) and the primary liver tumor (D) specimens.
Discussion
The vast majority of metastatic disease to the lungs presents as multiple solid, round, randomly distributed nodules of varying size secondary to hematogenous spread of malignant cells [2], [4]. Although classically associated with primary pulmonary adenocarcinoma, lepidic spread of metastatic malignancy along intact alveolar walls has been described in several case studies in the setting of extrathoracic adenocarcinomas, including gallbladder, pancreas, stomach, small bowel, colon, ovarian, and malignant melanoma [7], [8], [9], [10]. In 1 single-center retrospective analysis, approximately 10% of a single cohort of patients with primary gastrointestinal malignancies (n = 65) demonstrated a lepidic pattern of metastatic disease [6]. Although lepidic pulmonary metastases have also recently been described in the setting of cholangiocarcinoma and combined hepatocellular-cholangiocarcinoma (HCC-CC), to the best of our knowledge, this is the first reported case of lepidic spread of metastatic, poorly differentiated HCC with the thoracic metastases demonstrating acquisition of new pathologic features of cholangiocarcinoma [11], [12].
Radiographic features of lepidic metastases are often very nonspecific and manifest as areas of consolidation that may be associated with air bronchograms [2], [13], [14], [15]. Pleural effusions may be present in the setting of advanced disease [16]. HRCT findings of lepidic metastases are characterized by consolidation, ground-glass opacity, and centrilobular and tree-in-bud micronodules typically of ground-glass attenuation, reflecting cancer cells lining intact distal bronchioles with variable amounts of intra-alveolar involvement [6], [7], [13]. Although well-defined centrilobular and tree-in-bud micronodules of soft tissue attenuation have also been described, they are far less common [17], [18]. Focal and extensive areas of ground-glass opacification have been reported and with increased tumor burden may develop into regions of consolidation [6], [19]. Less common features such as nodules with surrounding ground-glass opacification, the so-called halo sign, and prominence of contrast opacified vessels traversing areas of lower attenuating consolidation, known as the “CT angiogram sign,” have been described [6], [20]. Development of malignant pleural effusions in the setting of disseminated disease has also been reported in at least 1 case of lepidic spread of malignant mesothelioma [16].
The presence of centrilobular and tree-in-bud micronodules most commonly reflects distal airway impaction by mucus, secretions, and micro-organisms in the setting of either infectious bronchiolitis or bronchiolitis secondary to aspiration or bronchiectasis [13], [14], [15]. Tree-in-bud nodules have also been reported in the endobronchial spread of pulmonary adenocarcinoma [21]. Ground-glass opacification and consolidation are also typically associated with acute conditions such as pneumonia, diffuse alveolar damage, pulmonary edema, and hemorrhage. The differential for chronic consolidation includes organizing pneumonia, chronic eosinophilic pneumonia, lymphoma, or primary pulmonary adenocarcinoma [15], [22], [23]. Thus, differentiating lepidic pulmonary metastases from these other more common entities relies heavily on clinical findings and the temporal evolution of imaging features. For example, lobar pneumonias are typically associated with fever, sputum production, and elevated white blood cell counts, and after antibiotic therapy, resolution over the following 2-4 weeks would be expected [2], [11]. On the other hand, lepidic metastasis should be considered when consolidation persists or worsens, or when there is absence of convincing evidence of infection, particularly in a patient with a known extrathoracic malignancy [13], [19], [24], [25]. In equivocal cases, definitive diagnosis ultimately requires biopsy.
In our patient, antibiotic treatment failure, the presence of atypical cells, and nonresolving consolidation raised concern for an unusual pattern of metastatic disease and a primary pulmonary adenocarcinoma. Studies have described high positive predictive values in diagnosing primary pulmonary adenocarcinoma with individual positivity of either TTF-1 (72%) or napsin A (83%) and higher specificity when positivity was demonstrated with both markers [26], [27], [28]. Our patient was negative for TTF-1 and napsin A, making primary pulmonary adenocarcinoma very unlikely. In addition, primary pulmonary adenocarcinomas typically do not suddenly appear on HRCT and rather slowly grow over the years [29].
Additional immunostaining with Hep Par 1 and CK19 was performed. Hep Par 1 has been reported as a sensitive marker for HCC with a positive predictive value up to 93% and has been positive on the pleural effusion specimen [30], [31]. CK19 is a marker of biliary differentiation and has been described as a promising prognostic marker for HCC with expression linked to a more aggressive clinical course and higher rates of disease recurrence [32], [33]. Although approximately 10%-27% of HCCs have been reported to express CK19, these tumors retain their typical morphologic characteristics, with CK19 more commonly expressed in cholangiocarcinoma and HCC-CC [34], [35]. In addition to CK19 expressed on both the pleural effusion and the pleural biopsy specimens, new glandular morphology was identified on the pleural biopsy specimen, a histologic morphology reported in cholangiocarcinoma [36]. These histologic findings, in addition to the overall radiographic appearance, suggest a lepidic spread of metastases from a poorly differentiated HCC with new pathologic features of cholangiocarcinoma as the unifying diagnosis.
In conclusion, this is the first reported case of rapidly progressing lepidic pulmonary metastases from a treated poorly differentiated HCC with acquisition of new pathologic features of cholangiocarcinoma. The rapid development and overlapping radiographic features with pneumonia may present as a potential diagnostic pitfall and could delay appropriate therapy. Therefore, although new consolidation and CT features of bronchiolitis are most commonly associated with acute inflammatory conditions, absence of infectious symptoms and failure of clearance after appropriate therapy should raise suspicion for lepidic metastasis in patients with known extrathoracic malignancies.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
No IRB approval was required for this article.
References
- 1.Mohammed T.L., Chowdhry A., Reddy G.P., Amorosa J.K., Brown K., Dyer D.S. ACR Appropriateness Criteria® screening for pulmonary metastases. J Thorac Imaging. 2011;26(1):W1–W3. doi: 10.1097/RTI.0b013e3182010bf9. [DOI] [PubMed] [Google Scholar]
- 2.Seo J., Im J., Goo J., Chung M., Kim M. Atypical pulmonary metastases: spectrum of radiologic findings. Radiographics. 2001;21(2):403–417. doi: 10.1148/radiographics.21.2.g01mr17403. [DOI] [PubMed] [Google Scholar]
- 3.Libshitz H.I., North L.B. Pulmonary metastases. Radiol Clin North Am. 1982;20:437–451. [PubMed] [Google Scholar]
- 4.Hirakata K., Nakata H., Nakagawa T. CT of metastases with pathological correlation. Semin Ultrasound CT MR. 1995;16:379–394. doi: 10.1016/0887-2171(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 5.Ikezoe J., Johkoh T., Takeuchi N., Ishida T., Morimoto S., Kitamura I. CT findings of endobronchial metastasis. Acta Radiol. 1991;32:455–460. [PubMed] [Google Scholar]
- 6.Gaeta M., Volta S. Air-space pattern in lung metastasis from adenocarcinoma of the GI tract. J Comput Assist Tomogr. 1996;20(2):300–304. doi: 10.1097/00004728-199603000-00025. [DOI] [PubMed] [Google Scholar]
- 7.Rosenblatt M.B., Lisa J.R., Collier F. Primary and metastatic bronchioloalveolar carcinoma. Dis Chest. 1967;52:147–152. doi: 10.1378/chest.52.2.147. [DOI] [PubMed] [Google Scholar]
- 8.Foster C.S. Mucus-secreting “alveolar-cell” tumour of the lung: a histochemical comparison of tumours arising within and outside the lung. Histopathology. 1980;4:567–577. doi: 10.1111/j.1365-2559.1980.tb02950.x. [DOI] [PubMed] [Google Scholar]
- 9.Tokunaga T., Arakawa H., Kuwashima Y. A case of lepidic pulmonary metastasis from adenocarcinoma of the gallbladder mimicking acute interstitial pneumonia. Clin Radiol. 2005;60:1213–1215. doi: 10.1016/j.crad.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Mizuuchi H., Suda K., Kitahara H., Shimamatsu S., Kohno M., Okamoto T. Solitary pulmonary metastasis from malignant melanoma of the bulbar conjunctiva presenting as a pulmonary ground glass nodule: report of a case. Thorac Cancer. 2015;6(1):97–100. doi: 10.1111/1759-7714.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishii T., Goto Y., Matsuzaki H., Ohishi N., Sakamoto Y., Saito R. Pulmonary metastasis of combined hepatocellular and cholangiocarcinoma: a unique radiographic presentation with air-space consolidation masquerading as pneumonia and primary pulmonary adenocarcinoma. Intern Med. 2015;54(11):1389–1392. doi: 10.2169/internalmedicine.54.3375. [DOI] [PubMed] [Google Scholar]
- 12.Nagayoshi Y., Yamamoto K., Hashimoto S., Hisatomi K., Doi S., Nagashima S. An autopsy case of lepidic pulmonary metastasis from cholangiocarcinoma. Intern Med. 2016;55(19):2849–2853. doi: 10.2169/internalmedicine.55.5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaikwad A., Souza C., Inacio J., Gupta A., Sekhon H., Seely J.M. Aerogenous metastases: a potential game changer in the diagnosis and management of primary lung adenocarcinoma. AJR Am J Roentgenol. 2014;203(6):W570–W582. doi: 10.2214/AJR.13.12088. [DOI] [PubMed] [Google Scholar]
- 14.Miller W., Panosian J. Causes and imaging patterns of tree-in-bud opacities. Chest. 2013;144(6):1883–1892. doi: 10.1378/chest.13-1270. [DOI] [PubMed] [Google Scholar]
- 15.Walker C., Abbott G., Greene R., Shepard J., Vummidi D., Digumarthy S. Imaging pulmonary infection: classic signs and patterns. AJR Am J Roentgenol. 2014;202(3):479–492. doi: 10.2214/AJR.13.11463. [DOI] [PubMed] [Google Scholar]
- 16.Rossi G., Cavazza A., Turrini E., Costantini M., Casali C., Morandi U. Exclusive intrapulmonary lepidic growth of a malignant pleural mesothelioma presenting with pneumothorax and involving the peritoneum. Int J Surg Pathol. 2006;14(3):234–237. doi: 10.1177/1066896906290360. [DOI] [PubMed] [Google Scholar]
- 17.Tateishi U., Müller N.L., Johkoh T., Maeshima A., Asamura H., Satake M. Mucin producing adenocarcinoma of the lung: thin-section computed tomography findings in 48 patients and their effect on prognosis. J Comput Assist Tomogr. 2005;29:361–368. doi: 10.1097/01.rct.0000162820.08909.e1. [DOI] [PubMed] [Google Scholar]
- 18.Gaeta M., Blandino A., Pergolizzi S., Mazziotti S., Caruso R., Barone M. Patterns of recurrence of bronchioloalveolar cell carcinoma after surgical resection: a radiological, histological, and immunohistochemical study. Lung Cancer. 2003;42:319–326. doi: 10.1016/s0169-5002(03)00362-3. [DOI] [PubMed] [Google Scholar]
- 19.Okafuji T., Sakai S., Yoshimitsu K., Soeda H., Furuya A., Matsuura S. Pulmonary metastasis from pancreatic cancer: a case showing biphasic radiological and histological pattern. CMIG Extra: Cases. 2004;28(7):68–71. [Google Scholar]
- 20.Shah N., Jubber A., Osman M., Syed I. Unusual pulmonary metastatic pattern in a case of pancreatic cancer. BMJ Case Rep. 2011;4365 doi: 10.1136/bcr.06.2011.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Webb R.W., Müller N.L., Naidich D.P. HRCT findings of lung disease. In: Webb R.W., Müller N.L., Naidich D.P., editors. High-resolution CT of the lung. 3rd ed. Lippincott-Raven Press; Philadelphia (PA): 2001. p. 120. [Google Scholar]
- 22.El-Sherief A., Gilman M., Healey T., Tambouret R., Shepard J., Abbott G. Clear vision through the haze: a practical approach to ground-glass opacity. Curr Probl Diagn Radiol. 2014;43(3):140–158. doi: 10.1067/j.cpradiol.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Boiselle P., Abbott G., McLoud T. 2nd ed. Mosby; Philadelphia (PA): 2011. Thoracic imaging: case review series; p. 356. [Google Scholar]
- 24.Donovan W.D., Yankelevitz D.F., Henschke C.I., Altorki N., Nash T.A. Endobronchial spread of bronchioloalveolar carcinoma. Chest. 1993;104:951–953. doi: 10.1378/chest.104.3.951. [DOI] [PubMed] [Google Scholar]
- 25.Aquino S.L., Chiles C., Halford P. Distinction of consolidative bronchioloalveolar carcinoma from pneumonia: do CT criteria work? AJR Am J Roentgenol. 1998;171:359–363. doi: 10.2214/ajr.171.2.9694451. [DOI] [PubMed] [Google Scholar]
- 26.Yatabe Y., Mitsudomi T., Takahashi T. TTF-1 expression in pulmonary adenocarcinomas. Am J Surg Pathol. 2002;26(6):767–773. doi: 10.1097/00000478-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Heymann J., Hoda R., Scognamiglio T. Polyclonal napsin A expression: a potential diagnostic pitfall in distinguishing primary from metastatic mucinous tumors in the lung. Arch Pathol Lab Med. 2014;138(8):1067–1071. doi: 10.5858/arpa.2013-0403-OA. [DOI] [PubMed] [Google Scholar]
- 28.Ye J., Findeis-Hosey J.J., Yang Q., McMahon L.A., Yao J.L., Li F. Combination of napsin A and TTF-1 immunohistochemistry helps in differentiating primary lung adenocarcinoma from metastatic carcinoma in the lung. Appl Immunohistochem Mol Morphol. 2011;19(4):313–317. doi: 10.1097/PAI.0b013e318205b059. [DOI] [PubMed] [Google Scholar]
- 29.Wilson D.O., Ryan A., Fuhrman C., Schuchert M., Shapiro S., Siegfried J.M. Doubling times and CT screen-detected lung cancers in the Pittsburgh Lung Screening Study. Am J Respir Crit Care Med. 2012;185(1):85–89. doi: 10.1164/rccm.201107-1223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan Z., van de Rijn M., Montgomery K., Rouse R. Hep par 1 antibody stain for the differential diagnosis of hepatocellular carcinoma: 676 tumors tested using tissue microarrays and conventional tissue sections. Mod Pathol. 2003;16(2):137–144. doi: 10.1097/01.MP.0000052103.13730.20. [DOI] [PubMed] [Google Scholar]
- 31.Kakar S., Muir T., Murphy L.M., Lloyd R.V., Burgart L.J. Immunoreactivity of Hep Par 1 in hepatic and extrahepatic tumors and its correlation with albumin in situ hybridization in hepatocellular carcinoma. Am J Clin Pathol. 2003;119(3):361–366. doi: 10.1309/8l872rphejrkf5jj. [DOI] [PubMed] [Google Scholar]
- 32.Uenishi T., Kubo S., Yamamoto T., Shuto T., Ogawa M., Tanaka H. Cytokeratin 19 expression in hepatocellular carcinoma predicts early postoperative recurrence. Cancer Sci. 2003;94:851–857. doi: 10.1111/j.1349-7006.2003.tb01366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhuang P.Y., Zhang J.B., Zhu X.D., Zhang W., Wu W.Z., Tan Y.S. Two pathologic types of hepatocellular carcinoma with lymph node metastasis with distinct prognosis on the basis of CK19 expression in tumor. Cancer. 2008;112:2740–2748. doi: 10.1002/cncr.23488. [DOI] [PubMed] [Google Scholar]
- 34.Lau S.K., Prakash S., Geller S.A., Alsabeh R. Comparative immunohistochemical profile of hepatocellular carcinoma, cholangiocarcinoma, and metastatic adenocarcinoma. Hum Pathol. 2002;33:1175–1181. doi: 10.1053/hupa.2002.130104. [DOI] [PubMed] [Google Scholar]
- 35.Wu P.C., Fang J.W., Lau V.K., Lai C.L., Lo C.K., Lau J.Y. Classification of hepatocellular carcinoma according to hepatocellular and biliary differentiation markers. Clinical and biological implications. Am J Pathol. 1996;149:1167–1175. [PMC free article] [PubMed] [Google Scholar]
- 36.Goodman Z. Neoplasms of the liver. Mod Pathol. 2007;20:S49–S60. doi: 10.1038/modpathol.3800682. [DOI] [PubMed] [Google Scholar]