Abstract
Objective
To identify potentially actionable dosimetric predictors of local control (LC) for non-small cell lung cancer (NSCLC) brain metastases treated with single-fraction stereotactic radiosurgery (SRS).
Methods and materials
Patients with NSCLC brain metastases treated with single-fraction SRS were identified. Eligible patients had at least 1 follow-up magnetic resonance imaging scan and were without prior metastasectomy or SRS to the same lesion. LC and overall survival (OS) were estimated using the Kaplan-Meier method. The Cox proportional hazards model was used for univariate (UVA) and multivariate analysis (MVA). Receiver operating characteristic (ROC) analysis was used to identify optimal cut points for dose-volume histogram metrics relative to LC.
Results
A total of 612 NSCLC brain metastasis were identified in 299 patients with single-fraction SRS between 1999 and 2014. Median follow-up was 10 months. Median OS from time of SRS was 11 months. Overall LC was 75% and 66% at 1 and 2 years, respectively. On UVA, increasing dose by any measure was associated with improved LC. On MVA, volume receiving at least 32 Gy (V32; hazard ratio [HR], 0.069; P < .000), along with higher prescription isodose (HR, 0.953; P = .031) and lower volume (HR, 1.359; P < .000), were independent predictors of improved LC. ROC analysis demonstrated a V32 of 24% to be most predictive for LC. For the entire cohort, 1-year LC for V32 ≥24% was 89% versus 67% for V32 <24% (P = .000). Stratifying by volume, lesions ≤2 cm (n = 323) had a 1-year LC of 95% versus 82% (P = .005) for V32 above and below 24%, respectively. For lesions 2.1 to 3 cm (n = 211), 1-year LC was 79% versus 59% (P = .003) for V32 above and below 24%, respectively. Total tumor volume alone was predictive for OS.
Conclusions
Volume, prescription isodose line, and V32 are independent predictors of LC. V32 represents an actionable SRS treatment planning parameter for NSCLC brain metastases.
Summary.
Examination of dose-volume histogram metrics as they relate to local control in stereotactic radiosurgery for brain metastasis has not been described. In retrospective review of 612 non-small cell lung cancer brain metastases treated with stereotactic radiosurgery, the volume of tumor receiving at least 32 Gy was associated with improved local control. This represents an actionable metric that may be modified at the time of treatment planning. Additional confirmatory studies are warranted.
Alt-text: Unlabelled box
Introduction
Lung cancer is the leading cause of cancer-related death1 and the most common source of brain metastases in the United States.2 Approximately 40% of patients diagnosed with non-small cell lung cancer (NSCLC) develop brain metastasis, with the central nervous system being the first site of failure in 34% of patients.3 Despite treatment, brain metastases remain the cause of mortality in 23% to 44% of patients.4
Local control (LC) of brain metastasis remains an important clinical end point. Brain metastasis control has been correlated with improved neurocognition at 3 months5; this has, in turn, been associated with improved quality of life.6 LC has also been associated with improved overall survival (OS).7
Actionable parameters predictive of LC have not been well studied in stereotactic radiosurgery (SRS), however. Several investigators have reported a positively correlated dose-LC relationship7, 8; however, prescription dose escalation is associated with increased treatment morbidity.9 The use of whole brain radiation therapy (WBRT) before SRS has also been associated with increased LC,10 but, as demonstrated by Chang et al, it is also associated with a significant detriment in neurocognition. Last, the use of an additional margin on the gross tumor volume has been associated with improved LC; however, the use of an additional margin is only applicable to small metastasis in locations distant from critical structures. Because of these factors, it is clear that other actionable parameters are needed. At present, the examination of dose-volume histogram (DVH) metrics as they relate to LC has not yet been described in the brain metastases SRS literature. The primary end point of this study was assessment of actionable DVH metrics predictive of LC that may be modified at the time of treatment planning. Secondary outcomes include patient-, tumor-, and treatment-related characteristics that are predictive of LC and OS.
Methods and materials
Data collection
Patients treated at Barnes Jewish Hospital and Washington University in St. Louis with pathologically confirmed NSCLC brain metastasis who received Gamma Knife SRS between June 1999 and January 2014 were identified from an institutional review board–approved registry. Eligible patients included those who underwent single-fraction SRS and had at least 1 follow-up brain magnetic resonance imaging (MRI) scan. Those who had brainstem metastases, radiosurgical boost to a metastasectomy cavity, or repeat SRS to a previously treated lesion were excluded. A total of 612 brain metastases in 299 patients met eligibility criteria and were included for analysis. Baseline patient, tumor, and treatment data were collected from the medical record in retrospective fashion.
Radiosurgery
All patients underwent single-fraction SRS with the Leksell Gamma Knife (Elekta AB, Stockholm, Sweden). Patients were treated using Model B, C, and Perfexion from June 1999 to August 2002, August 2002 to April 2008, and April 2008 to the present, respectively. All patients had intravenous access placed. The Leksell stereotactic frame was placed under local anesthetic to the pin sites, in combination with a low-dose anxiolytic. High-resolution contrast-enhanced MRI and noncontrast computed tomography imaging were obtained. Target volume delineation and treatment planning were performed in concert by a medical physicist, neurosurgeon, and radiation oncologist. The target volume was defined as the contrast-enhancing tumor on the T1-weighted MRI images without additional margin. Prescription dose was based generally on recommendations from Radiation Therapy Oncology Group (RTOG) 90-05 with frequent adjustments made at the discretion of the treating physician. Generally, lesions >3 cm were treated to 15 Gy, lesions 2.1 to 3 cm were treated to 18 Gy, and those ≤2 cm were treated to 20 to 24 Gy. Treatment planning parameters included tumor coverage by the prescription isodose line while attempting to maintain a gradient index (50% of prescription isodose volume/prescription isodose volume) < 3 and a conformality index (prescription isodose volume/tumor volume) < 2. No specific evaluation or optimization of the treatment plan was performed beyond these planning parameters.
Patient follow-up
Patients underwent a brain MRI scan every 2 to 3 months in follow-up. All brain MRI scans were reviewed from the time of SRS until death or last follow-up. Local failure was assessed in each treated lesion and was defined as an increase in the size of the treated metastasis at any time after SRS unless additional imaging studies or pathology from subsequent surgical resection suggested radiation necrosis. Radiation necrosis was assessed in each treated lesion and recorded if documented by any treating physician, radiologist, or pathologist.
Statistical analysis
Times were measured from the date of SRS. Clinical characteristics between groups were compared using the Mann-Whitney U test. DVH per lesion were manually exported from the treatment planning software (Leksell GammaPlan 10.1 Elekta, Stockholm, Sweden) and analyzed using a custom in-house DVH tool. DVH parameters extracted from each lesion included tumor volume in mm3, conformality index, minimum dose to xx% (Dxx) of the tumor volume (D10, D50, D90), minimum dose (Dmin), maximum dose, mean dose, median dose, and the percent of the tumor volume receiving at least xx Gy (Vxx) in 1-Gy increments from 20 to 40 Gy. Kaplan-Meier method was used to estimate LC and OS. Differences in LC and OS were analyzed with the log-rank test. Univariate and multivariate analysis was performed using the Cox proportional hazards model. For multivariate analysis, only variables significant on univariate analysis were included in the Cox proportional hazards model. Optimal cut points were determined using a combination of graphic diagnostic plots, a minimum P value approach, and receiver operating characteristic (ROC) curves. P < .05 was considered significant. All tests were 2-tailed. Statistical analyses were performed using SAS (version 9.2; SAS Institute, Cary, NC) and SPSS (version 21.0; IBM, Armonk, NY).
Results
Patient and treatment characteristics
Patient- and tumor-related characteristics are summarized in Table 1. The median age at the time of treatment was 61 years (range, 37-85), 55% were female, and the majority were white (86%). The majority of patients were recursive partitioning analysis class II (63%) and the median diagnosis specific graded prognostic assessment was 2. Two hundred patients had control of their primary disease at the time of radiosurgery. Most patients had adenocarcinoma (64%). Of those with adenocarcinoma, 5% were epidermal growth factor receptor-positive and <1% EML4-ALK were positive. All patients who were epidermal growth factor receptor or EML4-ALK were on targeted agents within 30 days of radiosurgery. Most patients had supratentorial tumors (78%). The median number of brain metastases was 1 (range, 1-8; mean, 1.7). A total of 68% (204) patients did not receive WBRT before SRS. Treatment-related characteristics are presented in Table 2. The median size of brain metastases was 431 mm3, with a median dose of 20 Gy and a median conformality index of 1.69.
Table 1.
Patient- and tumor-related characteristics
| Characteristic | Value |
|---|---|
| Age (y) | |
| Median (range) | 61 (37-85) |
| Histology (n = 299) | |
| Adenocarcinoma | 191 (64%) |
| EGFR mutant | 14 (5%) |
| EML4-ALK translocation | 1 (<1%) |
| Squamous cell carcinoma | 40 (13%) |
| Large cell | 2 (1%) |
| Adenosquamous | 6 (2%) |
| NSCLC-NOS | 60 (20%) |
| Gender | |
| Male | 136 (45%) |
| Female | 163 (55%) |
| Race | |
| Caucasian | 257 (86%) |
| African American | 38 (13%) |
| Other | 4 (1%) |
| KPS at time of radiosurgery | |
| 100 | 15 (5%) |
| 90 | 97 (33%) |
| 80 | 153 (53%) |
| 70 | 23 (8%) |
| 60 | 2 (1%) |
| Unknown | 9 (3%) |
| RPA class at time of radiosurgery | |
| 1 | 100 (33%) |
| 2 | 188 (63%) |
| 3 | 11 (4%) |
| GPA score at time of radiosurgery | |
| Median (range) | 2 (0-4) |
| Control of primary disease | |
| Yes | 200 |
| No | 99 |
| Number of brain metastases at time of radiosurgery | |
| Median (range) | 1 (1-8) |
| Tumor location | |
| Supratentorial | 232 (78%) |
| Cerebellar | 67 (22%) |
| Prior WBRT | |
| No | 204 (68%) |
| Yes | 95 (32%) |
EGFR, epidermal growth factor receptor; GPA, graded prognostic assessment; KPS, Karnofsky Performance Scale; NSCLC-NOS, non-small cell lung cancer, not otherwise specified; RPA, recursive partitioning analysis; WBRT, whole brain radiation therapy.
Table 2.
Treatment-related characteristics
| Characteristic | Value |
|---|---|
| Brain metastasis volume (mm3) | |
| Median (range) | 499 (3-21,400) |
| Interquartile range | 96-1815 |
| Total volume of brain metastases (mm3) | |
| Median (range) | 1930 (11-38,113) |
| Interquartile range | 649-4820 |
| Prescription dose (Gy) | |
| Median (range) | 20 (12-24) |
| Interquartile range | 18-21 |
| Prescription isodose line (%) | |
| Median (range) | 50 (45-90) |
| Interquartile range | 50-53 |
| Shots per lesion | |
| Median (range) | 3 (1-28) |
| Interquartile range | 2-5 |
| Conformality index | |
| Median | 1.7 |
| Interquartile range | 1.4-2.4 |
Outcomes
Median follow-up was 10 months. Median OS for all patients was 11 months, and the estimated survival at 1 and 2 years was 52% and 29%, respectively. Of 612 brain metastases, 123 (20%) were local failures. Overall LC was 75% and 66% at 1 and 2 years, respectively. Multiple follow-up MRI scans were obtained in 211 patients (71%). Failure was assigned to 16 metastases on the basis of a single follow-up MRI scan. There were 33 cases of radiation necrosis (5%) in 25 patients. With regard to salvage, 16 patients underwent surgical salvage after SRS, 52 patients underwent repeat SRS for new distant brain metastasis, and 55 patients received WBRT after SRS. Of those that received WBRT after SRS, the median time to WBRT was 6 months. Factors associated with LC on univariate analysis are summarized in Table 3. Improved LC was noted with use of the Perfexion model, prior WBRT, smaller tumor volume, larger (worse) conformality index, number of shots, and essentially every measure of dose to tumor. Specific to the DVH analysis, every measure from V20 to 40 Gy was significantly correlated with LC.
Table 3.
Complete list of univariate analysis of characteristics potentially predicting local control
| Characteristic | HR | 95% CI | P |
|---|---|---|---|
| Age | 1.01 | 0.988-1.027 | .452 |
| Female | 0.71 | 0.492-1.016 | .061 |
| African American | 1.00 | 0.582-1.721 | .998 |
| Asian | 0.73 | 0.180-2.970 | .662 |
| RPA class II | 0.92 | 0.634-1.342 | .675 |
| RPA class III | 1.12 | 0.478-2.634 | .791 |
| GPA | 1.03 | 0.817-1.295 | .809 |
| SIR class | 1.06 | 0.936-1.193 | .372 |
| Prior WBRT | 1.90 | 1.33-2.717 | <.001 |
| WBRT within 2 months | 1.87 | 1.03-3.407 | .039 |
| Histology | 1.40 | 0.874-2.240 | .162 |
| Tumor location | 1.42 | 0.938-2.141 | .097 |
| Tumor volume (mm3) | 1.57 | 1.40-1.759 | <.001 |
| Model C | 0.65 | 0.369-1.15 | .142 |
| Perfexion model | 0.59 | 0.357-0.985 | .043 |
| Dose (Gy) | 0.81 | 0.74-0.883 | <.001 |
| Isodose | 0.93 | 0.896-0.974 | .001 |
| Number of shots | 1.12 | 1.08-1.151 | <.001 |
| Conformality index | 0.75 | 0.611-0.913 | .004 |
| Gradient index | 0.88 | 0.672-1.159 | .368 |
| Dmean | 1.00 | 0.998-0.999 | <.001 |
| Dmedian | 1.00 | 0.998-0.999 | <.001 |
| Dmin | 1.00 | 0.998-0.999 | <.001 |
| D10 | 1.00 | 0.999-1.00 | <.001 |
| D50 | 1.00 | 0.998-0.999 | <.001 |
| D90 | 1.00 | 0.997-0.999 | <.001 |
| V15 | 0.55 | 0.000-798.9 | .872 |
| V18 | 0.04 | 0.007-0.255 | <.001 |
| V20 | 0.11 | 0.04-0.301 | <.001 |
| V21 | 0.16 | 0.07-0.330 | <.001 |
| V22 | 0.20 | 0.107-0.355 | <.001 |
| V23 | 0.19 | 0.107-0.321 | <.001 |
| V24 | 0.19 | 0.108-0.321 | <.001 |
| V25 | 0.19 | 0.107-0.322 | <.001 |
| V26 | 0.19 | 0.106-0.329 | <.001 |
| V27 | 0.17 | 0.092-0.310 | <.001 |
| V28 | 0.16 | 0.084-0.310 | <.001 |
| V29 | 0.14 | 0.068-0.291 | <.001 |
| V30 | 0.13 | 0.055-0.280 | <.001 |
| V31 | 0.09 | 0.035-0.229 | <.001 |
| V32 | 0.06 | 0.018-0.175 | <.001 |
| V33 | 0.03 | 0.008-0.135 | <.001 |
| V34 | 0.02 | 0.003-0.105 | <.001 |
| V35 | 0.01 | 0.001-0.075 | <.001 |
| V36 | 0.00 | 0.000-0.064 | <.001 |
| V37 | 0.00 | 0.000-0.042 | <.001 |
| V38 | 0.00 | 0.000-0.020 | .001 |
| V39 | 0.00 | 0.000-0.009 | .007 |
| V40 | 0.00 | 0.000-0.011 | .235 |
| V50 | 0.00 | 0.000-0 | .983 |
CI, confidence interval; conformality index, prescription isodose volume/tumor volume; D(xx), minimum dose to xx% of the tumor volume; HR, hazard ratio; V(xx), percentage of tumor volume receiving xx Gy; SIR, score index for radiosurgery class; WBRT, whole brain radiation therapy. Other abbreviations as in Table 1.
Bold text indicates statistical significance.
On multivariate analysis (Table 4), only prescription isodose (hazard ratio [HR], 0.953; P = .031), volume (HR, 1.359; P < .000), and V32 Gy (HR, 0.069; P < .000) remained significant predictors of LC. In the multivariate model building, it is notable that V22 to V39 remained within the model until V32 was entered. V32 was chosen by the model by virtue of it having the lowest P value (P = .0004). After entry of V32 into the model, all remaining Vxx were no longer significant. Prescription dose was not significant on multivariate analysis.
Table 4.
Candidate variables significant on univariate and multivariate analyses of characteristics predicting local control
| Characteristic | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Isodose | 0.93 | 0.896-0.974 | .001 | 0.953 | 0.911-0.996 | .031 |
| Tumor volume (mm3) | 1.57 | 1.40-1.759 | <.001 | 1.359 | 1.197-1.543 | <.001 |
| V32 | 0.06 | 0.018-0.175 | <.001 | 0.069 | 0.018-0.264 | <.001 |
| Prior WBRT | 1.90 | 1.33-2.717 | <.001 | |||
| WBRT within 2 months | 1.87 | 1.03-3.407 | .039 | |||
| Perfexion model | 0.59 | 0.357-0.985 | .043 | |||
| Dose (Gy) | 0.81 | 0.74-0.883 | <.001 | |||
| Number of shots | 1.12 | 1.08-1.151 | <.001 | |||
| CI | 0.75 | 0.611-0.913 | .004 | |||
| Dmean | 1.00 | 0.998-0.999 | <.001 | |||
| Dmedian | 1.00 | 0.998-0.999 | <.001 | |||
| Dmin | 1.00 | 0.998-0.999 | <.001 | |||
| D10 | 1.00 | 0.999-1.00 | <.001 | |||
| D50 | 1.00 | 0.998-0.999 | <.001 | |||
| D90 | 1.00 | 0.997-0.999 | <.001 | |||
| V18 | 0.04 | 0.007-0.255 | <.001 | |||
| V20 | 0.11 | 0.04-0.301 | <.001 | |||
| V21 | 0.16 | 0.07-0.330 | <.001 | |||
| V22 | 0.20 | 0.107-0.355 | <.001 | |||
| V23 | 0.19 | 0.107-0.321 | <.001 | |||
| V24 | 0.19 | 0.108-0.321 | <.001 | |||
| V25 | 0.19 | 0.107-0.322 | <.001 | |||
| V26 | 0.19 | 0.106-0.329 | <.001 | |||
| V27 | 0.17 | 0.092-0.310 | <.001 | |||
| V28 | 0.16 | 0.084-0.310 | <.001 | |||
| V29 | 0.14 | 0.068-0.291 | <.001 | |||
| V30 | 0.13 | 0.055-0.280 | <.001 | |||
| V31 | 0.09 | 0.035-0.229 | <.001 | |||
| V33 | 0.03 | 0.008-0.135 | <.001 | |||
| V34 | 0.02 | 0.003-0.105 | <.001 | |||
| V35 | 0.01 | 0.001-0.075 | <.001 | |||
| V36 | 0.00 | 0.000-0.064 | <.001 | |||
| V37 | 0.00 | 0.000-0.042 | <.001 | |||
| V38 | 0.00 | 0.000-0.020 | .001 | |||
| V39 | 0.00 | 0.000-0.009 | .007 | |||
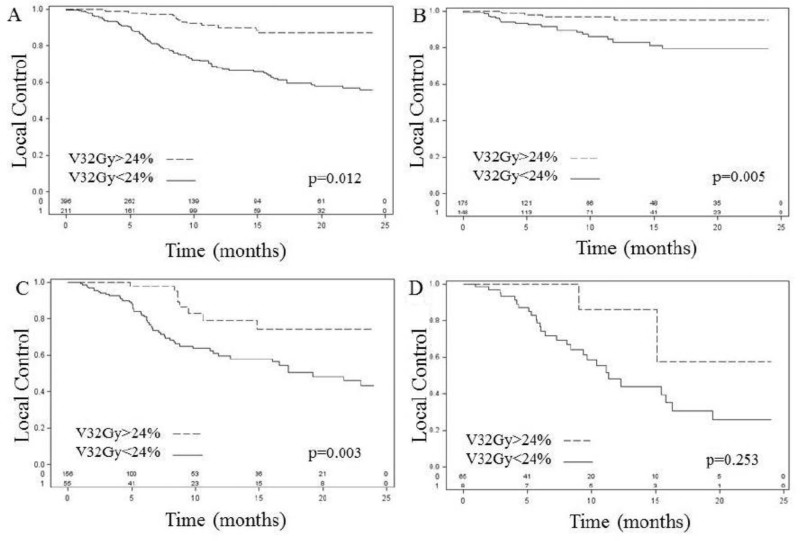
ROC analysis was performed for V32 Gy, which demonstrated that the most predictive cut point was 24% for LC, although 5% to 63% were statistically significant. Overall, the LC rate for V32 ≥24% was 89% and 67% for V32 <24% at 1 year (Fig 1A). ROC analysis was not performed for volume. Instead, volume was stratified into 3 clinically meaningful maximal axial dimension equivalent (diameter = 2 × cuberoot (3V/4π) size categories: 3cm. The 1-year LC was 88%, 64%, and 53% for lesions less than 2cm, 2.1 to 3cm, and greater than 3cm, respectively. For lesions ≤2 cm (n = 323), 1-year LC for V32 ≥24% versus V32 <24% was 79% versus 59% (P = .003), respectively (Fig 1C). For lesions >3 cm (n = 73), 1-year LC for V32 ≥24% versus V32 <24% was 85% versus 47% (P = .253), respectively (Fig 1D). The overall rate of radiation necrosis was 5%, with no indication of higher rates in the higher V32 group (3 cases [0.4%] for V32 ≥24% versus 30 cases [4.9%] for V32 <24%). Additional analysis did not reveal V32 to be a significant predictor of radiation necrosis; however, given the small number of cases of V32 ≥24%, it is difficult to derive clinically meaningful conclusions on the incidence of radiation necrosis. Univariate analysis demonstrated that prior WBRT, age, dose, maximum dose, graded prognostic assessment, volume of the largest brain metastasis, number of brain metastases, and total brain metastasis volume were predictive of OS; however, on multivariate analysis, only total brain metastasis volume remained significant (HR 1.76; P < .001) (Table 5).
Figure 1.
Kaplan-Meier curves of local control. (A) V32 overall, (B) V32 for lesions <2 cm, (C) V32 for lesions 2.1 to 3 cm, and (D) V32 for lesions >3 cm. V32, volume of tumor receiving at least 32 Gy.
Table 5.
Candidate variables significant on univariate and multivariate analyses of characteristics predicting overall survival
| Characteristic | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Prior WBRT | 1.41 | 1.06-1.86 | .018 | |||
| Age | 0.98 | 0.966-0.955 | .013 | |||
| Dose | 0.90 | 0.843-0.960 | .002 | |||
| Maximum dose | 0.99 | 0.9995-0.9997 | .029 | |||
| GPA | 0.79 | 0.659-0.969 | .023 | |||
| Number of metastases | 1.12 | 1.01-1.25 | .033 | |||
| Total metastases volume (mm3) | 1.00 | 1.00001-1.00006 | .003 | 1.76 | 1.38-2.25 | <.001 |
| Largest lesion volume | 1.00 | 1.00001-1.00006 | .019 | |||
Discussion
We report the results of a large population of patients with NSCLC brain metastasis treated with single-fraction SRS. In general, these patients had a limited burden of intracranial disease, good performance status, and preferential prognostic assessment scores. In examining determinants of LC, we found that decreasing tumor volume, higher prescription isodose, and higher V32 were independent predictors of LC on multivariate analysis. Prescription to a higher isodose line was found to be associated with improved LC. The most obvious explanation is that very small lesions are often treated to a higher prescription isodose line because of excessive coverage at the standard 50% isodose level; however, higher prescription isodose line remained a significant predictor for LC even after accounting for volume in multivariate analysis. The most predictive cutpoint was 57%, although 58% to 60% were significant as well. These results are in concordance with those found by Sheehan et al,11 who analyzed 627 NSCLC metastases in 273 patients treated with SRS. With respect to local failure, they found that lower prescription isodose was a significant predictor of failure; however, the clinical explanation for such a phenomenon is not clear and may relate to difficult to characterize idiosyncratic differences in planning for more irregularly shaped tumors that necessitate lower isodose line prescriptions for a given volume. In contrast to our findings, Shiue et al12 analyzed 496 brain metastases and found that prescription isodose line selection was not predictive of local failure. Of the covariates associated with LC, isodose was the least significantly correlated in our analysis and does not appear to have sound clinical rationale for manipulation in an effort to improve LC.
We also found that increasing brain metastasis volume is a risk factor for local failure. Such a phenomenon has been well described.3, 5, 9, 11, 13 RTOG protocol 90-05 established current single-fraction SRS prescription maximum tolerated dose guidelines based on tumor volume. In these guidelines, brain metastasis volume and maximum tolerated dose are inversely related because dose escalation in larger tumors was associated with increasing acute and chronic central nervous system toxicities. As such, it is not surprising that larger brain metastases have poor LC. To date, there has been no clear way to alter the SRS plan in a manner to safely improve LC while still respecting the need to paradoxically deliver smaller prescription doses as tumor volume increases.
Most notably, we found that doses in excess of prescription dose were highly predictive of LC, even after accounting for tumor volume and prescription dose. In fact, prescription dose was not significantly associated with LC in the final multivariate model. Although V32 was selected by the multivariate model, this was done purely through selection of the variable with the lowest P value, which by definition also comes at the expense of excluding other Vxx values. In fact, before selection of V32, all of the values ranging from V31-V35 had a P value <.001. Thus, the ultimate metric used to evaluate hotspots is less important than the phenomenon being described; that is, idiosyncratic differences in radiation planning leading to larger hotspots (“internal dose escalation”) within the target, even for a given prescription dose and volume of tumor, are associated with improved LC. Moreover, this increase in LC is not associated with a clear increase in radiation necrosis.
The underlying reason why larger hotspots increased LC is not well understood. It is possible that this observation is due to the underlying nature of Gamma Knife SRS treatment planning, in that the dosimetry is inherently inhomogeneous. It is possible areas of internal dose escalation lead to increased dose to high-risk, more radioresistant volumes within the tumor, such as the hypoxic subvolume. Interestingly, this was the hypothesized explanation for the decrease in local progression with Gamma Knife radiosurgery in RTOG 90-05. A similar theory has been postulated by Tome et al,14 who reported that boosting of a tumor subvolume has the potential to yield an increase in tumor control probability (TCP). They noted, however, that to achieve an increase in TCP a significant portion of the tumor (60%-80%) must be boosted. When specifically modeling hypoxic subvolumes, Popple et al15 noted that boosting modest volumes of geometrically stable hypoxia leads to significant increases in TCP. They noted that boost doses of 120% to 150% of the primary dose to hypoxic subvolume increased TCP to that found in the absence of permanent hypoxia. Building on these theories, Kim et al16 performed a prostate cancer planning study evaluating the use of selective high-risk subvolume boosting intensity modulated radiation therapy (IMRT) against that of homogenous dose escalation IMRT for plans delivering the same equivalent uniform dose. They demonstrated that selective high-risk area boosting IMRT increased the TCP without significant dose increases to surrounding normal structures as compared with dose-escalated IMRT. Similarly, using positron emission tomography (PET) imaging, Vanderstaeten et al17 performed a study in head and neck cancer comparing PET contour-based IMRT to that of PET voxel intensity-based IMRT. Using PET voxel-based intensity maps allows for dose escalation within the most radioresistant regions of the tumor. The planning study demonstrated that PET voxel intensity-based IMRT has the potential to improve LC without increasing toxicity to surrounding organs. Our findings require validation against underlying imaging data to examine whether there is a correlation between radioresistant subvolumes and that of internal dose escalation.
The principal limitation of our work is its single-institution, retrospective design; furthermore, our definition of LC may over-report failures. Last, at present, we have not yet explored the correlation between internal dose escalation and underlying radiomics, which may further explain our findings. Despite these limitations, however, we have identified specific factors correlated with individual tumor LC after SRS. These findings will aid in physician-patient discussion and counseling before treatment. Moreover, the finding that a larger hotspot leads to increased LC represents an actionable metric at the time of treatment planning, with little increase in treatment-related morbidity. These results merit validation in independent datasets using multiple treatment platforms or in future prospective studies.
Conclusions
In patients with NSCLC brain metastasis treated with single-fraction SRS, higher volume, lower isodose line, and lower V32 were independent predictors of local failure. V32 represents a potentially actionable metric that may be altered at the time of treatment planning to improve LC. These results warrant further investigation in future prospective studies.
Footnotes
Presented as an oral abstract presentation at the 2015 Annual Meeting, American Society for Radiation Oncology, San Antonio, Texas.
Conflict of interest: None.
References
- 1.Seigel R., Ma J., Zou Z., Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Nayak L., Lee E., Wen P. Epidemiology of brain metastasis. Curr Oncol Rep. 2012;14:48–54. doi: 10.1007/s11912-011-0203-y. [DOI] [PubMed] [Google Scholar]
- 3.Mamon H., Yeap B., Janne P. High risk of brain metastases in surgically staged IIIA nonsmall-cell lung cancer patients treated with surgery, chemotherapy, and radiation. J Clin Oncol. 2005;23:1530–1537. doi: 10.1200/JCO.2005.04.123. [DOI] [PubMed] [Google Scholar]
- 4.Kocher M., Soffietti R., Abacioglu U. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Regine W., Scott C., Murray K., Curran W. Neurocognitive outcome in brain metastasis patients treated with accelerated-fractionation vs. accelerated hyperfractionated radiotherapy: An analysis from radiation therapy oncology group study 91-04. Int J Radiat Oncol Biol Phys. 2001;51:711–717. doi: 10.1016/s0360-3016(01)01676-5. [DOI] [PubMed] [Google Scholar]
- 6.Li J., Benzten S., Li J., Renschler M., Mehta M. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int J Radiat Oncol Biol Phys. 2008;70:64–70. doi: 10.1016/j.ijrobp.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 7.Shiau C., Sneed P., Shu H. Radiosurgery for brain metastases: Relationship of dose and pattern of enhancement to local control. Int J Radiat Oncol Biol Phys. 1997;37:375–383. doi: 10.1016/s0360-3016(96)00497-x. [DOI] [PubMed] [Google Scholar]
- 8.Vogelbaum M., Angelov L., Lee S., Li L., Barnett G., Suh J. Local control of brain metastases by stereotactic radiosurgery in relation to dose to the tumor margin. J Neurosurg. 2006;104:907–912. doi: 10.3171/jns.2006.104.6.907. [DOI] [PubMed] [Google Scholar]
- 9.Shaw E., Scott C., Souhami L. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final Report of RTOG Protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47:291–298. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 10.Andrews D., Scott C., Sperduto P. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomized trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 11.Sheehan J., Sun M., Kondziolka D., Flickinger J., Lunsford L. Radiosurgery for non-small cell lung carcinoma metastatic to the brain: Long-term outcomes and prognostic factors influencing patient survival time and local tumor control. J Neurosurg. 2002;97:1276–1281. doi: 10.3171/jns.2002.97.6.1276. [DOI] [PubMed] [Google Scholar]
- 12.Shiue K., Barnett G., Suh J. Using higher isodose lines for gamma knife treatment of 1 to 3 brain metastases is safe and effective. Neurosurgery. 2014;74:360–365. doi: 10.1227/NEU.0000000000000289. [DOI] [PubMed] [Google Scholar]
- 13.Molenaar R., Wiggenraad R., Verbeek-de Kanter A., Walchenbach R., Vecht C. Relationship between volume, dose and local control in stereotactic radiosurgery of brain metastasis. Br J Neurosurg. 2009;23:170–178. doi: 10.1080/02688690902755613. [DOI] [PubMed] [Google Scholar]
- 14.Tome W., Fowler J. Selective boosting of tumor subvolumes. Int J Radiat Oncol Biol Phys. 2000;48:593–599. doi: 10.1016/s0360-3016(00)00666-0. [DOI] [PubMed] [Google Scholar]
- 15.Popple R., Ove R., Shen S. Tumor control probability for selective boosting of hypoxic subvolumes, including the effect of reoxygenation. Int J Radiat Oncol Biol Phys. 2002;54:921–927. doi: 10.1016/s0360-3016(02)03007-9. [DOI] [PubMed] [Google Scholar]
- 16.Kim Y., Tome W. Is it beneficial to selectively boost high-risk tumor subvolumes? A comparison of selectively boosting high-risk tumor subvolumes versus homogeneous dose escalation of the entire tumor based on equivalent EUD plans. Acta Oncol. 2008;47:906–916. doi: 10.1080/02841860701843050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanderstraeten B., Duthoy W., De Gersem W., De Neve W., Thierens H. 18F]fluoro-deoxy-glucose positron emission tomography ([18F]FDG-PET) voxel intensity-based intensity-modulated radiation therapy (IMRT) for head and neck cancer. Radiother Oncol. 2006;79:249–258. doi: 10.1016/j.radonc.2006.03.003. [DOI] [PubMed] [Google Scholar]