Abstract
Purpose
For high-risk prostate cancer (HR-PCa) in men with a life expectancy of at least 10 years, the National Comprehensive Cancer Network recommends radiation therapy (RT) plus androgen deprivation therapy (ADT) with category 1 evidence or radical prostatectomy (RP) as an acceptable initial therapy. Randomized evidence regarding which therapy is optimal for disease control is lacking for men with HR-PCa. We performed a propensity-score-matched comparison of outcomes for men with localized HR-PCa treated with primary RT or RP.
Methods and materials
The medical records of patients with localized HR-PCa who were treated at our institution between 2002 and 2011 were reviewed. Patient and disease characteristics, treatment details, and outcomes were collected. A combination of nearest-neighbor propensity score matching on age, Adult Comorbidity Evaluation-27 comorbidity index, prostate-specific antigen, biopsy Gleason scores, and clinical T-stage as well as exact matching on prostate-specific antigen, biopsy Gleason scores, and clinical T-stage was performed. Outcomes were measured from diagnosis. Multivariate Cox proportional hazards regression was used to compare metastasis-free and overall survival.
Results
A total of 246 patients were identified with 62 propensity-score-matched pairs. ADT was administered to 6.5% and 80.6% of patients receiving RP and RT, respectively. Five-year rates of metastasis for RP and RT were 33% and 8.9%, respectively (P = .003). Overall survival was not different. Delay of salvage therapy was longer for patients undergoing primary RT (P < .001). Findings were similar when only those patients who did not receive ADT were compared.
Conclusions
At our institution, treatment with primary RT resulted in superior metastasis-free survival over RP. This was not accompanied by an improvement in OS.
Summary.
The best upfront treatment for high-risk prostate cancer is unclear. We performed a propensity score-matched analysis of patients with high-risk prostate cancer treated with primary radiation therapy (RT) or primary radical prostatectomy and measured distant metastasis-free survival and overall survival. Distant metastasis-free survival was superior in the RT cohort compared with the radical prostatectomy cohort and overall survival was not different, suggesting that primary RT may result in delayed or overall lower development of metastatic disease.
Alt-text: Unlabelled box
Introduction
High-risk prostate cancer (HR-PCa) is defined by the National Comprehensive Cancer Center (NCCN) as adenocarcinoma of the prostate with one or more of the following high-risk features: initial prostate-specific antigen (PSA) >20 ng/mL, clinical T stage 3a or higher, and biopsy sum Gleason score (GS) ≥8. Despite controversy over active treatment versus conservative management for prostate cancer, patients with HR-PCa have a high rate of distant metastases and 15% to 30% cancer-specific death without primary treatment.1, 2 Therefore, the relevant question for this patient population is which primary treatment modality is the most appropriate and effective.
Per the NCCN guidelines, category 1 evidence supports the use of definitive radiation therapy (RT) plus long-term (defined as 2-3 years) androgen deprivation therapy (ADT). Radical prostatectomy (RP) plus pelvic lymph node dissection is also recommended for initial management of HR-PCa with category 2A evidence. Randomized evidence comparing RP or definitive RT as the primary treatment modality for localized prostate cancer is limited to a few small trials that did not meet accrual and therefore were not powered for primary endpoints including metastasis-free survival and cause-specific survival.3, 4, 5
The recently reported ProtecT trial randomized 1643 men with a new diagnosis of prostate cancer and a life expectancy of at least 10 years to undergo active monitoring, RP, or RT.6 The 10-year results of the trial showed no difference in prostate cancer–specific survival with any of the treatments and no difference in freedom from disease progression between RP or RT, which were both superior to active monitoring. Of the men enrolled on this trial, only 2% had high-risk disease as defined by the NCCN, so it is not clear how readily these findings can be applied to men with HR-PCa.7
Therefore, in the absence of meaningful prospective, randomized evidence, we performed a carefully matched paired comparison of patients treated for HR-PCa at our institution per NCCN treatment guidelines for either external beam RT with ADT or RP with adjuvant therapy, depending on pathologic features.
Methods and materials
Patients and treatments
Patients with HR-PCa as defined by the NCCN risk grouping who received either definitive RT or RP at our institution between 2001 and 2011 were identified. After institutional review board approval, medical records of these patients were reviewed for patient and disease characteristics and clinical outcomes including biochemical or clinical recurrence, development of metastatic disease, overall survival (OS), and salvage therapy. After comparison of the unmatched cohorts revealed a biased distribution of known adverse risk factors, a propensity-score-matching algorithm was applied as described in the Statistics section.
Survival was determined by a review of the chart and an Internet search for confirmation of death, including published obituaries and the social security death index. When it could be ascertained with certainty, the cause of death was included.
RP was either open retropubic or minimally invasive (i.e., laparoscopic or robotic-assisted). Lymph node dissection was performed in all cases and commonly limited to the obturator fossa.
Definitive RT was delivered using modern techniques of external beam RT to a median total dose of 75.6 Gy (range, 73.8-77.4 Gy). Intensity modulated RT was used in 60 of 62 patients using 18 MV x-rays. Two patients received 3-dimensional, conformal RT. Fifty-five patients were estimated to have ≥15% risk of pelvic lymph node involvement as determined by the Memorial Sloan Kettering Cancer Center pre-radical prostatectomy nomogram and received radiation to the pelvic lymph nodes followed by a sequential boost to the prostate. The remaining 7 patients received radiation to the prostate and seminal vesicles followed by a boost to the prostate. The planning target volume was determined by the mode of localization and has been described previously.8, 9
ADT was administered to a proportion of patients in each group. When delivered in conjunction with primary RT, ADT consisted of injectable luteinizing hormone releasing hormone administered beginning 2 months prior to the start of RT for a total of 2 years. Of the patients receiving upfront RP, 1 patient received adjuvant RT, 1 patient received adjuvant ADT, and 3 patients received adjuvant concurrent RT/ADT.
Statistics
A propensity-score-matching method was used to reduce the imbalance between the RP and RT cohorts. A combination of exact matching and nearest-neighbor matching was used. Exact matching was used on categorized primary prostate cancer risk stratification variables, including clinical T stage, PSA, and biopsy GS. Clinical T stage was categorized as T1, T2, and T3. PSA was categorized as <10, 10 to 20, and ≥20. Biopsy GS was categorized as Gleason 6 or 7, Gleason 8, and Gleason >8. The variables used in the nearest-neighbor matching process were numeric age in years and a categorized Adult Comorbidity Evaluation-27 (ACE-27) index score (a validated comorbidity index)10 with scores of 0, 1, and ≥2 to represent patient comorbidity. The entire matching process was done in R, Version 3.2.2 using the package MatchIt.11 Using the date of diagnosis as the starting time point, multivariate Cox proportional hazards regression was used to compare metastasis-free survival, OS, and time to salvage therapy.
Results
Patient characteristics
A total of 246 patients with HR-PCa treated with upfront RT (n = 86) or RP (n = 160) were identified. Patient characteristics are shown in Supplemental Table S1 (available as supplementary material online only at www.advancesradonc.org) and demonstrate an imbalance in known adverse risk factors including clinical T stage and present PSA in favor of RP. Therefore, we used a propensity-score-matching approach to identify a paired patient cohort to minimize these biases. The resulting propensity scores were well matched (Supplemental Table S2 and Supplemental Fig. S1).
As a result of the propensity-score matching, patient and disease features including age and ACE-27 comorbidity score were not significantly different, and initial PSA, biopsy GleasonGS, and clinical T stage were matched exactly (Table 1). Follow-up time and the interval from diagnosis to the start of RT or RP were both significantly longer for the RT group (mean: 41 vs 51.4 months [P = .004] and 56 vs 131.5 days [P < .001], respectively; Table 1). ADT was administered as a component of initial therapy to 6.5% and 80.6% of patients receiving RP and RT, respectively. The pathologic findings after RP are shown in Supplemental Table S4.
Table 1.
Patient characteristics
| RP (n = 62) | RT (n = 62) | P-value | |
|---|---|---|---|
| Clinical T stage | NA | ||
| T1 | 36 | 36 | |
| T2 | 23 | 23 | |
| T3 | 3 | 3 | |
| PSA | NA | ||
| <10 | 30 | 30 | |
| 10-20 | 5 | 5 | |
| >20 | 27 | 27 | |
| Biopsy Gleason score | NA | ||
| 6 or 7 | 17 | 17 | |
| 8 | 30 | 30 | |
| 9 or 10 | 15 | 15 | |
| ACE-27 | .52 | ||
| 0 | 15 | 10 | |
| 1 | 30 | 32 | |
| ≥2 | 17 | 20 | |
| Age (years) | .492 | ||
| Mean | 62.9 | 64.2 | |
| Standard deviation | 7.1 | 9.1 | |
| Follow-up (months)* | .004 | ||
| Median | 41 | 51.4 | |
| Standard deviation | 26.5 | 29.8 | |
| Days to Treatment from Dx | < .001 | ||
| Median | 56 | 131.5 | |
| Standard deviation | 29 | 299 |
ACE-27, Adult Comorbidity Evaluation-27; Dx, diagnosis; NA, not applicable; PSA, prostate-specific antigen; RP, radical prostatectomy; RT, radiation therapy.
Calculated from the completion of RT or date of RP.
Disease outcomes
Despite the more adverse features seen in patients treated with RT in the unmatched cohort of 246 patients, distant metastasis–free survival (DMFS) was significantly longer (hazard ratio [HR]: 0.27; 95% confidence interval [CI], 0.10-0.73; P = .01) and OS was not different (HR: 1.9; 95% CI, 0.83-4.32; P = .128). PSA >20 and biopsy GS of ≥8 was also associated with worse DMFS. Additional factors that were associated with worse OS included PSA >20 and biopsy GS >8 (Supplemental Table S3).
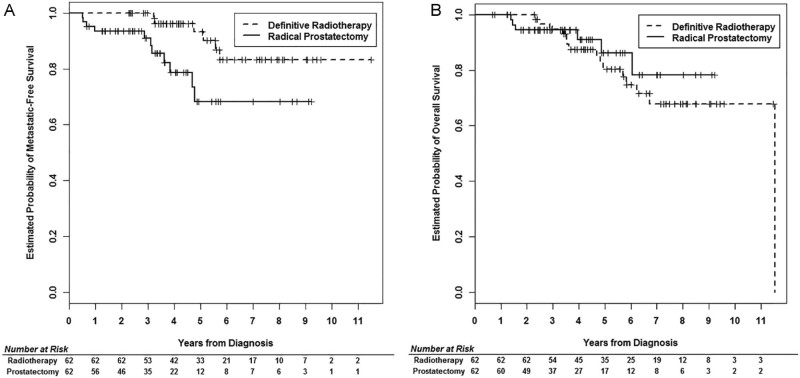
For the propensity-score-matched cohort of 124 patients, DMFS was again significantly longer for patients in the RT group compared with those in the RP group (HR: 0.023; 95% CI, 0.07-0.71; P = .011; Fig 1A). The only other clinical factor that was significantly associated with lower DMFS was PSA ≥20 ng/mL (Table 2). OS was not statistically different between the 2 groups (HR: 1.58; 95% CI, 0.56-4.48; P = .38; Fig 1B), and no single factor was significantly associated with OS in the Cox proportional hazards multivariate model (Table 2). After review of the medical charts, the cause of death was unknown for a large proportion of patients and was attributed to prostate cancer for 2 of 15 patients in the RT group and 2 of 6 patients in the RP group. Prostate cancer–specific death could not be compared given the scarcity of events. Crude biochemical failure rates were 21% and 58% after a median of 51.4 months and 41 months for RT and RP, respectively. Biochemical failure was not formally compared between the treatment groups.
Figure 1.
Propensity-score-matched outcomes. Kaplan Meier plot of (A) distant metastasis–free survival and (B) overall survival in matched patients undergoing RP or RT for primary treatment of high-risk prostate cancer.
Table 2.
Cox proportional hazards models
| Metastasis free survival |
Overall survival |
|||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value | |
| RT vs RP | 0.23 | 0.07-0.71 | .011 | 1.58 | 0.56-4.48 | .385 |
| Age | 1.00 | 0.93-1.07 | .949 | 1.02 | 0.97-1.07 | .532 |
| Clinical T Stage T2 vs T1 | 0.73 | 0.17-3.08 | .667 | 0.44 | 0.12-1.65 | .225 |
| Clinical T Stage T3 vs T1 | 1.68 | 0.35-8.02 | .515 | 0.48 | 0.06-4.17 | .507 |
| PSA 20+ vs <20 | 5.22 | 1.18-23.12 | .029 | 1.47 | 0.51-4.27 | .476 |
| Gleason score 8 vs 6-7 | 2.59 | 0.46-14.61 | .283 | 0.99 | 0.19-5.23 | .995 |
| Gleason score 8+ vs 6-7 | 3.53 | 0.66-18.96 | .142 | 4.22 | 0.86-20.7 | .076 |
| ACE 1 vs 0 | 1.04 | 0.30-3.62 | .956 | 0.70 | 0.19-2.6 | .590 |
| ACE 2+ vs 0 | 1.21 | 0.25-5.9 | .817 | 2.48 | 0.67-9.12 | .173 |
ACE, Adult Comorbidity Evaluation; CI, confidence interval; PSA, prostate-specific antigen; RP, radical prostatectomy; RT, radiation therapy.
Salvage therapies
Despite the worse adverse features for patients receiving RT in the unmatched cohort, salvage therapy administration was significantly lower (HR: 0.15; 95% CI, 0.07-0.33; P < .001). Age, pretreatment PSA >20, and ACE comorbidity score ≥2 were also associated with a higher risk of salvage treatment (Supplemental Table S3).
For the propensity-score-matched cohort, administration of salvage therapy was 16.1% in the RT group compared with 40.3% in the RP group (HR: 0.19; 95% CI, 0.08-0.43; P < .001). The median time to salvage therapy for the RT cohort was 36.3 months compared with 13.1 months for RP. Of 8 patients receiving salvage therapy in the RT group, 1 patient underwent salvage RP, and 7 patients received ADT. Of patients in the RP group, salvage therapy consisted of RT in 68% (most with concurrent ADT), ADT alone in 24%, and chemotherapy in 8%.
Patients treated without ADT
Because of the known benefit of ADT for delay in development of biochemical failure, development of distant metastases. and OS, we compared the 20% of patients who underwent RT without ADT to those who underwent RP without ADT and found no difference in OS (P = .098) or DM (P = .061) but a significantly lower risk of salvage therapy for patients undergoing RT compared with RP (P = .032; Supplemental Fig. S2).
Discussion
Selection of the best treatment option for patients with localized HR-PCa is important not only for optimal disease control, but also for avoiding undue therapy-related toxicity. In the absence of robust randomized data for these patients, many men undergo RP for high-risk disease despite the risk of adverse features on surgical pathology for which the NCCN recommends consideration of adjuvant RT.12 Moreover, between 32% and 70% of patients will experience biochemical failure after RP for high-risk prostate cancer and will thus be considered for salvage pelvic RT if their disease remains localized.13 In our patient population, 4 patients received adjuvant therapy and 23 patients (37%) received salvage therapy.
Although salvage therapy after either RP or RT is thought to be generally comparable, in any scenario, long-term salvage rates are generally low. Stephenson et al found that salvage RT after RP achieved 32% progression-free probability at 6 years, lower for men with pre-RT PSA of >1 ng/mL.14 Ongoing randomized trials comparing adjuvant RT for high-risk pathologic features to early-salvage RT ± ADT aim to minimize the number of patients requiring adjuvant RT without sacrificing disease control and survival.15, 16 With the recognition of suboptimal salvage rates, management for HR-PCa does appear to be evolving, and increased use of extended lymph node dissection17 and vigilant post-RP surveillance with serial ultrasensitive PSA may help improve rates of successful salvage.18
In the setting of salvage RT, efforts to improve success include trials testing concurrent and adjuvant short-term ADT with chemotherapy,19 pelvic lymph node RT,20 and hypofractionated RT.21 A phase 2 study of patients with localized failure after definitive RT for HR-PCa showed a 5-year biochemical relapse-free survival and distant metastases-free survival of 68.5% and 81.5%, respectively, after salvage with prostate brachytherapy.22, 23 Focal cryotherapy showed a 2-year freedom from biochemical recurrence for 44%,24, 25 and salvage prostatectomy resulted in approximately 45% of patients who were free from biochemical recurrence at a median of 34 months.26
Perhaps as important, all salvage therapies carry additional risk for toxicity, which often compound that experienced from the primary therapy.27, 28 In our analysis, 35% of men in the RP group received 2 local therapies (5 adjuvant and 17 salvage therapy for biochemical failure) compared with 1 patient in the RT cohort (salvage RP). If adjuvant RT had been administered to all patients with adverse pathologic features (≥pT3 disease, positive surgical margins), more patients in our study would have received adjuvant RT after RP. Toxicities from adjuvant or salvage therapy are additive, as demonstrated with significantly more patient-reported urinary toxicity and sexual dysfunction in men who require RT after prostatectomy.29
Here we show that both on initial comparison and after propensity score correction for age, clinical T stage, PSA, GS, and a comorbidity index, the rate of distant metastatic disease is higher in patients receiving RP as primary therapy compared with definitive RT. The follow-up for our cohort was relatively short (median: 5.2 years; alive without metastases: 5.6 years), and men with prostate cancer can experience recurrence decades after initial diagnosis.
More patients received long-term ADT as upfront therapy with definitive RT than with RP (80.6% vs 6.5%). We purposefully included patients who received ADT with RT to represent the standard of care for HR-PCa. Similarly, most patients who undergo RP for HR-PCa do not receive ADT as a part of initial management. This approach was also similar in the ProtecT trial design.7 Nevertheless, we believed it was important to remove this variable and repeated the analysis including only patients who did not receive ADT as part of upfront therapy. Of the 20% of patients who did not receive ADT with RT, no patients developed metastatic disease during the follow-up period (mean: 4.5 years for this subgroup; range, 2.4-8.9 years).
A limitation of this study is the small sample size available for analysis. For the initial nonmatched analysis, we included all patients with clinically HR-PCa treated at our institution with either RP or definitive RT; thus, the analysis is representative of treatment patterns at our institution.
Additionally, one might expect a decrease in the manifestation of metastatic disease with ADT on the order of 5% to 10% as seen in randomized trials, including Radiation Therapy Oncology Group 9202.30 However, we saw a 25% absolute lower rate of distant metastasis with RT, with only 80% of patients receiving ADT. If ADT is indeed responsible for the improved outcomes seen in our analysis, perhaps it should be considered for more patients undergoing RP for HR-PCa.
It is well-documented in nonrandomized settings that patients undergoing RP have less aggressive prostate cancer features, fewer comorbid conditions, and better health than men undergoing RT.31 In an attempt to control for possible imbalances in confounding comorbidities, we included the ACE Comorbidity Index in our propensity score calculation. Because men undergoing primary RT for prostate cancer as a whole have significantly more comorbid conditions that are expected to affect survival, we would expect worse OS in the group undergoing RT. However, we found that survival was not significantly different even among unmatched patient groups (Supplemental Table S3). In the matched comparison, although DMFS was significantly higher in patients treated with primary RT, OS was not. Prostate cancer metastases are strongly linked to prostate cancer–specific mortality32; however, the follow-up time of this study is relatively short and this, along with a small sample size and small event numbers, likely explain the lack of difference in OS.
Time from diagnosis to treatment was significantly longer in the RT group, which highlights the path that many patients take from primary care physician to urologist and eventually, in a proportion of patients, to a radiation oncologist. The connection between referral bias and treatment decision is well documented.33 One might assume that delayed treatment would be associated with worse outcomes; however, we observed the opposite. Moreover, we chose to calculate outcomes from the date of diagnosis to development of metastatic disease, salvage treatment, and death to account for any delay in treatment and for the inherent nature of RP versus RT delivered over approximately 8 weeks.
Given the retrospective nature of the study, toxicity related to either treatment could not be reliably assessed. This is an important consideration when comparing therapies and should be addressed ideally in a prospective manner.
In contrast to several prior studies comparing RP with RT for prostate cancer, the current study included contemporary treatment methods, with only 2 patients receiving <75 Gy RT (dose-escalation associated with superior local and biochemical control), 97% being treated with intensity modulated RT, and most patients receiving long-term ADT.
Conclusions
This propensity-score-matched analysis from a single institution using contemporary treatments corroborates prior reports that dose-escalated RT as primary treatment for HR-PCa is at least comparable with RP for disease control.34, 35, 36 Our data suggest that RT with concurrent/adjuvant ADT may be superior for preventing development of distant metastases. When considering the higher toxicity associated with RP followed by either adjuvant or salvage RT and the higher rates of salvage therapy overall, definitive dose-escalated RT and ADT may be an overall better primary therapy for men with high-risk prostate cancer.
Acknowledgements
The authors thank Jennifer German for administrative assistance in the preparation and submission of the manuscript.
Footnotes
Sources of support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest: The authors have no relevant conflicts of interest.
Supplementary material for this article (https://doi.org/10.1016/j.adro.2017.12.001) can be found at www.advancesradonc.org.
Supplementary data
The following is the supplementary data to this article:
Figures S1 and S2, Tables S1-S4.
References
- 1.Han M., Partin A.W., Pound C.R., Epstein J.I., Walsh P.C. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28:555–565. doi: 10.1016/s0094-0143(05)70163-4. [DOI] [PubMed] [Google Scholar]
- 2.D'Amico A.V., Whittington R., Malkowicz S.B. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 3.Lennernäs B., Majumder K., Damber J.E. Radical prostatectomy versus high-dose irradiation in localized/locally advanced prostate cancer: A Swedish multicenter randomized trial with patient-reported outcomes. Acta Oncol. 2015;54:875–881. doi: 10.3109/0284186X.2014.974827. [DOI] [PubMed] [Google Scholar]
- 4.Akakura K., Suzuki H., Ichikawa T. A randomized trial comparing radical prostatectomy plus endocrine therapy versus external beam radiotherapy plus endocrine therapy for locally advanced prostate cancer: Results at median follow-up of 102 months. Jpn J Clin Oncol. 2006;36:789–793. doi: 10.1093/jjco/hyl115. [DOI] [PubMed] [Google Scholar]
- 5.Wallace K., Fleshner N., Jewett M., Basiuk J., Crook J. Impact of a multi-disciplinary patient education session on accrual to a difficult clinical trial: The Toronto experience with the surgical prostatectomy versus interstitial radiation intervention trial. J Clin Oncol. 2006;24:4158–4162. doi: 10.1200/JCO.2006.06.3875. [DOI] [PubMed] [Google Scholar]
- 6.Hamdy F.C., Donovan J.L., Lane J.A. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415–1424. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
- 7.Lane J.A., Donovan J.L., Davis M. Active monitoring, radical prostatectomy, or radiotherapy for localised prostate cancer: Study design and diagnostic and baseline results of the ProtecT randomised phase 3 trial. Lancet Oncol. 2014;15:1109–1118. doi: 10.1016/S1470-2045(14)70361-4. [DOI] [PubMed] [Google Scholar]
- 8.Olsen J.R., Noel C.E., Baker K., Santanam L., Michalski J.M., Parikh P.J. Practical method of adaptive radiotherapy for prostate cancer using real-time electromagnetic tracking. Int J Radiat Oncol Biol Phys. 2012;82:1903–1911. doi: 10.1016/j.ijrobp.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michalski J.M., Lawton C., El Naqa I. Development of RTOG consensus guidelines for the definition of the clinical target volume for postoperative conformal radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;76:361–368. doi: 10.1016/j.ijrobp.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanabria A., Carvalho A.L., Vartanian J.G., Magrin J., Ikeda M.K., Kowalski L.P. Validation of the Washington University Head and Neck Comorbidity Index in a cohort of older patients. Arch Otolaryngol Head Neck Surg. 2008;134:603–607. doi: 10.1001/archotol.134.6.603. [DOI] [PubMed] [Google Scholar]
- 11.Stuart E.A., King G., Imai K., Ho D.E. MatchIT: Nonparametric preprocessing for parametric causal inference. Polit Anal. 2011;42(i08) [Google Scholar]
- 12.Prostate Cancer NCCN Evidence Blocks Version 2.2017. https://www.nccn.org/professionals/physician_gls/pdf/prostate_blocks.pdf Available at.
- 13.Cooper B.T., Sanfilippo N.J. Concurrent chemoradiation for high-risk prostate cancer. World J Clin Oncol. 2015;6:35–42. doi: 10.5306/wjco.v6.i4.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stephenson A.J., Scardino P.T., Kattan M.W. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25:2035–2041. doi: 10.1200/JCO.2006.08.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parker C., Clarke N., Logue J. RADICALS (radiotherapy and androgen deprivation in combination after local surgery) Clin Oncol (R Coll Radiol) 2007;19:167–171. doi: 10.1016/j.clon.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Australian New Zealand Clinical Trials Registry Radiotherapy following radical prostatectomy—Adjuvant versus early salvage. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=369003 Available at.
- 17.Harbin A.C., Eun D.D. The role of extended pelvic lymphadenectomy with radical prostatectomy for high-risk prostate cancer. Urol Oncol. 2015;33:208–216. doi: 10.1016/j.urolonc.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Rodin D., Drumm M., Clayman R. Risk factors for disease progression after postprostatectomy salvage radiation: Long-term results of a single-institution experience. Clin Genitourin Cancer. 2017 doi: 10.1016/j.clgc.2017.07.026. pii: S1558-7673(17)30236-7. [DOI] [PubMed] [Google Scholar]
- 19.Hurwitz M.D., Harris J., Sartor O. Adjuvant radiation therapy, androgen deprivation, and docetaxel for high-risk prostate cancer postprostatectomy: Results of NRG Oncology/RTOG study 0621. Cancer. 2017;123:2489–2496. doi: 10.1002/cncr.30620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.RTOG Clinical Trials, Study Number 0534. https://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0534 Available at.
- 21.Kruser T.J., Jarrard D.F., Graf A.K. Early hypofractionated salvage radiotherapy for postprostatectomy biochemical recurrence. Cancer. 2011;117:2629–2636. doi: 10.1002/cncr.25824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamada Y., Kollmeier M.A., Pei X. A Phase II study of salvage high-dose-rate brachytherapy for the treatment of locally recurrent prostate cancer after definitive external beam radiotherapy. Brachytherapy. 2014;13:111–116. doi: 10.1016/j.brachy.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lahmer G., Lotter M., Kreppner S., Fietkau R., Strnad V. Protocol-based image-guided salvage brachytherapy. Early results in patients with local failure of prostate cancer after radiation therapy. Strahlenther Onkol. 2013;189:668–674. doi: 10.1007/s00066-013-0373-7. [DOI] [PubMed] [Google Scholar]
- 24.Donnelly B.J., Saliken J.C., Ernst D.S. Role of transrectal ultrasound guided salvage cryosurgery for recurrent prostate carcinoma after radiotherapy. Prostate Cancer Prostatic Dis. 2005;8:235–242. doi: 10.1038/sj.pcan.4500811. [DOI] [PubMed] [Google Scholar]
- 25.Pisters L.L., von Eschenbach A.C., Scott S.M. The efficacy and complications of salvage cryotherapy of the prostate. J Urol. 1997;157:921–925. [PubMed] [Google Scholar]
- 26.Tefilli M.V., Gheiler E.L., Tiguert R. Salvage surgery or salvage radiotherapy for locally recurrent prostate cancer. Urology. 1998;52:224–229. doi: 10.1016/s0090-4295(98)00151-4. [DOI] [PubMed] [Google Scholar]
- 27.Bates A.S., Samavedi S., Kumar A. Salvage robot assisted radical prostatectomy: A propensity matched study of perioperative, oncological and functional outcomes. Eur J Surg Oncol. 2015;41:1540–1546. doi: 10.1016/j.ejso.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Matei D.V., Ferro M., Jereczek-Fossa B.A. Salvage radical prostatectomy after external beam radiation therapy: A systematic review of current approaches. Urol Int. 2015;94:373–382. doi: 10.1159/000371893. [DOI] [PubMed] [Google Scholar]
- 29.Daly T., Hickey B.E., Lehman M., Francis D.P., See A.M. Adjuvant radiotherapy following radical prostatectomy for prostate cancer. Cochrane Database Syst Rev. 2011;(12) doi: 10.1002/14651858.CD007234.pub2. CD007234. [DOI] [PubMed] [Google Scholar]
- 30.Horwitz E.M., Bae K., Hanks G.E. Ten-year follow-up of radiation therapy oncology group protocol 92-02: A phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26:2497–2504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 31.Markovina S., Michalski J. Counterpoint: Unfair comparisons lead to unwarranted conclusions-Can treatment modalities for localized prostate cancer truly be compared without bias? Brachytherapy. 2015;14:756–760. doi: 10.1016/j.brachy.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Popiolek M., Rider J.R., Andrén O. Natural history of early, localized prostate cancer: A final report from three decades of follow-up. Eur Urol. 2013;63:428–435. doi: 10.1016/j.eururo.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Jang T.L., Bekelman J.E., Liu Y. Physician visits prior to treatment for clinically localized prostate cancer. Arch Intern Med. 2010;170:440–450. doi: 10.1001/archinternmed.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stattin P., Holmberg E., Johansson J.-E. Outcomes in localized prostate cancer: National Prostate Cancer Register of Sweden follow-up study. J Natl Cancer Inst. 2010;102:950–958. doi: 10.1093/jnci/djq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boorjian S.A., Karnes R.J., Viterbo R. Long-term survival after radical prostatectomy versus external-beam radiotherapy for patients with high-risk prostate cancer. Cancer. 2011;117:2883–2891. doi: 10.1002/cncr.25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kibel A.S., Ciezki J.P., Klein E.A. Survival among men with clinically localized prostate cancer treated with radical prostatectomy or radiation therapy in the prostate specific antigen era. J Urol. 2012;187:1259–1265. doi: 10.1016/j.juro.2011.11.084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1 and S2, Tables S1-S4.