Abstract
Pancreatic arteriovenous malformation (pAVM) is a very rare entity, as less than 100 cases are reported in the international literature. Patients with pAVM may be asymptomatic or may present a wide range of symptoms, such as vague pain, feeling of fullness, gastrointestinal bleeding, or even portal hypertension. We present the multimodality approach in the diagnosis of a patient with pAVM and treatment via transcatheter arterial embolization of the lesion using steel coils. The patient was free of symptoms 12 months later.
Keywords: Pancreas, Arteriovenous malformation, Multimodality imaging
Introduction
Pancreatic arteriovenous malformation (pAVM) is defined as an abnormal vascular net, developed from one or more feeding arteries and enlarged early draining veins, creating an arteriovenous shunting.
The incidence of pAVM is extremely rare, with less than 100 reported cases in the international literature. Arteriovenous malformation (AVM) of the pancreas causes a wide range of symptoms such as vague pain, feeling of fullness, or even portal hypertension, gastrointestinal (GI) tract bleeding, and pancreatitis. Nevertheless, the majority of the patients remain asymptomatic.
This case describes the use of multimodality imaging including ultrasound (US), computed tomography (CT) scan, magnetic resonance imaging (MRI), and digital subtraction angiography (DSA) in the precise diagnosis of pAVM. Additionally, therapy of this entity via transcatheter arterial embolization (TAE) using steel coils is presented.
Case report
A 54-year-old man was referred to our hospital for evaluation of melena and vague pain at the right upper quadrant, after laparoscopic cholecystecomy 2 months ago and hospitalization for 2 weeks. Laboratory data revealed a normal hepatic and pancreatic function. The patient tested negative for hepatitis B and C viruses. Hemoglobin levels were 6.2 g/dL and the patient received blood transfusion.
Upper GI endoscopy revealed small red spots in the mucosa of the anterior wall of the second part of the duodenum but no active bleeding. Colonoscopy did not reveal any pathology. Transabdominal color Doppler ultrasound demonstrated an area of turbulent flow just lateral to the proximal superior mesenteric vein (Fig. 1), where an endoscopic ultrasound of the pancreas showed no pancreatic lesion. An abdominal CT revealed multiple vascular formations in the anatomic area between the uncinate process of the pancreas, the second part of the duodenum, and the superior mesenteric vein. The largest vascular formation drained into the superior mesenteric vein (Fig. 2, Fig. 3). Additional abdomen MRI showed multiple small flow voids in the area of the aforementioned lesion with mixed signal after gadolinium contrast injection (Fig. 4).
Fig. 1.
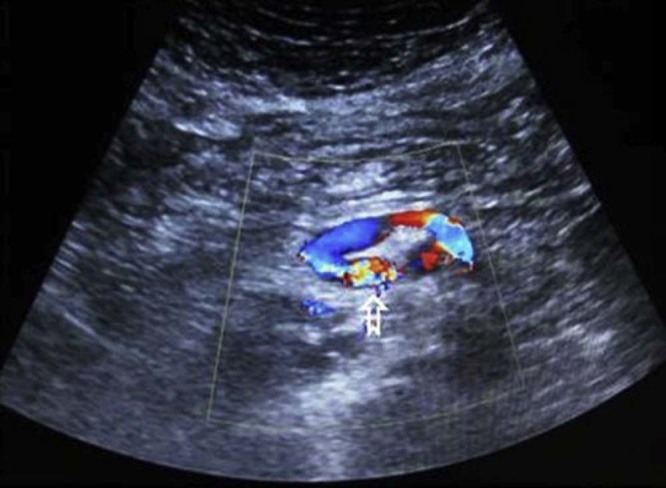
Color Doppler transabdominal ultrasound transverse image demonstrates a vessel with turbulent flow (open arrow) communicating with the proximal superior mesenteric vein.
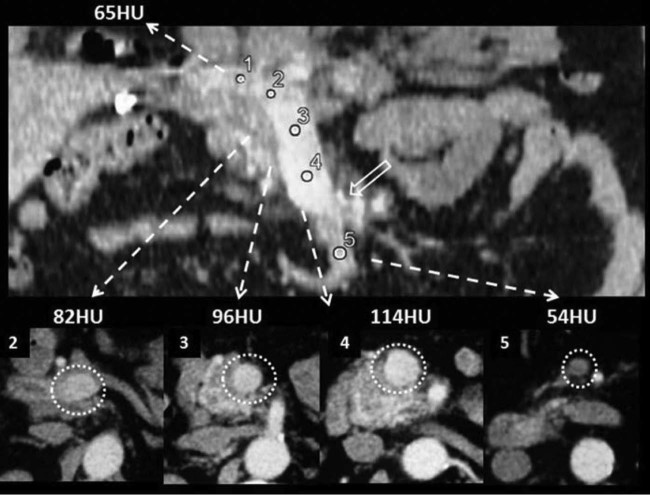
Fig. 2.
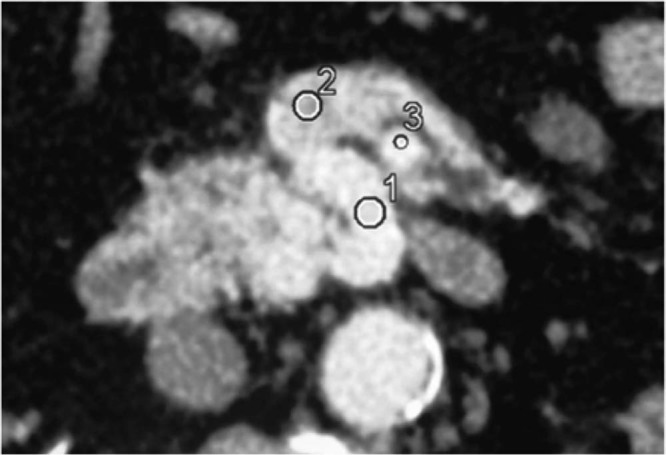
The pancreatic arteriovenous malformation is located in the anatomic area between the uncinate process of the pancreas and the second part of the duodenum. Note that the enlarged draining vein has a similar enhancement (1 = 129 HU) as the superior mesenteric artery (3 = 130 HU) has, and the superior mesenteric vein shows early opacification (2 = 79 HU), indicating the presence of an arteriovenous shunt.
Fig. 3.
Coronal and axial multiplanar reformation images show the early enhancement of the portal vein (measurement 1) and the superior mesenteric vein (measurements 2, 3, and 4) in the early arterial phase, because of the drainage of the afferent veins of the pancreatic arteriovenous malformation at the level of the arrows. Note the acute increase of Hounsfield units in the superior mesenteric vein (measurement 4) compared to the unenhanced superior mesenteric vein distally (measurement 5).
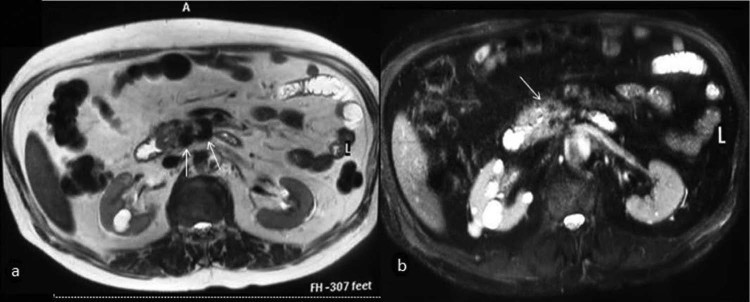
Fig. 4.
T2 TSE (a) and T2 fat-suppressed (b) axial images show multiple flow voids in the pancreatic head (arrows).
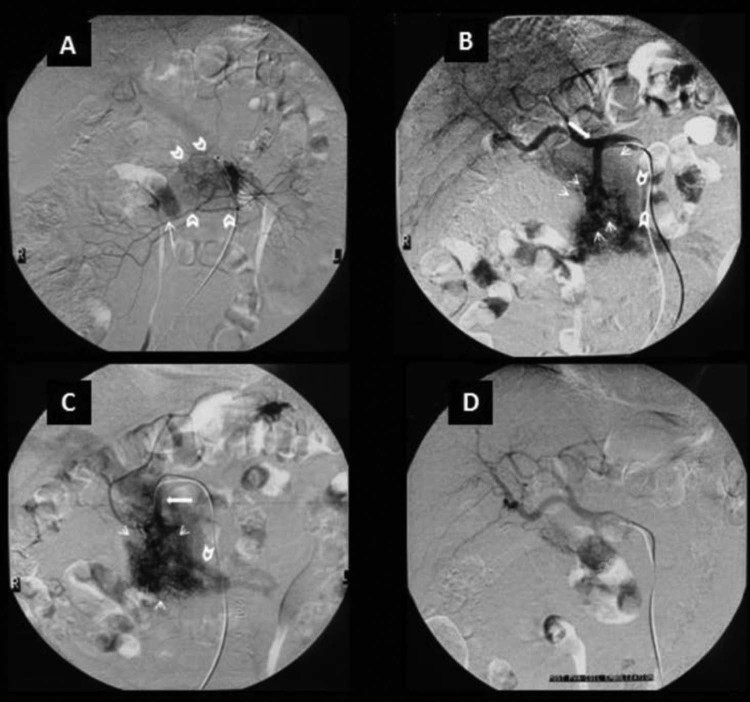
Finally, TAE was indicated for diagnostic and therapeutic reasons. DSA angiography revealed the vascular nidus supplied by branches of the gastroduodenal artery and an instant outline of the splenoportal axis (Fig. 5A-C). Embolization of the main feeding arteriole was performed. Postembolization infusion showed a smaller shunt between the arteriole and the splenic vein, which was treated with a coil placement, and no further venous enhancement was seen (Fig. 5D).
Fig. 5.
(A) Superior mesenteric angiogram depicts the nidus (open arrows), which consists of multiple shunting arterioles. Note early opacification of the portal vein (arrow) in the arterial phase. (B) Celiac angiogram demonstrating dilatation of the gastroduodenal artery (arrow) and a tightly packed mass of enlarged feeding arteries, which represent the pancreatic arteriovenous malformation (small arrows). Note the visible draining vein (open arrows). (C) Selective angiogram of the gastroduodenal artery shows that it is the main feeding artery. Note the early opacification of the superior mesenteric vein and of the portal vein. (D) Celiac angiogram post PVA embolization of the main feeding arteriole and after additional coil placement, showing a reduction of the nidus size and no early venous return to the portal system.
After 6 and 12 months of follow-up, the patient was asymptomatic.
Discussion
Although it can occur anywhere in the body, AVM of the GI tract and particularly in the pancreas is extremely rare. Only 0.9% of all GI vascular malformations are located in the pancreas [1] and 90% of them are classified as congenital. GI vascular malformations can be found sporadically, indicating a persistent remnant of the fetal pancreatic vascular network, or being part of the wide spectrum of hereditary hemorrhagic telangiectasia, also known as the Osler-Weber-Rendu syndrome [2]. Acquired pAVMs (10% of the cases), derive as a consequence of trauma, tumor, or inflammation of the pancreas [1]. Our patient did not reveal any other telangiectasia, and his personal and family histories were negative.
Although pAVM can occur everywhere in the pancreatic parenchyma, the commonest affected part is the head [3]. They tend to grow progressively in size. Large pAVMs usually lead to portal hypertension [4].
The splenic artery is most commonly involved in a pAVM (47%), followed by the gastroduodenal (22%) and small pancreatic arteries (25%) [5].
Small lesions do not cause symptoms. Larger lesions usually cause abdominal pain most possibly caused by steal syndrome as the pAVM shunts away the blood from the mesenteric circulation and, mainly, GI bleeding. In our case, the main cause of the patient's GI bleeding was erosion into the adjacent duodenum. Other causes of bleeding include the rupture of gastroesophageal varices caused by portal hypertension and the erosion of the AVM into the pancreatic duct or the bile ducts [6]. Symptoms usually occur in the fifth decade of life (mean age 49.8 years), and there is a clear male predilection [7].
US as part of the multimodality imaging is a first-line, cheap modality that may reveal the presence of small hypoechoic nodules in the pancreatic parenchyma, whereas the Doppler color flow detects the mosaic pattern of the arteriovenous shunt [8], the direction of the blood flow, and the relation of the pAVM to the portal vein [9].
Multimodality computed tomography (MDCT) may accurately, reveal the strong enhancement of multiple small hypervascular spots, which represent the discrete intrapancreatic vessels and, of course, the early contrast filling of the portal vein during the early arterial phase. Additionally, MDCT can reveal the relation of the lesion to the adjacent organs [7]. MRI depicts the “honeycomb”-like flow voids, demonstrating the abnormal vascular network, in all pulse sequences.
The differential diagnosis should include hypervascular pancreatic lesions, such as islet cell pancreatic tumors, cystadenocarcinoma, sarcoma, metastasis, and, rarely, chronic pancreatitis [10].
Angiography is the gold standard confirming the diagnosis of a pAVM and excluding any other possible differential diagnoses [11]. DSA depicts the complex intrapancreatic vascular network, the dilated tortues feeding arteries and the early draining veins. Furthermore, DSA can help determine, whether a pAVM will be treated via TAE and transjugular intrahepatic portosystemic shunts, or whether a surgical resection treatment will be preferred. TAE is recommended in small lesions with a few feeding arteries and no portal hypertension. On the other hand, surgical resection is advisable in larger pAVM lesions with multiple feeding arteries and drainage veins and when portal hypertension has already been established [12].
Conclusion
Pancreatic AVMs constitute rare entities. DSA remains the gold standard not only in the diagnosis but also in the treatment of pAVMs that have not yet caused portal hypertension like in our case. Nevertheless, radiologists must be familiar with imaging features of this entity in different imaging modalities.
References
- 1.Butte J.M., San Francisco I.F., Pacheco F., Solar A., Crovari F.J., Jarufe N.P. Arteriovenous malformation of the pancreas: report of a case. Surg Today. 2007;37:604–607. doi: 10.1007/s00595-006-3459-3. [DOI] [PubMed] [Google Scholar]
- 2.Nishiyama R., Kawanishi Y., Mitsuhashi H., Kanai T., Ohba K., Mori T. Management of pancreatic arteriovenous malformation. J Hepatobiliary Pancreat Surg. 2000;7(4):438–442. doi: 10.1007/s005340070041. [DOI] [PubMed] [Google Scholar]
- 3.Sharma M., Bedi M.M.S., Mahesh S., Gandhi M.D., Antony R., Mukkada R.J. Arteriovenous malformation of the pancreatic head—difficulties in diagnosis and treatment. Indian J Gastroenterol. 2011;30(1):46–48. doi: 10.1007/s12664-010-0070-8. [DOI] [PubMed] [Google Scholar]
- 4.Makhoul F., Kaur P., Johnston T.D., Jeon H., Gedaly R., Ranjan D. Arteriovenous malformation of the pancreas: a case report and review of literature. Int J Angiol. 2008;17(4):211–213. doi: 10.1055/s-0031-1278312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang S., Lim H.K., Lee W.J., Choi D., Jang K.-T. Arteriovenous malformation of the pancreas in a patient with gastrointestinal bleeding: helical CT findings. Abdom Imaging. 2004;29(2):259–262. doi: 10.1007/s00261-003-0093-z. [DOI] [PubMed] [Google Scholar]
- 6.Aida K., Nakamura H., Kihara Y., Abe S., Okamoto K., Otsuki M. Duodenal ulcer and pancreatitis associated with pancreatic arteriovenous malformation. Eur J Gastroenterol Hepatol. 2002;14(5):551–554. doi: 10.1097/00042737-200205000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Song K.B., Kim S.C., Park J.B., Kim Y.H., Jung Y.S., Kim M.H. Surgical outcomes of pancreatic arteriovenous malformation in a single center and review of literature. Pancreas. 2012;41(3):388–396. doi: 10.1097/MPA.0b013e31822a25cc. [DOI] [PubMed] [Google Scholar]
- 8.Koito K., Namieno T., Nagakawa T., Ichimura T., Hirokawa N., Mukaiya M. Congenital arteriovenous malformation of the pancreas: its diagnostic features on images. Pancreas. 2001;22:267–273. doi: 10.1097/00006676-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Kurosaki M., Hattori K., Minato Y., Shiigai T., Ohashi I., Umehara I. Asymptomatic arteriovenous malformation of the pancreas. Demonstration by Doppler ultrasonography and magnetic resonance imaging. Dig Dis Sci. 1993;38(7):1342–1346. doi: 10.1007/BF01296088. [DOI] [PubMed] [Google Scholar]
- 10.Shearer D.D., Demos T.C., Sichlau M.J. Pancreatic Arteriovenous Malformation: a case report and literature review. J Radiol Case Rep. 2011;5(8):8–13. doi: 10.3941/jrcr.v5i8.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanno A., Satoh K., Kimura K., Masamune A., Asakura T., Egawa S. Acute pancreatitis due to pancreatic arteriovenous malformation: 2 case reports and review of the literature. Pancreas. 2006;32(4):422–425. doi: 10.1097/01.mpa.0000220869.72411.39. [DOI] [PubMed] [Google Scholar]
- 12.Takemoto I., Tsuda M., Yano Y., Miyazaki H., Yamada H., Azuma T. Pancreatic arteriovenous malformation combined with portal thrombosis. Intern Med. 2007;46:233–236. doi: 10.2169/internalmedicine.46.1852. [DOI] [PubMed] [Google Scholar]